Spatiotemporal Localisation of Heparan Sulphate Proteoglycans throughout Mouse Lens Morphogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunolabelling of Heparan Sulphate Proteoglycans
3. Results
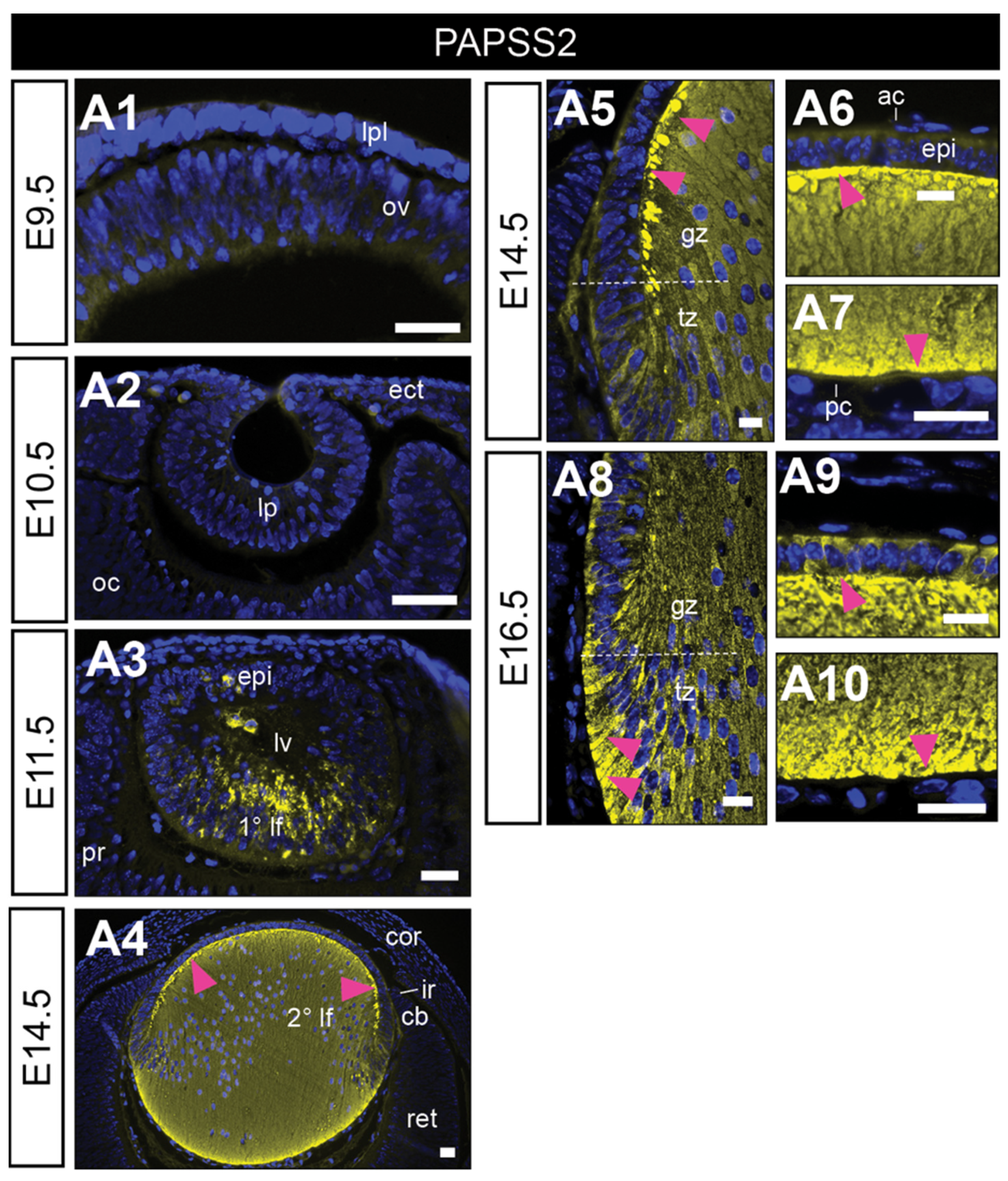
3.1. PAPS Synthetase-2 Is Localised to the Developing Mouse Lens
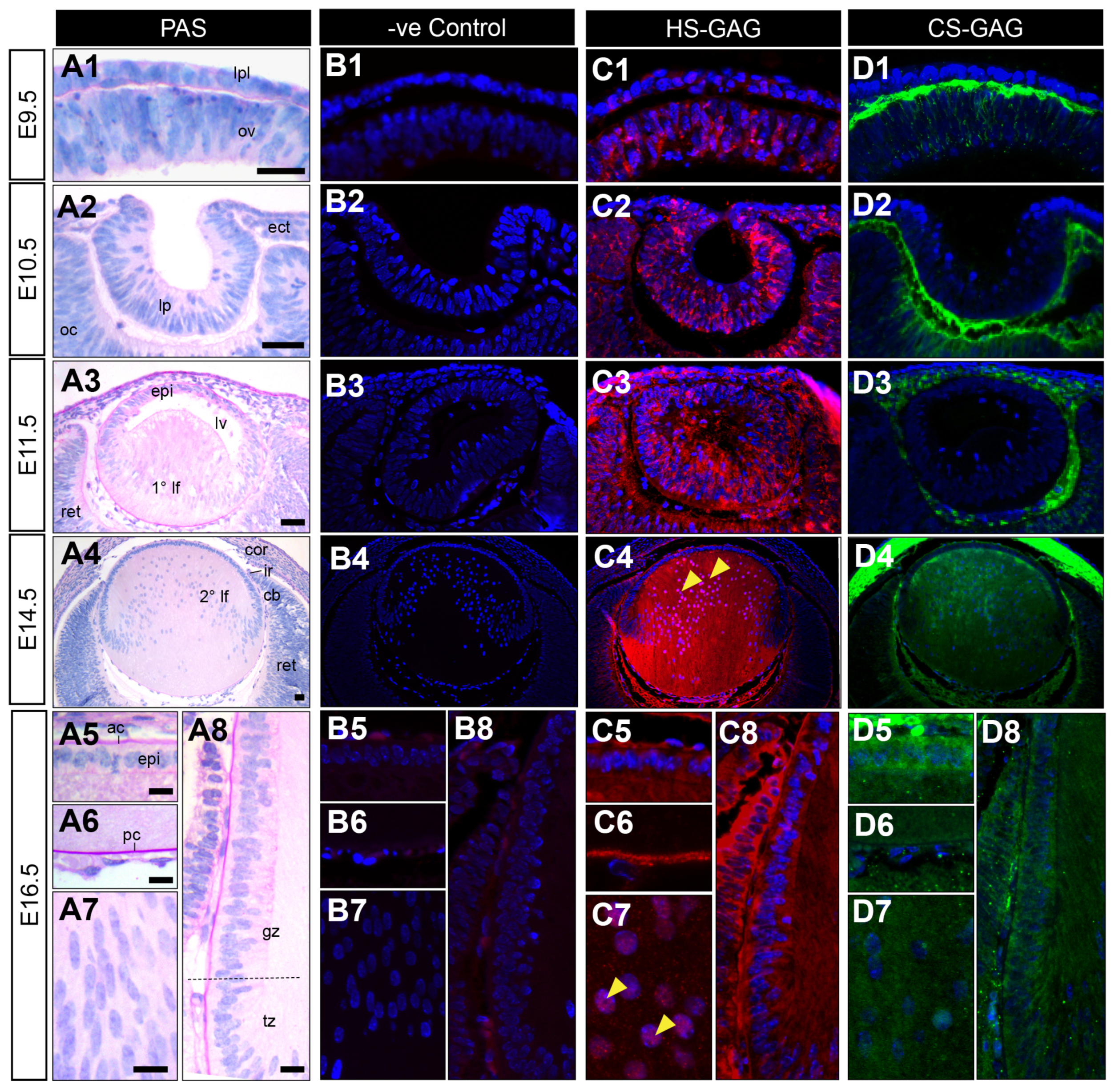
3.2. Heparan Sulphate Is the Predominant Sulphated Glycosaminoglycan Expressed throughout Murine Lens Development
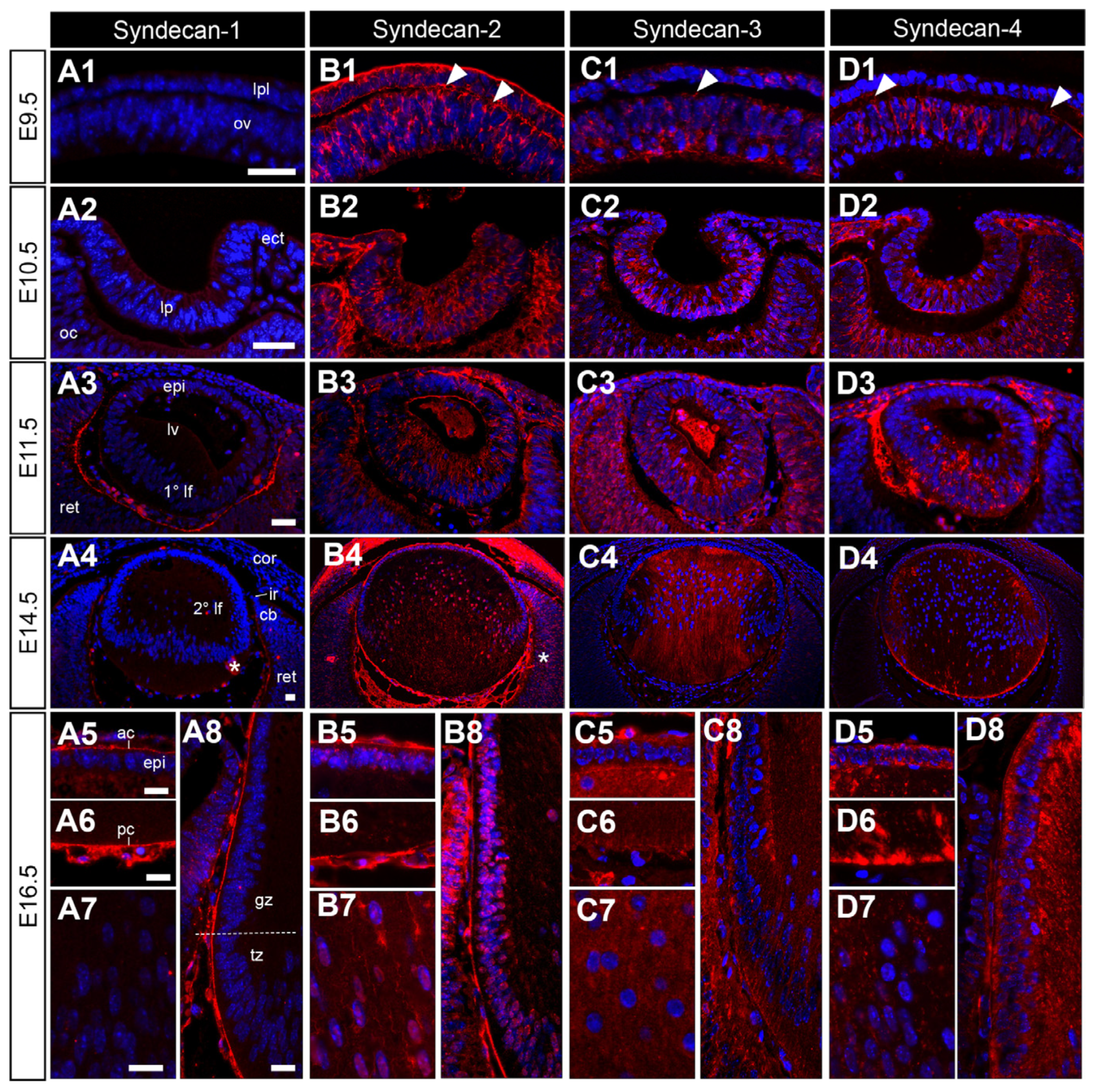
3.3. Syndecan HSPGs Are Differentially Spatially Localised in the Lens throughout Development
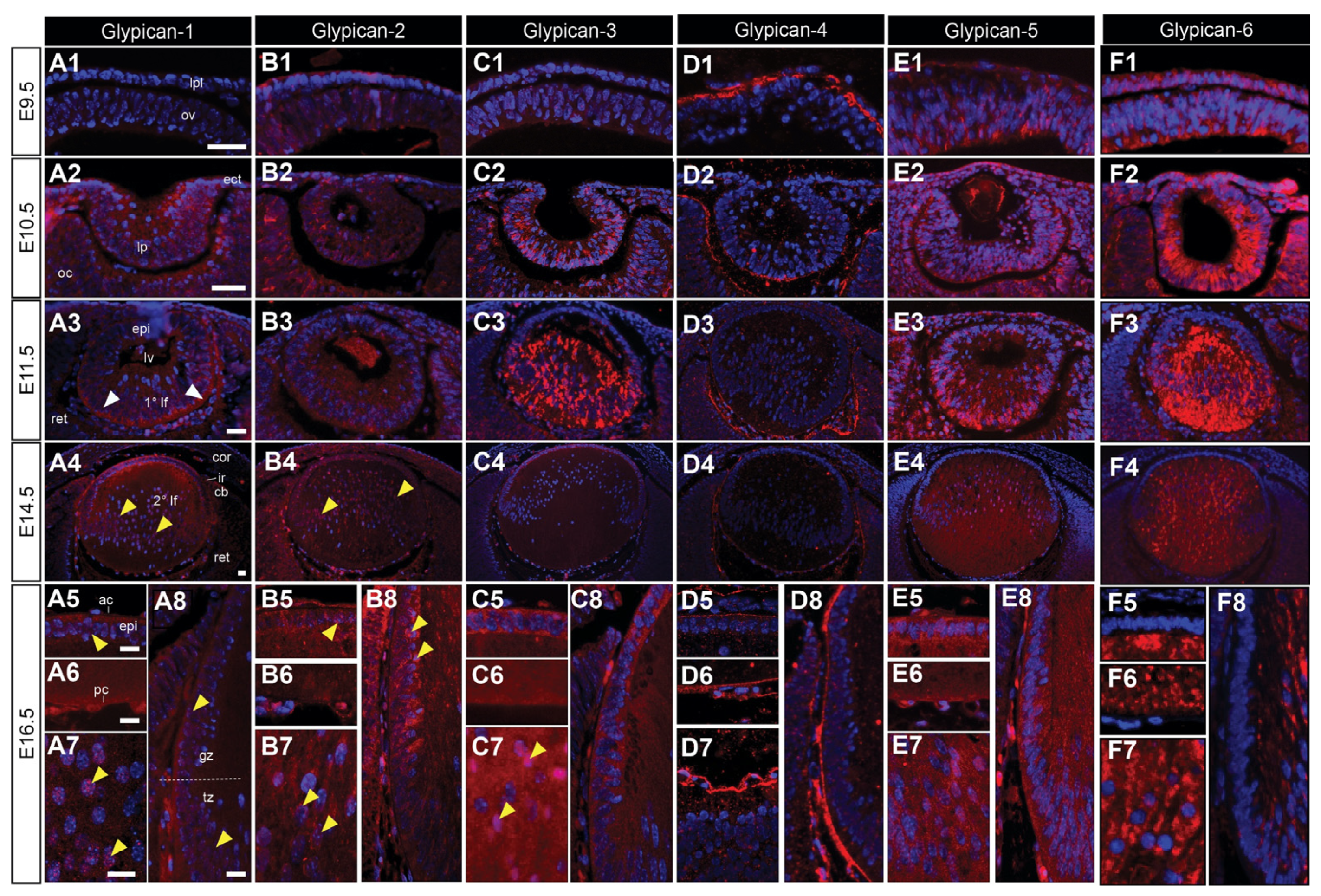
3.4. Glypican HSPGs Differ in Temporal Expression at Key Stages of Lens/Eye Development
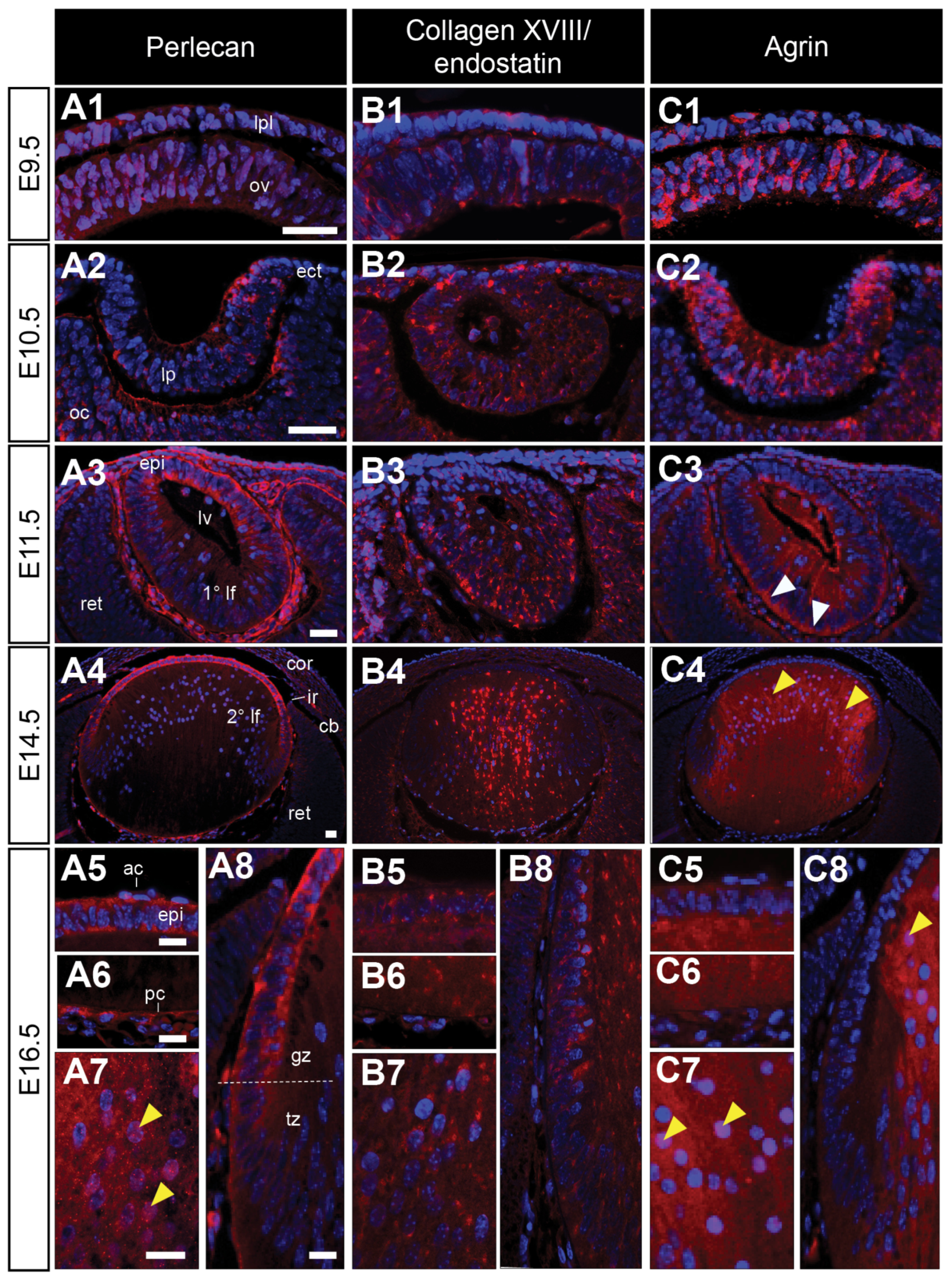
3.5. Localisation of High-Molecular-Weight HSPGs Extends beyond the Lens Capsule
| Embryonic Day | GAG | Other | Core Proteins | |
|---|---|---|---|---|
| E9.5 | Lens placode | HS | Sdc2, Sdc3, Sdc4, Gpc2, Gpc4, Gpc5, Gpc6, perlecan, collagen XVIII, agrin | |
| Optic vesicle | HS, CS | Sdc2, Sdc3, Sdc4, Gpc2, Gpc5, Gpc6, perlecan, collagen XVIII, agrin | ||
| Preplacodal matrix | CS | Sdc2, Sdc4, Gpc4, Agrin | ||
| E10.5 | Lens pit | HS | Sdc2, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, Gpc5, Gpc6, perlecan, collagen XVIII, agrin | |
| Head ectoderm | HS | Sdc2, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, Gpc4, Gpc5, Gpc6, perlecan, agrin | ||
| Optic cup | HS, CS | Sdc2, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, Gpc4, Gpc5, Gpc6, perlecan, collagen XVIII, agrin | ||
| Extracellular matrix (lens pit-optic cup) | CS | Sdc2, Gpc4, perlecan? | ||
| E11.5 | Anterior lens epithelium | HS | PAPSS2 | Sdc2, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, perlecan, collagen XVIII, agrin |
| 1° lens fibre cells | HS | PAPSS2 | Sdc2, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, Gpc5, Gpc6, perlecan, collagen XVIII, agrin | |
| Lens capsule | HS | Sdc1, Sdc2, Gpc4, perlecan | ||
| E14.5 | Anterior lens epithelium | HS *, CS | Sdc2 *, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, Gpc4, perlecan, collagen XVIII | |
| 2° lens fibre cells | HS *, CS | PAPSS2 | Sdc2 *, Sdc3, Sdc4, Gpc1, Gpc2, Gpc3, Gpc5, Gpc6, collagen XVIII, agrin | |
| Lens capsule | HS, CS | Sdc1, Sdc2, Gpc2, Gpc4, perlecan | ||
| Tunica vasculosa lentis | HS, CS | Sdc1, Sdc2, Gpc1, Gpc2, Gpc4, Gpc5, perlecan | ||
| E16.5 | Anterior lens epithelium | HS *, CS | Sdc2 *, Sdc3, Sdc4, Gpc1 *, Gpc2 *, Gpc3 *, Gpc5, perlecan, collagen XVIII, agrin | |
| 2° lens fibre cells | HS *, CS | PAPSS2 | Sdc2 *, Sdc3, Sdc4, Gpc1 *, Gpc2 *, Gpc3 *, Gpc5, Gpc6, perlecan *, collagen XVIII, agrin * | |
| Lens capsule | HS | Sdc1, Sdc2, Gpc4, perlecan | ||
| Tunica vasculosa lentis | HS, CS | Sdc1, Sdc2, Gpc1, Gpc2, Gpc4, Gpc5, perlecan |
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- McAvoy, J.W.; Chamberlain, C.G.; de Longh, R.U.; Hales, A.M.; Lovicu, F.J. Lens development. Eye 1999, 13, 425–437. [Google Scholar] [CrossRef]
- Lovicu, F.J.; Ang, S.; Chorazyczewska, M.; McAvoy, J.W. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFβ-induced anterior subcapsular cataract. Dev. Neurosci. 2004, 26, 446–455. [Google Scholar] [CrossRef]
- Wormstone, M.I.; Wride, M.A. The ocular lens: A classic model for development, physiology and disease. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1190–1192. [Google Scholar] [CrossRef]
- Lovicu, F.J.; McAvoy, J.W. Growth factor regulation of lens development. Dev. Biology 2005, 280, 1–14. [Google Scholar] [CrossRef]
- Cvekl, A.; Ashery-Padan, R. The cellular and molecular mechanisms of vertebrate lens development. Development 2014, 141, 4432–4447. [Google Scholar] [CrossRef]
- Cvekl, A.; Zhang, X. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet. 2017, 33, 677–702. [Google Scholar] [CrossRef]
- McAvoy, J.W.; Chamberlain, C.G.; de Iongh, R.U.; Richardson, N.A.; Lovicu, F.J. The role of fibroblast growth factor in eye lens development. Ann. N.Y. Acad. Sci. 1991, 638, 256–274. [Google Scholar] [CrossRef]
- Faber, S.C.; Dimanlig, P.; Makarenkova, H.P.; Shirke, S.; Ko, K.; Lang, R.A. Fgf receptor signaling plays a role in lens induction. Development 2001, 128, 4425–4438. [Google Scholar] [CrossRef]
- Robinson, M.L. An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 2006, 17, 726–740. [Google Scholar] [CrossRef]
- De Iongh, R.U.; Abud, H.E.; Hime, G.R. WNT/Frizzled signaling in eye development and disease. Front. Bioscience 2006, 11, 2442–2464. [Google Scholar] [CrossRef]
- Chen, Y.; Stump, R.J.W.; Lovicu, F.J.; Shimono, A.; McAvoy, J.W. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev. Biol. 2008, 324, 161–176. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Lovicu, F.J.; McAvoy, J.W. Planar cell polarity in the mammalian eye lens. Organogenesis 2011, 7, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Faber, S.C.; Robinson, M.L.; Makarenkova, H.P.; Lang, R.A. Bmp signaling is required for development of primary lens fiber cells. Development 2002, 129, 3727–3737. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Insights into Bone Morphogenetic Protein—(BMP-) Signaling in Ocular Lens Biology and Pathology. Cells 2021, 10, 2604. [Google Scholar] [CrossRef]
- Tsonis, P.A.; Vergara, M.N.; Spence, J.R.; Madhavan, M.; Kramer, E.L.; Call, M.K.; Santiago, W.G.; Vallance, J.E.; Robbins, D.J.; Del Rio-Tsonis, K. A novel role of the hedgehog pathway in lens regeneration. Dev. Biol. 2004, 267, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Pillai-Kastoori, L.; Wen, W.; Wilson, S.G.; Strachan, E.; Lo-Castro, A.; Fichera, M.; Musumeci, S.A.; Lehmann, O.J.; Morris, A.C. Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis. PLoS Genet. 2014, 10, e1004491. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. An. Acad. Bras. Cienc. 2009, 81, 409–429. [Google Scholar] [CrossRef]
- Wong, K.O.; Wong, K.P. Direct measurement and regulation of 3“-phosphoadenosine 5-”phosphosulfate (PAPS) generation in vitro. Biochem. Pharmacol. 1996, 52, 1187–1194. [Google Scholar] [CrossRef]
- Schröder, E.; Gebel, L.; Eremeev, A.A.; Morgner, J.; Grum, D.; Knauer, S.K.; Bayer, P.; Mueller, J.W. Human PAPS Synthase Isoforms Are Dynamically Regulated Enzymes with Access to Nucleus and Cytoplasm. PLoS ONE 2012, 7, e29559. [Google Scholar] [CrossRef]
- Quarto, N.; Amalric, F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J. Cell Sci. 1994, 107 Pt 11, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. BioEssays 2000, 22, 108–112. [Google Scholar] [CrossRef]
- Ashikari-Hada, S.; Habuchi, H.; Kariya, Y.; Itoh, N.; Reddi, A.H.; Kimata, K. Characterization of Growth Factor-binding Structures in Heparin/Heparan Sulfate Using an Octasaccharide Library. J. Biol. Chem. 2004, 279, 12346–12354. [Google Scholar] [CrossRef]
- Pegge, J.; Tatsinkam, A.J.; Rider, C.C.; Bell, E. Heparan sulfate proteoglycans regulate BMP signalling during neural crest induction. Dev. Biol. 2020, 460, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Fuerer, C.; Habib, S.J.; Nusse, R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev. Dyn. 2010, 239, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mii, Y.; Takada, S. Heparan Sulfate Proteoglycan Clustering in Wnt Signaling and Dispersal. Front. Cell Dev. Biol. 2020, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, H.; Olivares, G.H.; Faunes, F.; Oliva, C.; Larraín, J. Heparan sulfate proteoglycans exert positive and negative effects in Shh activity. J. Cell. Biochem. 2005, 96, 831–838. [Google Scholar] [CrossRef]
- Palma, V.; Carrasco, H.; Reinchisi, G.; Olivares, G.; Faunes, F.; Larraín, J. SHh activity and localization is regulated by perlecan. Biol. Res. 2011, 44, 63–67. [Google Scholar] [CrossRef]
- Couchman, J.R.; Woods, A. Syndecan-4 and integrins: Combinatorial signaling in cell adhesion. J. Cell Sci. 1999, 112, 3415–3420. [Google Scholar] [CrossRef]
- Yang, N. Syndecan-1-induced ECM fiber alignment requires integrin αvβ3 and syndecan-1 ectodomain and heparan sulfate chains. PLoS ONE 2016, 11, e0150132. [Google Scholar] [CrossRef]
- Carulli, S.; Beck, K.; Dayan, G.; Boulesteix, S.; Lortat-Jacob, H.; Rousselle, P. Cell surface proteoglycans syndecan-1 and -4 bind overlapping but distinct sites in laminin α3 LG45 protein domain. J. Biol. Chem. 2012, 287, 12204–12216. [Google Scholar] [CrossRef] [PubMed]
- Tumova, S.; Woods, A.; Couchman, J.R. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J. Biol. Chem. 2000, 275, 9410–9417. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Schuksz, M.; Esko, J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007, 446, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, L.; Kreuger, J.; Cohen, S.M.; Shraiman, B.I. On the role of glypicans in the process of morphogen gradient formation. Dev. Biol. 2006, 300, 512–522. [Google Scholar] [CrossRef]
- Wishart, T.F.L.; Lovicu, F.J. An atlas of heparan sulfate proteoglycans in the postnatal rat lens. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- Rossi, M. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003, 22, 236–245. [Google Scholar] [CrossRef]
- Pan, Y.; Woodbury, A.; Esko, J.D.; Grobe, K.; Zhang, X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 2006, 133, 4933–4944. [Google Scholar] [CrossRef]
- Wishart, T.F.L.; Lovicu, F.J. Heparan sulfate proteoglycans (HSPGs) of the ocular lens. Prog. Retin. Eye Res. 2023, 93, 101118. [Google Scholar] [CrossRef]
- Qu, X.; Carbe, C.; Tao, C.; Powers, A.; Lawrence, R.; van Kuppevelt, T.H.; Cardoso, W.V.; Grobe, K.; Esko, J.D.; Zhang, X. Lacrimal gland development and Fgf10-Fgfr2b signaling are controlled by 2-O- and 6-O-sulfated heparan sulfate. J. Biol. Chem. 2011, 286, 14435–14444. [Google Scholar] [CrossRef]
- Kakrana, A.; Yang, A.; Anand, D.; Djordjevic, D.; Ramachandruni, D.; Singh, A.; Huang, H.; Ho, J.W.K.; Lachke, S.A. ISyTE 2.0: A database for expression-based gene discovery in the eye. Nucleic Acids Res. 2018, 46, D875–D885. [Google Scholar] [CrossRef]
- Khan, S.Y.; Hackett, S.F.; Lee, M.-C.W.; Pourmand, N.; Talbot, C.C.; Riazuddin, S.A. Transcriptome Profiling of Developing Murine Lens Through RNA Sequencing. Investig. Opthalmol. Vis. Sci. 2015, 56, 4919–4926. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.V.; Kumar, P.K.R.; Sutharzan, S.; Tsonis, P.A.; Liang, C.; Robinson, M.L. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol. Vis. 2014, 20, 1491–1517. [Google Scholar] [PubMed]
- Cavalheiro, G.R.; Matos-Rodrigues, G.E.; Zhao, Y.; Gomes, A.L.; Anand, D.; Predes, D.; de Lima, S.; Abreu, J.G.; Zheng, D.; Lachke, S.A.; et al. N-myc regulates growth and fiber cell differentiation in lens development. Dev. Biol. 2017, 429, 105–117. [Google Scholar] [CrossRef]
- Audette, D.S.; Anand, D.; So, T.; Rubenstein, T.B.; Lachke, S.A.; Lovicu, F.J.; Duncan, M.K. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development 2016, 143, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Kokenyesi, R.; Bernfield, M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J. Biol. Chem. 1994, 269, 12304–12309. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.E.; Upholt, W.B.; Kosher, R.A. Characterization of Chicken Syndecan-3 as a Heparan Sulfate Proteoglycan and Its Expression during Embryogenesis. Dev. Biol. 1995, 168, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Winzen, U.; Cole, G.J.; Halfter, W. Agrin is a chimeric proteoglycan with the attachment sites for heparan sulfate/chondroitin sulfate located in two multiple serine-glycine clusters. J. Biol. Chem. 2003, 278, 30106–30114. [Google Scholar] [CrossRef]
- Dong, S.; Cole, G.J.; Halfter, W. Expression of collagen XVIII and localization of its glycosaminoglycan attachment sites. J. Biol. Chem. 2003, 278, 1700–1707. [Google Scholar] [CrossRef]
- Heathcote, J.G.; Orkin, R.W. Biosynthesis of Sulphated Macromolecules by Rabbit Lens Epithelium. I. Identification of the Major Macromolecules Synthesized by Lens Epithelial Cells in vitro. J. Cell. Biol. 1984, 99, 852–860. [Google Scholar] [CrossRef]
- Mohan, P.S.; Spiro, R.G. Characterization of heparan sulfate proteoglycan from calf lens capsule and proteoglycans synthesized by cultured lens epithelial cells. Comparison with other basement membrane proteoglycans. J. Biol. Chem. 1991, 266, 8567–8575. [Google Scholar] [CrossRef]
- Maeda, N.; Ishii, M.; Nishimura, K.; Kamimura, K. Functions of chondroitin sulfate and heparan sulfate in the developing brain. Neurochem. Res. 2011, 36, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Clerc, O.; Ricard-Blum, S. Glycosaminoglycan-Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem. 2020, 69, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Keenan, T.D.L.; Fielder, H.L.; Collinson, L.J.; Holley, R.J.; Merry, C.L.R.; van Kuppevelt, T.H.; Day, A.J.; Bishop, P.N. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Investig. Opthalmol. Vis. Sci. 2011, 52, 6511–6521. [Google Scholar] [CrossRef] [PubMed]
- Smetsers, T.F.C.M.; van de Westerlo, E.M.A.; ten Dam, G.B.; Clarijs, R.; Versteeg, E.M.M.; van Geloof, W.L.; Veerkamp, J.H.; van Muijen, G.N.P.; van Kuppevelt, T.H. Localization and characterization of melanoma-associated glycosaminoglycans: Differential expression of chondroitin and heparan sulfate epitopes in melanoma. Cancer Res. 2003, 63, 2965–2970. [Google Scholar] [PubMed]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; David, G.; Weinreb, R.N.; Kaufman, P.L.; Peters, D.M. Distribution of syndecans 1-4 within the anterior segment of the human eye: Expression of a variant syndecan-3 and matrix-associated syndecan-2. Exp. Eye Res. 2004, 79, 61–74. [Google Scholar] [CrossRef]
- Huang, J.; Rajagopal, R.; Liu, Y.; Dattilo, L.K.; Shaham, O.; Ashery-Padan, R.; Beebe, D.C. The mechanism of lens placode formation: A case of matrix-mediated morphogenesis. Dev. Biol. 2011, 355, 32–42. [Google Scholar] [CrossRef]
- Lang, R.A. Pathways regulating lens induction in the mouse. Int. J. Dev. Biol. 2004, 48, 783–791. [Google Scholar] [CrossRef]
- Gunhaga, L. The lens: A classical model of embryonic induction providing new insights into cell determination in early development. Philos. Trans. Biol. Sci. 2011, 366, 1193–1203. [Google Scholar] [CrossRef]
- Rapraeger, A.C. Syndecan-Regulated Receptor Signaling. J. Cell. Biol. 2000, 149, 995–997. [Google Scholar] [CrossRef]
- Capurro, M.I.; Xu, P.; Shi, W.; Li, F.; Jia, A.; Filmus, J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev. Cell 2008, 14, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Bányai, L.; Sonderegger, P.; Patthy, L. Agrin binds BMP2, BMP4 and TGFβ1. PLoS ONE 2010, 5, e10758. [Google Scholar] [CrossRef]
- Seppinen, L.; Pihlajaniemi, T. The multiple functions of collagen XVIII in development and disease. Matrix Biol. 2011, 30, 83–92. [Google Scholar] [CrossRef]
- Kerever, A.; Mercier, F.; Nonaka, R.; de Vega, S.; Oda, Y.; Zalc, B.; Okada, Y.; Hattori, N.; Yamada, Y.; Arikawa Hirasawa, E. Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res. 2014, 12, 492–505. [Google Scholar] [CrossRef]
- Jin, H.; Fisher, M.; Grainger, R.M. Defining progressive stages in the commitment process leading to embryonic lens formation. Genesis 2012, 50, 728–740. [Google Scholar] [CrossRef]
- Fujimura, N. WNT/β-Catenin Signaling in Vertebrate Eye Development. Front. Cell. Dev. Biol. 2016, 4, 138. [Google Scholar] [CrossRef]
- Barth, K.A.; Wilson, S.W. Expression of zebrafish nk2. 2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development 1995, 121, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Stump, R.; Ang, S.; Chen, Y.J.; von Bahr, T.; Lovicu, F.J.; Pinson, K.; de Iongh, R.U.; Yamaguchi, T.P.; Sassoon, D.A.; McAvoy, J.W. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev. Biol. 2003, 259, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Dawes, L.J.; Sugiyama, Y.; Tanedo, A.S.; Lovicu, F.J.; McAvoy, J.W. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Investig. Opthalmol. Vis. Sci. 2013, 54, 1582–1590. [Google Scholar] [CrossRef]
- Kerr, C.L.; Huang, J.; Williams, T.; West-Mays, J.A. Activation of the hedgehog signaling pathway in the developing lens stimulates ectopic FoxE3 expression and disruption in fiber cell differentiation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3316–3330. [Google Scholar] [CrossRef]
- Boswell, B.A.; Overbeek, P.A.; Musil, L.S. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev. Biol. 2008, 324, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Wederell, E.D.; De Iongh, R.U. Extracellular matrix and integrin signaling in lens development and cataract. Semin. Cell. Dev. Biol. 2006, 17, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell. Biol. 2007, 8, 957–969. [Google Scholar] [CrossRef]
- Kim, M.-S.; Saunders, A.M.; Hamaoka, B.Y.; Beachy, P.A.; Leahy, D.J. Structure of the protein core of the glypican Dally-like and localization of a region important for hedgehog signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.A.; Williamson, R.C.; Bass, M.D. Syndecan and integrin interactomes: Large complexes in small spaces. Curr. Opin. Struct. Biol. 2012, 22, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, C.; McAvoy, J. Localisation of laminin and fibronectin during rat lens morphogenesis. Differentiation 1984, 28, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zelenka, P.S. Regulation of cell adhesion and migration in lens development. Int. J. Dev. Biol. 2004, 48, 857–865. [Google Scholar] [CrossRef]
- Simirskii, V.N.; Wang, Y.; Duncan, M.K. Conditional deletion of β1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev. Biol. 2007, 306, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Pathania, M.; Wang, Y.; Simirskii, V.N.; Duncan, M.K. β1-integrin controls cell fate specification in early lens development. Differentiation 2016, 92, 133–147. [Google Scholar] [CrossRef]
- Filmus, J. The function of Glypicans in the mammalian embryo. Am. J. Physiol. Cell. Physiol. 2022, 322, C694–C698. [Google Scholar] [CrossRef]
- Strate, I.; Tessadori, F.; Bakkers, J. Glypican4 promotes cardiac specification and differentiation by attenuating canonical Wnt and Bmp signaling. Development 2015, 142, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ho, M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am. J. Physiol. Cell. Physiol. 2021, 321, C846–C858. [Google Scholar] [CrossRef] [PubMed]
- Jidigam, V.K.; Srinivasan, R.C.; Patthey, C.; Gunhaga, L. Apical constriction and epithelial invagination are regulated by BMP activity. Biol. Open 2015, 4, 1782–1791. [Google Scholar] [CrossRef]
- Chauhan, B.K.; Lou, M.; Zheng, Y.; Lang, R.A. Balanced Rac1 and RhoA activities regulate cell shape and drive invagination morphogenesis in epithelia. Proc. Natl. Acad. Sci. USA 2011, 108, 18289–18294. [Google Scholar] [CrossRef]
- Grisaru, S. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev. Biol. 2001, 231, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.T.P.; Lopes, H.B.; Oliveira, F.S.; Weffort, D.; Freitas, G.P.; Adolpho, L.F.; Fernandes, R.R.; Rosa, A.L.; Beloti, M.M. The extracellular matrix protein Agrin is expressed by osteoblasts and contributes to their differentiation. Cell Tissue Res. 2021, 386, 335–347. [Google Scholar] [CrossRef]
- Fisher, M.C.; Li, Y.; Seghatoleslami, M.R.; Dealy, C.N.; Kosher, R.A. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. 2006, 25, 27–39. [Google Scholar] [CrossRef]
- Bass, M.D.; Morgan, M.R.; Humphries, M.J. Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation. Soft Matter 2007, 3, 372–376. [Google Scholar] [CrossRef]
- Okina, E.; Manon-Jensen, T.; Whiteford, J.R.; Couchman, J.R. Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand. J. Med. Sci. Sports 2009, 19, 479–489. [Google Scholar] [CrossRef]
- Tsoyi, K.; Chu, S.G.; Patino-Jaramillo, N.G.; Wilder, J.; Villalba, J.; Doyle-Eisele, M.; McDonald, J.; Liu, X.; El-Chemaly, S.; Perrella, M.A.; et al. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am. J. Cell. Mol. Biol. 2018, 58, 208–215. [Google Scholar] [CrossRef]
- Kolluri, A.; Ho, M. The Role of Glypican-3 in Regulating Wnt, YAP, and Hedgehog in Liver Cancer. Front. Oncol. 2019, 9, 708. [Google Scholar] [CrossRef]
- Uechi, G.; Sun, Z.; Schreiber, E.M.; Halfter, W.; Balasubramani, M. Proteomic View of Basement Membranes from Human Retinal Blood Vessels, Inner Limiting Membranes, and Lens Capsules. J. Proteome Res. 2014, 13, 3693–3705. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tholozan, F.M.; Goldberg, M.W.; Bowen, L.; Wu, J.; Quinlan, R.A. A gradient of matrix-bound FGF-2 and perlecan is available to lens epithelial cells. Exp. Eye Res. 2014, 120, 10–14. [Google Scholar] [CrossRef] [PubMed]
- DeDreu, J.; Walker, J.L.; Menko, A.S. Dynamics of the lens basement membrane capsule and its interaction with connective tissue-like extracapsular matrix proteins. Matrix Biol. 2021, 96, 18–46. [Google Scholar] [CrossRef]
- Fuerst, P.G.; Rauch, S.M.; Burgess, R.W. Defects in eye development in transgenic mice overexpressing the heparan sulfate proteoglycan agrin. Dev. Biol. 2007, 303, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.G.; McAvoy, J.W. Fibre differentiation and polarity in the mammalian lens: A key role for FGF. Prog. Retin. Eye Res. 1997, 16, 443–478. [Google Scholar] [CrossRef]
- Hubka, K.M.; Carson, D.D.; Harrington, D.A.; Farach-Carson, M.C. Perlecan domain I gradients establish stable biomimetic heparin binding growth factor gradients for cell migration in hydrogels. Acta Biomater. 2019, 97, 385–398. [Google Scholar] [CrossRef]
- Soulintzi, N.; Zagris, N. Spatial and temporal expression of perlecan in the early chick embryo. Cells Tissues Organs 2007, 186, 243–256. [Google Scholar] [CrossRef]
- Fukai, N.; Eklund, L.; Marneros, A.G.; Oh, S.P.; Keene, D.R.; Tamarkin, L.; Niemelä, M.; Ilves, M.; Li, E.; Pihlajaniemi, T.; et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002, 21, 1535–1544. [Google Scholar] [CrossRef]
- Marneros, A.G.; Keene, D.R.; Hansen, U.; Fukai, N.; Moulton, K.; Goletz, P.L.; Moiseyev, G.; Pawlyk, B.S.; Halfter, W.; Dong, S.; et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004, 23, 89–99. [Google Scholar] [CrossRef]
- Elamaa, H.; Sormunen, R.; Rehn, M.; Soininen, R.; Pihlajaniemi, T. Endostatin Overexpression Specifically in the Lens and Skin Leads to Cataract and Ultrastructural Alterations in Basement Membranes. Am. J. Pathol. 2005, 166, 221–229. [Google Scholar] [CrossRef]
- Aikio, M.; Hurskainen, M.; Brideau, G.; Hägg, P.; Sormunen, R.; Heljasvaara, R.; Gould, D.B.; Pihlajaniemi, T. Collagen XVIII short isoform is critical for retinal vascularization, and overexpression of the Tsp-1 domain affects eye growth and cataract formation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7450–7462. [Google Scholar] [CrossRef]
- Liu, I.H.; Zhang, C.; Min, J.K.; Cole, G.J. Retina development in zebraf ish requires the heparan sulfate proteoglycan agrin. Dev. Neurobiol. 2008, 68, 877–898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, T.; Madakashira, B.P.; Thiels, C.A.; Bechtle, C.A.; Garcia, C.M.; Zhang, H.; Yu, K.; Ornitz, D.M.; Beebe, D.C.; et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 2008, 318, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Lovicu, F.J.; McAvoy, J.W. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development 2001, 128, 5075–5084. [Google Scholar] [CrossRef]
- Liang, Y.; Häring, M.; Roughley, P.J.; Margolis, R.K.; Margolis, R.U. Glypican and biglycan in the nuclei of neurons and glioma cells: Presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. J. Cell. Biol. 1997, 139, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Expression of agrin in the developing and adult rat brain. Neuroscience 1997, 76, 581–596. [CrossRef]
- Stewart, M.D.; Sanderson, R.D. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol. 2014, 35, 56–59. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wishart, T.F.L.; Lovicu, F.J. Spatiotemporal Localisation of Heparan Sulphate Proteoglycans throughout Mouse Lens Morphogenesis. Cells 2023, 12, 1364. https://doi.org/10.3390/cells12101364
Wishart TFL, Lovicu FJ. Spatiotemporal Localisation of Heparan Sulphate Proteoglycans throughout Mouse Lens Morphogenesis. Cells. 2023; 12(10):1364. https://doi.org/10.3390/cells12101364
Chicago/Turabian StyleWishart, Tayler F. L., and Frank J. Lovicu. 2023. "Spatiotemporal Localisation of Heparan Sulphate Proteoglycans throughout Mouse Lens Morphogenesis" Cells 12, no. 10: 1364. https://doi.org/10.3390/cells12101364






