Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells
Abstract
:1. Introduction
2. Hypothesis
3. Potential Sources of Human STZ Exposure
3.1. STZ-Producing Bacteria
3.2. Gut Microbiota
3.3. Environmental Sources of STZ
3.4. Opportunistic Infections with STZ-Producing Bacteria
4. STZ Can Readily Induce Autoimmune Diabetes in Animal Models
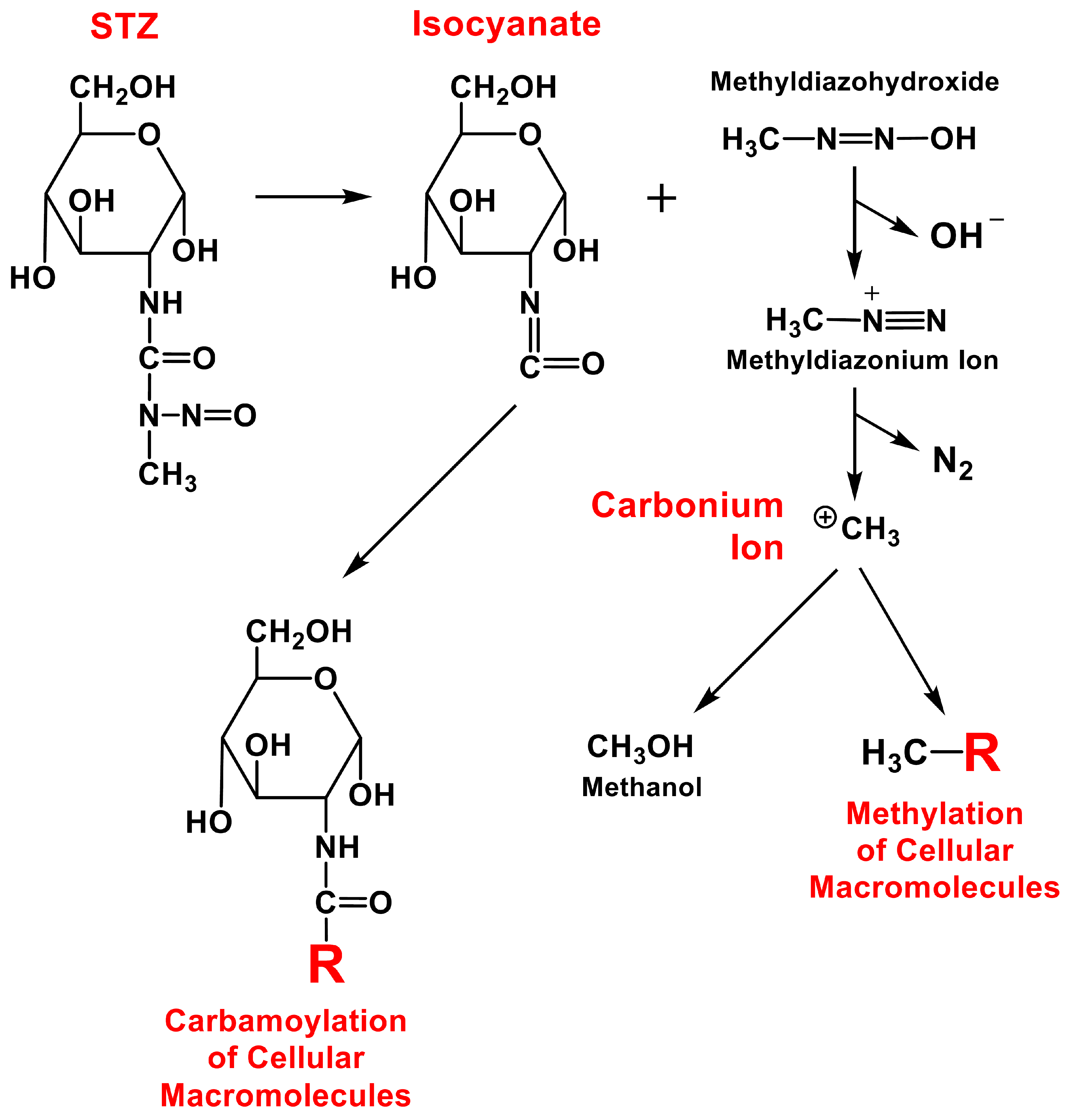
5. STZ Can Covalently Modify Cellular Components to Form Autoimmune Antigens
5.1. Covalent Modifications of Cellular DNA
5.2. Covalent Modifications of Cellular Proteins
5.2.1. Possibility 1. STZ and the O-Glycosylation Process in Islet β-Cells
5.2.2. Possibility 2. STZ May Covalently Modify GLUT2
6. STZ Can Induce Cellular Immune Responses in Pancreatic Islets
6.1. Observations Made in Immune Function-Intact Laboratory Animals
6.2. Observations Made in Immunodeficient Mice
6.3. Insights Gained from Transplantation Studies
7. STZ Can Induce the Production of Autoantibodies against Cellular Components of Pancreatic Islets
7.1. Clinically Identified T1DM-Related Autoantibodies
7.2. Antibodies Identified in STZ-Induced Diabetic Animal Models
8. Environmental and Genetic Factors Affecting the Development of T1DM
8.1. Environmental Factors
8.2. Genetic Factors
9. Other Potential Factors Related to T1DM
9.1. Potential Role of Gut Microbiota in T1DM
9.2. Potential Relationship between Cow’s Milk Feeding and Autoimmune T1DM
9.3. A Potential Strategy for Reducing the Risk of Developing T1DM
10. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes–2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knip, M.; Siljander, H. Autoimmune mechanisms in type 1 diabetes. Autoimmun. Rev. 2008, 7, 550–557. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Insulin Resistance in type 2 diabetes. In Textbook of Diabetes, 4th ed.; Holt, R.I.G., Cockram, C.S., Flyvbjerg, A., Goldstein, B.J., Eds.; Blackwell Pubishing Ltd.: Chichester, UK, 2010; pp. 174–190. [Google Scholar]
- Garrison, A. Screening, diagnosis, and management of gestational diabetes mellitus. Am. Fam. Physician 2015, 91, 460–467. [Google Scholar]
- Spaight, C.; Gross, J.; Horsch, A.; Puder, J.J. Gestational Diabetes Mellitus. Endocr. Dev. 2016, 31, 163–178. [Google Scholar]
- Forlenza, G.P.; Moran, A.; Nathan, B. Other Specific Types of Diabetes. In Diabetes in America, 3rd ed.; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567985/ (accessed on 2 January 2022).
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar]
- Rewers, M.; Ludvigsson, J. Genetic risk factors for type 1 diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef] [Green Version]
- Vavra, J.J.; Deboer, C.; Dietz, A.; Hanka, L.J.; Sokolski, W.T. Streptozotocin, a new antibacterial antibiotic. Antibiot. Annu. 1959, 7, 230–235. [Google Scholar]
- Conville, P.S.; Witebsky, F.G. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and Other Aerobic Actinomycetes. In Manual of Clinical Microbiology, 11th ed.; Jorgensen, J.H., Carroll, K.C., Funke, G., Pfaller, M.A., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 504–535. [Google Scholar]
- Hopwood, D.A. Streptomyces in Nature and Medicine: The Antibiotic Makers; Oxford University Press: Oxford, UK, 2007; ISBN 978-0-19-515066-7. [Google Scholar]
- Kämpfer, P. The family Streptomycetaceae, part I: Taxonomy. Prokaryotes 2006, 3, 538–604. [Google Scholar]
- Korn-Wendish, F. The family streptomycetaceae. In The Prokaryotes: A Handbook on the Biology of Bacteria, Ecophysiology, Identification, Applications; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Schnedl, W.J.; Ferber, S.; Johnson, J.H.; Newgard, C.B. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 1994, 43, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gleichmann, H. GLUT2 in pancreatic islets: Crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes 1998, 47, 50–56. [Google Scholar] [CrossRef]
- Murray-Lyon, I.M.; Eddleston, A.L.; Williams, R.; Brown, M.; Hogbin, B.M.; Bennett, A.; Edwards, J.C.; Taylor, K.W. Treatment of multiple-hormone-producing malignant islet cell tumour with streptozotocin. Lancet 1969, 2, 895–898. [Google Scholar]
- Brentjens, R.; Saltz, L. Islet cell tumors of the pancreas: The medical oncologist’s perspective. Surg. Clin. N. Am. 2001, 81, 527–542. [Google Scholar] [CrossRef]
- Bolzán, A.D.; Bianchi, M.S. Genotoxicity of streptozotocin. Mutat. Res. 2002, 512, 121–134. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Wu, K.K.; Huan, Y. Diabetic atherosclerosis mouse models. Atherosclerosis 2007, 191, 241–249. [Google Scholar] [CrossRef]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Like, A.A.; Rossini, A.A. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science 1976, 193, 415–417. [Google Scholar] [CrossRef]
- Gäbel, H.; Bitter-Suermann, H.; Henriksson, C.; Säve-Söderbergh, J.; Lundholm, K.; Brynger, H. Streptozotocin diabetes in juvenile pigs. Evaluation of an experimental model. Horm. Metab. Res. 1985, 17, 275–280. [Google Scholar] [CrossRef]
- Roep, B.O. The role of T-cells in the pathogenesis of Type 1 diabetes: From cause to cure. Diabetologia 2003, 46, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.P.; Stolp, H.; Trüper, H.G.; Balows, A.; Schlegel, H.G. The Prokaryotes: A Handbook on Habitats, Isolation and Identification of Bacteria; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Sommer, F.; Backhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Klatter, F.A.; Visser, J.T.; Wildeboer-Veloo, A.C.; Harmsen, H.J.; Rozing, J.; Bos, N.A. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 2006, 49, 2105–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef]
- Valladares, R.; Sankar, D.; Li, N.; Williams, E.; Lai, K.K.; Abdelgeliel, A.S.; Gonzalez, C.F.; Wasserfall, C.H.; Larkin, J.; Schatz, D.; et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS ONE 2010, 5, e10507. [Google Scholar] [CrossRef]
- Maclaren, N.K.; Atkinson, M.A. Insulin-dependent diabetes mellitus: The hypothesis of molecular mimicry between islet cell antigens and microorganisms. Mol. Med. Today 1997, 3, 76–83. [Google Scholar] [CrossRef]
- Virtanen, S.M.; Räsänen, L.; Ylönen, K.; Aro, A.; Clayton, D.; Langholz, B.; Pitkäniemi, J.; Savilahti, E.; Lounamaa, R.; Tuomilehto, J.; et al. Early introduction of dairy products associated with increased risk of IDDM in Finnish children. The Childhood in Diabetes in Finland Study Group. Diabetes 1993, 42, 1786–1790. [Google Scholar] [CrossRef]
- Patelarou, E.; Girvalaki, C.; Brokalaki, H.; Patelarou, A.; Androulaki, Z.; Vardavas, C. Current evidence on the associations of breastfeeding, infant formula, and cow’s milk introduction with type 1 diabetes mellitus: A systematic review. Nutr. Rev. 2012, 70, 509–519. [Google Scholar] [CrossRef]
- Dunne, E.F.; Burman, W.J.; Wilson, M.L. Streptomyces pneumonia in a patient with human immunodeficiency virus infection: Case report and review of the literature on invasive Streptomyces infections. Clin. Infect. Dis. 1998, 27, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.J.; Ali, S.T.; Weinbaum, D.; Goldberg, E. Streptomyces infection in AIDS presenting with pneumonia and monarthritis. Infect. Dis. Clin. Pract. 1996, 5, 207. [Google Scholar] [CrossRef]
- Kapadia, M.; Rolston, K.V.; Han, X.Y. Invasive Streptomyces infections: Six cases and literature review. Am. J. Clin. Pathol. 2007, 127, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Caron, F. Streptomyces sp as a cause of nodular pneumonia in a HIV infected patient? Med. Microbiol. Lett. 1992, 1, 297–303. [Google Scholar]
- Riviere, E.; Neau, D.; Roux, X.; Lippa, N.; Roger-Schmeltz, J.; Mercie, P.; Longy-Boursier, M. Pulmonary streptomyces infection in patient with sarcoidosis, France, 2012. Emerg. Infect. Dis. 2012, 18, 1907. [Google Scholar] [CrossRef]
- Wilson, G.; Leiter, E. Streptozotocin interactions with pancreatic β cells and the induction of insulin-dependent diabetes. In The Role of Viruses and the Immune System in Diabetes Mellitus; Springer: Berlin/Heidelberg, Germany, 1990; pp. 27–54. [Google Scholar]
- Wilson, G.L.; Hartig, P.C.; Patton, N.J.; LeDoux, S.P. Mechanisms of nitrosourea-induced β-cell damage: Activation of poly (ADP-ribose) synthetase and cellular distribution. Diabetes 1988, 37, 213–216. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Nahdi, A.M.T.A.; John, A.; Raza, H. Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β-cells. Oxid. Med. Cell. Longev. 2017, 2017, 7054272. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.T.; Steinberg, C. Immunologic studies on the induction of diabetes in experimental animals: Cellular basis for the induction of diabetes by streptozotocin. Diabetes 1984, 33, 771–777. [Google Scholar] [CrossRef]
- Szkudelski, T.; Zywert, A.; Szkudelska, K. Metabolic disturbances and defects in insulin secretion in rats with streptozotocin-nicotinamide-induced diabetes. Physiol. Res. 2013, 62, 663–670. [Google Scholar] [CrossRef]
- Wang-Fischer, Y.; Garyantes, T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef]
- Ar’Rajab, A.; AhréN, B. Long-term diabetogenic effect of streptozotocin in rats. Pancreas 1993, 8, 50–57. [Google Scholar] [CrossRef]
- Howard, C.F. Nonhuman primates as models for the study of human diabetes mellitus. Diabetes 1982, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tancrede, G.; Rousseau-Migneron, S.; Nadeau, A. Long-term changes in the diabetic state induced by different doses of streptozotocin in rats. Br. J. Exp. Pathol. 1983, 64, 117. [Google Scholar] [PubMed]
- Schlosser, M.J.; Kapeghian, J.C.; Verlangieri, A.J. Selected physical and biochemical parameters in the streptozotocin-treated guinea pig: Insights into the diabetic guinea pig model. Life Sci. 1987, 41, 1345–1353. [Google Scholar] [CrossRef]
- Wilson, J.D.; Dhall, D.P.; Simeonovic, C.J.; Lafferty, K.J. Induction and management of diabetes mellitus in the pig. Aust. J. Exp. Biol. Med. Sci. 1986, 64, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kaminski, N.E.; Fischer, L.J. Examination of the immunosuppressive effect of Δ9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes. Int. Immunopharmacol. 2001, 1, 699–712. [Google Scholar] [CrossRef]
- Dufrane, D.; van Steenberghe, M.; Guiot, Y.; Goebbels, R.M.; Saliez, A.; Gianello, P. Streptozotocin-induced diabetes in large animals (pigs/primates): Role of GLUT2 transporter and β-cell plasticity. Transplantation 2006, 81, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.I.; Peterson, R.G.; Stecker, M.S.; Patel, N.H.; Willis, L.R.; Galley, P.; Eclavea, A.C.; Dreesen, R.G. Suprarenal intraarterial infusion of alloxan and streptozotocin during balloon occlusion of the juxtarenal abdominal aorta: A simple technique for inducing diabetes mellitus in canines with reduced mortality. Acad. Radiol. 2001, 8, 473–477. [Google Scholar] [CrossRef]
- Lazarus, S.S.; Shapiro, S.H. Streptozotocin-induced diabetes and islet cell alterations in rabbits. Diabetes 1972, 21, 129–137. [Google Scholar] [CrossRef]
- Gerling, I.C.; Friedman, H.; Greiner, D.L.; Shultz, L.D.; Leiter, E.H. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes 1994, 43, 433–440. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pipeleers, D.G.; Ling, Z.; Welsh, N.; Hellerström, C.; Andersson, A. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc. Natl. Acad. Sci. USA 1994, 91, 9253–9256. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wright, J.R. Human β cells are exceedingly resistant to streptozotocin in vivo. Endocrinology 2002, 143, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, L.J.; van de Bunt, M.; Braun, M.; Frayn, K.N.; Clark, A.; Gloyn, A.L. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: Implications for understanding genetic association signals at this locus. Mol. Genet. Metab. 2011, 104, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.; Bhonde, R.R. Combined effect of nicotinamide and streptozotocin on diabetic status in partially pancreatectomized adult BALB/c mice. Horm. Metab. Res. 2000, 32, 330–334. [Google Scholar] [CrossRef]
- LeDoux, S.; Woodley, S.; Patton, N.; Wilson, G. Mechanisms of nitrosourea-induced β-cell damage: Alterations in DNA. Diabetes 1986, 35, 866–872. [Google Scholar] [CrossRef]
- Lampasona, V.; Liberati, D. Islet autoantibodies. Curr. Diab. Rep. 2016, 16, 53. [Google Scholar] [CrossRef]
- Konrad, R.J.; Kudlow, J.E. The role of O-linked protein glycosylation in β-cell dysfunction. Int. J. Mol. Med. 2002, 10, 535–539. [Google Scholar]
- Hanover, J.A.; Lai, Z.; Lee, G.; Lubas, W.A.; Sato, S.M. Elevated O-linked N-acetylglucosamine metabolism in pancreatic β-cells. Arch. Biochem. Biophys. 1999, 362, 38–45. [Google Scholar] [CrossRef]
- Elsner, M.; Guldbakke, B.; Tiedge, M.; Munday, R.; Lenzen, S. Relative importance of transport and alkylation for pancreatic β-cell toxicity of streptozotocin. Diabetologia 2000, 43, 1528–1533. [Google Scholar] [CrossRef] [Green Version]
- Gai, W.; Schott-Ohly, P.; Walde, S.S.; Gleichmann, H. Differential target molecules for toxicity induced by streptozotocin and alloxan in pancreatic islets of mice in vitro. Exp. Clin. Endocrinol. Diabetes 2004, 112, 29–37. [Google Scholar] [CrossRef]
- Van De Winkel, M.; Smets, G.; Gepts, W.; Pipeleers, D. Islet cell surface antibodies from insulin-dependent diabetics bind specifically to pancreatic B cells. J. Clin. Investig 1982, 70, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kniola, B.; Hellström, S.; Ludvigsson, J. Islet cell surface antibodies (ICSA) do bind to human pancreas: Computerised, quantitative determination of ICSA using both pre- and postembedding immunocolloidal techniques. Diabetes Res. Clin. Pract. 1988, 39, 173–183. [Google Scholar] [CrossRef]
- Stošić-Grujičić, S.; Maksimović, D.; Badovinac, V.; Samardžić, T.; Trajković, V.; Lukić, M.; Stojković, M.M. Antidiabetogenic effect of pentoxifylline is associated with systemic and target tissue modulation of cytokines and nitric oxide production. J. Autoimmun. 2001, 16, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Esensten, J.H.; Lee, M.R.; Glimcher, L.H.; Bluestone, J.A. T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J. Immunol. 2009, 183, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Weide, L.G.; Lacy, P.E. Low-dose streptozocin-induced autoimmune diabetes in islet transplantation model. Diabetes 1991, 40, 1157–1162. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Richardson, C.C.; Ravishankar, A.; Brigatti, C.; Liberati, D.; Lampasona, V.; Piemonti, L.; Morgan, D.; Feltbower, R.G.; Christie, M.R. Identification of tetraspanin-7 as a target of autoantibodies in type 1 diabetes. Diabetes 2016, 65, 1690–1698. [Google Scholar] [CrossRef] [Green Version]
- Krischer, J.P.; Lynch, K.F.; Schatz, D.A.; Ilonen, J.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; Simell, O.G.; Toppari, J. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: The TEDDY study. Diabetologia 2015, 58, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet 2001, 358, 221–229. [Google Scholar] [CrossRef]
- Ilonen, J.; Hammais, A.; Laine, A.-P.; Lempainen, J.; Vaarala, O.; Veijola, R.; Simell, O.; Knip, M. Patterns of β cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013, 62, 3636–3640. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-W.; Maclaren, N.K. Antibodies to nucleic acids in juvenile-onset diabetes. Diabetes 1978, 27, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Aitken, R.J.; Wilson, I.; Williams, A.J.; Bingley, P.J. Early onset of diabetes in the proband is the major determinant of risk in HLA DR3-DQ2/DR4-DQ8 siblings. Diabetes 2014, 63, 1041–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, T.A.; Ide, A.; Jahromi, M.M.; Barker, J.M.; Fernando, M.S.; Babu, S.R.; Yu, L.; Miao, D.; Erlich, H.A.; Fain, P.R.; et al. Extreme genetic risk for type 1A diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 14074–14079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, T.A.; Ide, A.; Humphrey, K.; Barker, J.M.; Steck, A.; Erlich, H.A.; Yu, L.; Miao, D.; Redondo, M.J.; McFann, K.; et al. Genetic prediction of autoimmunity: Initial oligogenic prediction of anti-islet autoimmunity amongst DR3/DR4-DQ8 relatives of patients with type 1A diabetes. J. Autoimmun. 2005, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Taylor, G. Immune insulitis and antibodies to nucleic acids induced with streptozotocin in mice. Clin. Exp. Immunol. 1981, 43, 425. [Google Scholar] [PubMed]
- Op de Beeck, A.; Eizirik, D.L. Viral infections in type 1 diabetes mellitus—Why the β cells? Nat. Rev. Endocrinol. 2016, 12, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Lönnrot, M.; Lynch, K.F.; Larsson, H.E.; Lernmark, Å.; Rewers, M.J.; Törn, C.; Burkhardt, B.R.; Briese, T.; Hagopian, W.A.; She, J.-X. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: The TEDDY study. Diabetologia 2017, 60, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Yki-Järvinen, H.; Sammalkorpi, K.; Koivisto, V.A.; Nikkilä, E.A. Severity, duration, and mechanisms of insulin resistance during acute infections. J. Clin. Endocrinol. Metab. 1989, 69, 317–323. [Google Scholar] [CrossRef]
- Clark, A.L.; Urano, F. Endoplasmic reticulum stress in beta cells and autoimmune diabetes. Curr. Opin. Immunol. 2016, 43, 60–66. [Google Scholar] [CrossRef] [Green Version]
- van Lummel, M.; Duinkerken, G.; van Veelen, P.A.; de Ru, A.; Cordfunke, R.; Zaldumbide, A.; Gomez-Touriño, I.; Arif, S.; Peakman, M.; Drijfhout, J.W. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes 2014, 63, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Oikarinen, S.; Martiskainen, M.; Tauriainen, S.; Huhtala, H.; Ilonen, J.; Veijola, R.; Simell, O.; Knip, M. Hyöty H: Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 2011, 60, 276–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, W.-C.G.; Rawlinson, W.D.; Craig, M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ 2011, 342, d35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knip, M. Environmental triggers and determinants of β cell autoimmunity and type 1 diabetes. Rev. Endocr. Metab. Disorders 2003, 4, 213–223. [Google Scholar] [CrossRef]
- Concannon, P.; Rich, S.S.; Nepom, G.T. Genetics of type 1A diabetes. N. Engl. J. Med. 2009, 360, 1646–1654. [Google Scholar] [CrossRef] [Green Version]
- Todd, J.A.; Walker, N.M.; Cooper, J.D.; Smyth, D.J.; Downes, K.; Plagnol, V.; Bailey, R.; Nejentsev, S.; Field, S.F.; Payne, F. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 2007, 39, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Jeffrey, J.; Fain, P.R.; Eisenbarth, G.S.; Orban, T. Concordance for islet autoimmunity among monozygotic twins. N. Engl. J. Med. 2008, 359, 2849–2850. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A. Immunogenetics of type 1 diabetes: A comprehensive review. J. Autoimmun. 2015, 64, 101–112. [Google Scholar] [CrossRef]
- Pociot, F.; Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 2016, 387, 2331–2339. [Google Scholar] [CrossRef]
- Frederiksen, B.N.; Steck, A.K.; Kroehl, M.; Lamb, M.M.; Wong, R.; Rewers, M.; Norris, J.M. Evidence of stage- and age-related heterogeneity of non-HLA SNPs and risk of islet autoimmunity and type 1 diabetes: The diabetes autoimmunity study in the young. Clin. Dev. Immunol. 2013, 2013, 417657. [Google Scholar] [CrossRef]
- Roesch, L.F.; Lorca, G.L.; Casella, G.; Giongo, A.; Naranjo, A.; Pionzio, A.M.; Li, N.; Mai, V.; Wasserfall, C.H.; Schatz, D.; et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J. 2009, 3, 536–548. [Google Scholar] [CrossRef] [Green Version]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuno, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Savilahti, E.; Saukkonen, T.T.; Virtala, E.T.; Tuomilehto, J.; Akerblom, H.K. Increased levels of cow’s milk and β-lactoglobulin antibodies in young children with newly diagnosed IDDM. The Childhood Diabetes in Finland Study Group. Diabetes Care 1993, 16, 984–989. [Google Scholar] [CrossRef]
- Lévy-Marchal, C.; Karjalainen, J.; Dubois, F.; Karges, W.; Czernichow, P.; Dosch, H.-M. Antibodies against bovine albumin and other diabetes markers in French children. Diabetes Care 1995, 18, 1089–1094. [Google Scholar] [CrossRef]
- Vaarala, O.; Klemetti, P.; Savilahti, E.; Reijonen, H.; Ilonen, J.; Åkerblom, H.K. Cellular immune response to cow’s milk β-lactoglobulin in patients with newly diagnosed IDDM. Diabetes 1996, 45, 178–182. [Google Scholar] [CrossRef]
- Cavallo, M.G.; Fava, D.; Monetini, L.; Barone, F.; Pozzilli, P. Cell-mediated immune response to β casein in recent-onset insulin-dependent diabetes: Implications for disease pathogenesis. Lancet 1996, 348, 926–928. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Stene, L.C.; Ludvigsson, J.; Rosenbauer, J.; Cinek, O.; Svensson, J.; Perez-Bravo, F.; Memon, A.; Gimeno, S.G.; Wadsworth, E.J.; et al. Breast-feeding and childhood-onset type 1 diabetes: A pooled analysis of individual participant data from 43 observational studies. Diabetes Care 2012, 35, 2215–2225. [Google Scholar] [CrossRef] [Green Version]
- Rosenbauer, J.; Herzig, P.; Kaiser, P.; Giani, G. Early nutrition and risk of type 1 diabetes mellitus—A nationwide case-control study in preschool children. Exp. Clin. Endocrinol. Diabetes 2007, 115, 502–508. [Google Scholar] [CrossRef]
- Goldberg, J.P.; Folta, S.C.; Must, A. Milk: Can a “good” food be so bad? Pediatrics 2002, 110, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Turck, D. Is cow’s milk harmful to a child’s health? J. Pediatr. Gastroenterol. Nutr. 2011, 53, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dohle, C.; Friemann, J.; Green, B.S.; Gleichmann, H. Prevention of high- and low-dose STZ-induced diabetes with D-glucose and 5-thio-D-glucose. Diabetes 1993, 42, 420–428. [Google Scholar] [CrossRef]
- Wang, Z.; Gleichmann, H. Glucose transporter 2 expression: Prevention of streptozotocin-induced reduction in β-cells with 5-thio-D-glucose. Exp. Clin. Endocrinol. Diabetes 1995, 103 (Suppl. 2), 83–97. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008, 8, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Ait-Omar, A.; Monteiro-Sepulveda, M.; Poitou, C.; Le Gall, M.; Cotillard, A.; Gilet, J. GLUT2 accumulation in enterocyte apical and intracellular membranes: A study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 2011, 60, 2598–2607. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, C.D. Casarett & Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; Klaassen, C.D., Ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Altomare, E.; Vendemiale, G.; Albano, O. Hepatic glutathione content in patients with alcoholic and non-alcoholic liver diseases. Life Sci. 1988, 43, 991–998. [Google Scholar] [CrossRef]
- Broder, L.E.; Carter, S.K. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann. Intern. Med. 1973, 79, 108–118. [Google Scholar] [CrossRef]
- Moertel, C.G.; Hanley, J.A.; Johnson, L.A. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1980, 303, 1189–1194. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.T. Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells. Cells 2022, 11, 492. https://doi.org/10.3390/cells11030492
Zhu BT. Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells. Cells. 2022; 11(3):492. https://doi.org/10.3390/cells11030492
Chicago/Turabian StyleZhu, Bao Ting. 2022. "Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells" Cells 11, no. 3: 492. https://doi.org/10.3390/cells11030492





