Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Isolation and Culture of Vdr−/− Retinal EC
2.3. RNA Purification and Real Time Quantitative PCR (qPCR) Analysis
2.4. Western Blot Analysis
2.5. Flow Cytometry
2.6. Indirect Immunofluorescence Staining
2.7. Scratch Wound Assays
2.8. Transwell Migration Assays
2.9. Cell Adhesion Assays
2.10. Cell Proliferation Assays
2.11. Cell Viability Assay
2.12. Measuring Intracellular Iron Levels
2.13. Statistical Analysis
3. Results
3.1. Isolation and Characterization of Vdr−/− Retinal EC
3.2. Vdr−/− Retinal EC Were Less Migratory
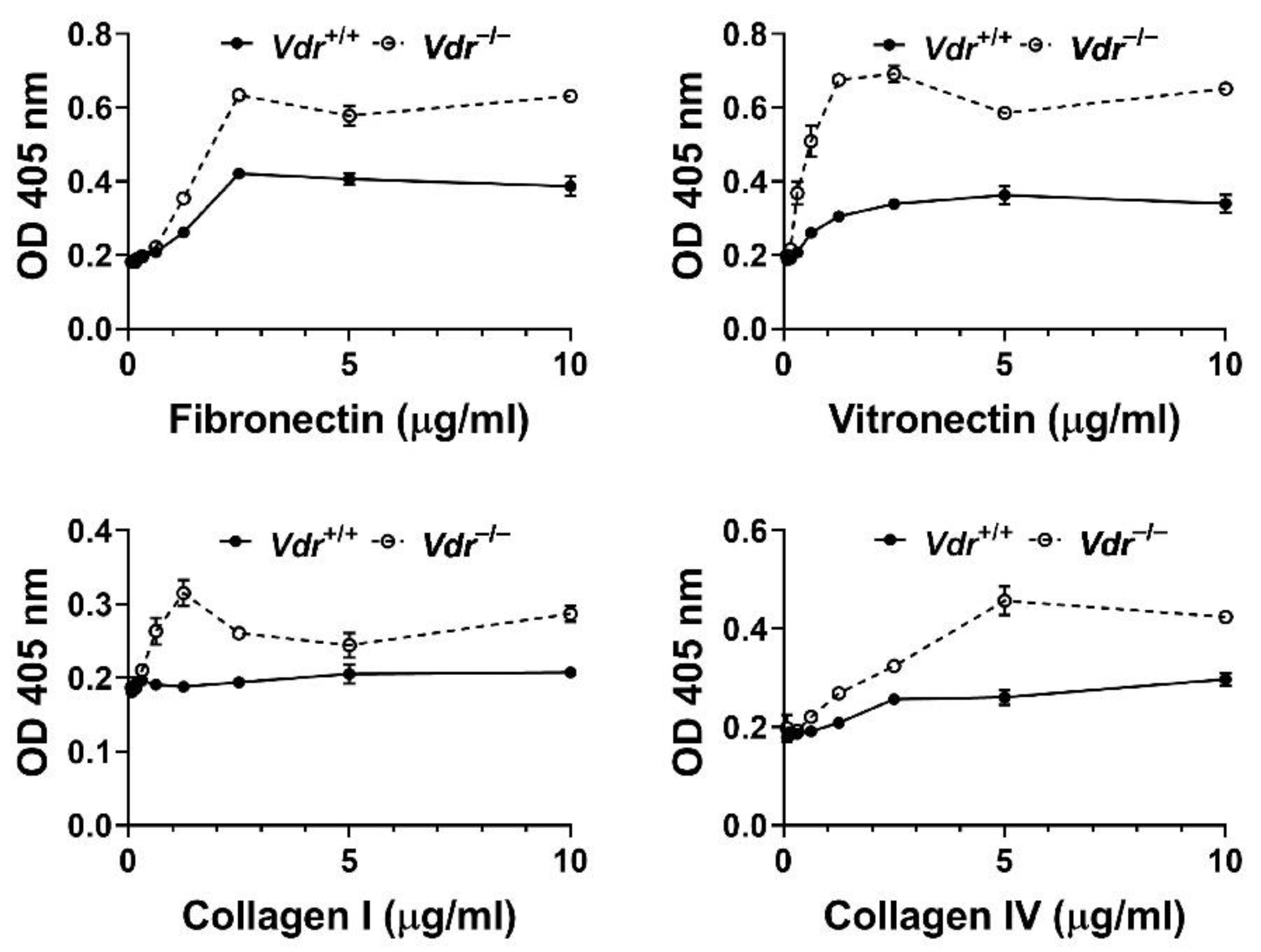
3.3. Vdr−/− Retinal EC Were More Adherent
3.4. Altered Expression of ECM Proteins in Vdr−/− Retinal EC
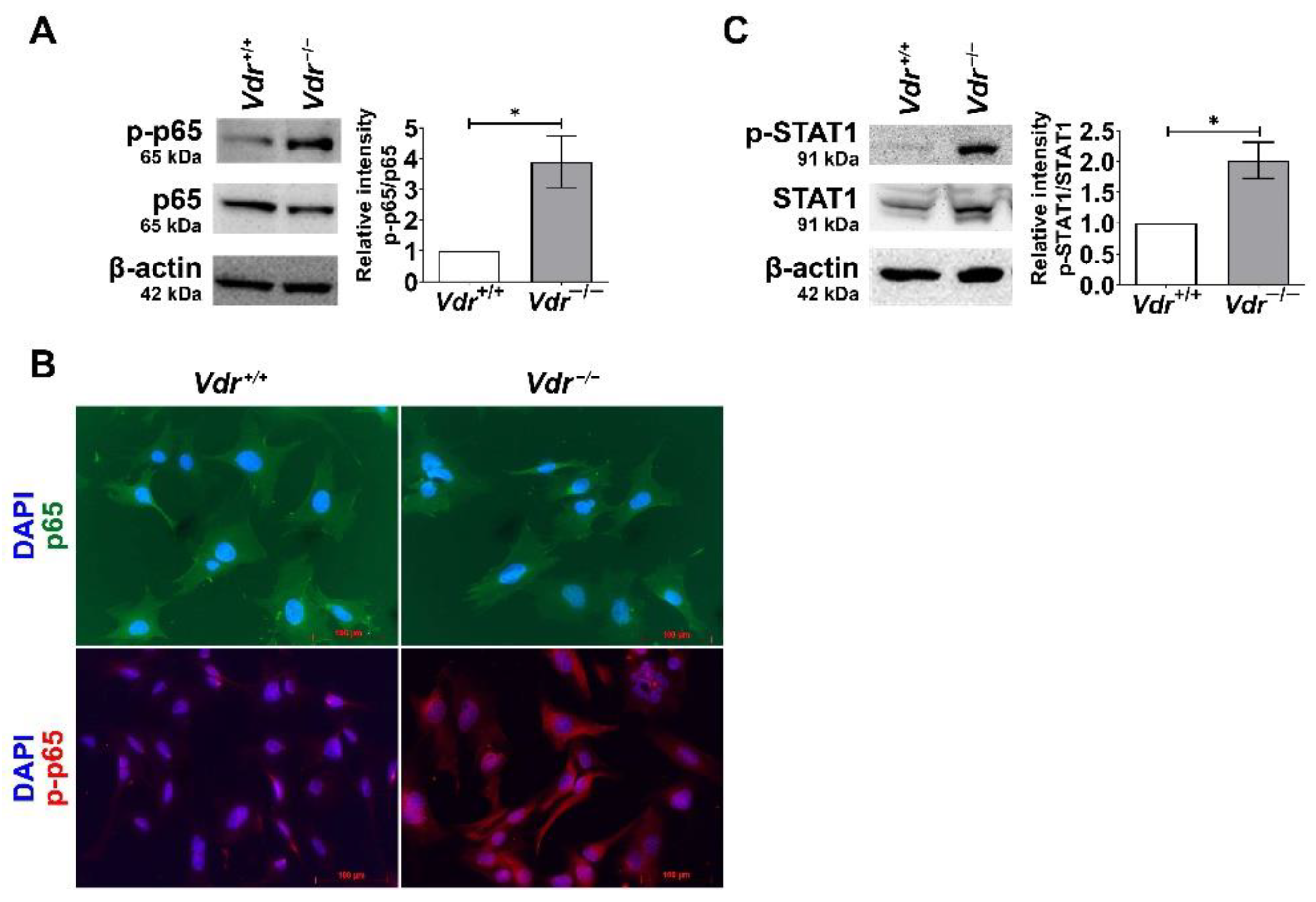
3.5. Increased Expression of Pro-Inflammatory Mediators and Activation of Proinflammatory Transcription Factors in Vdr−/− Retinal EC
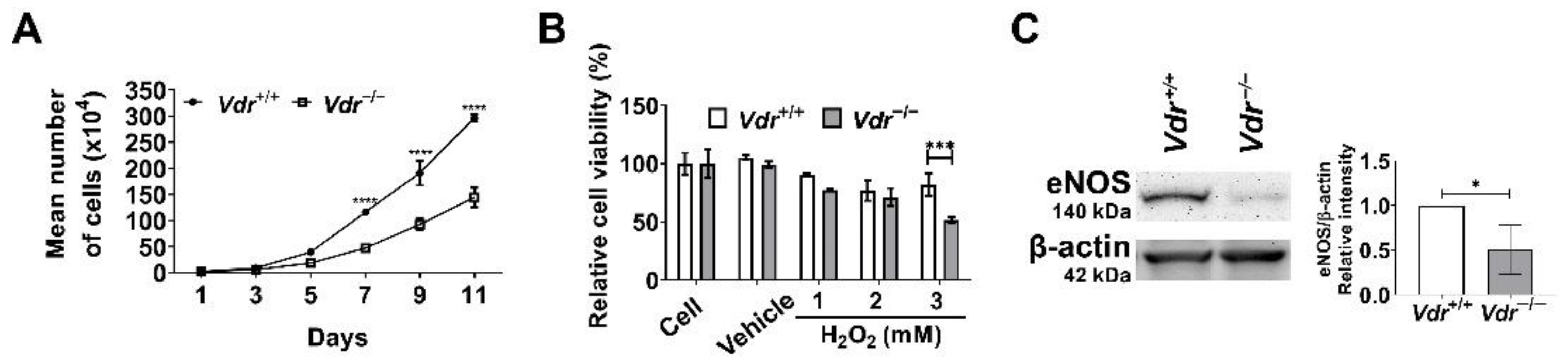
3.6. Altered Cell Proliferation and Increased Oxidative Stress in Vdr−/− Retinal EC
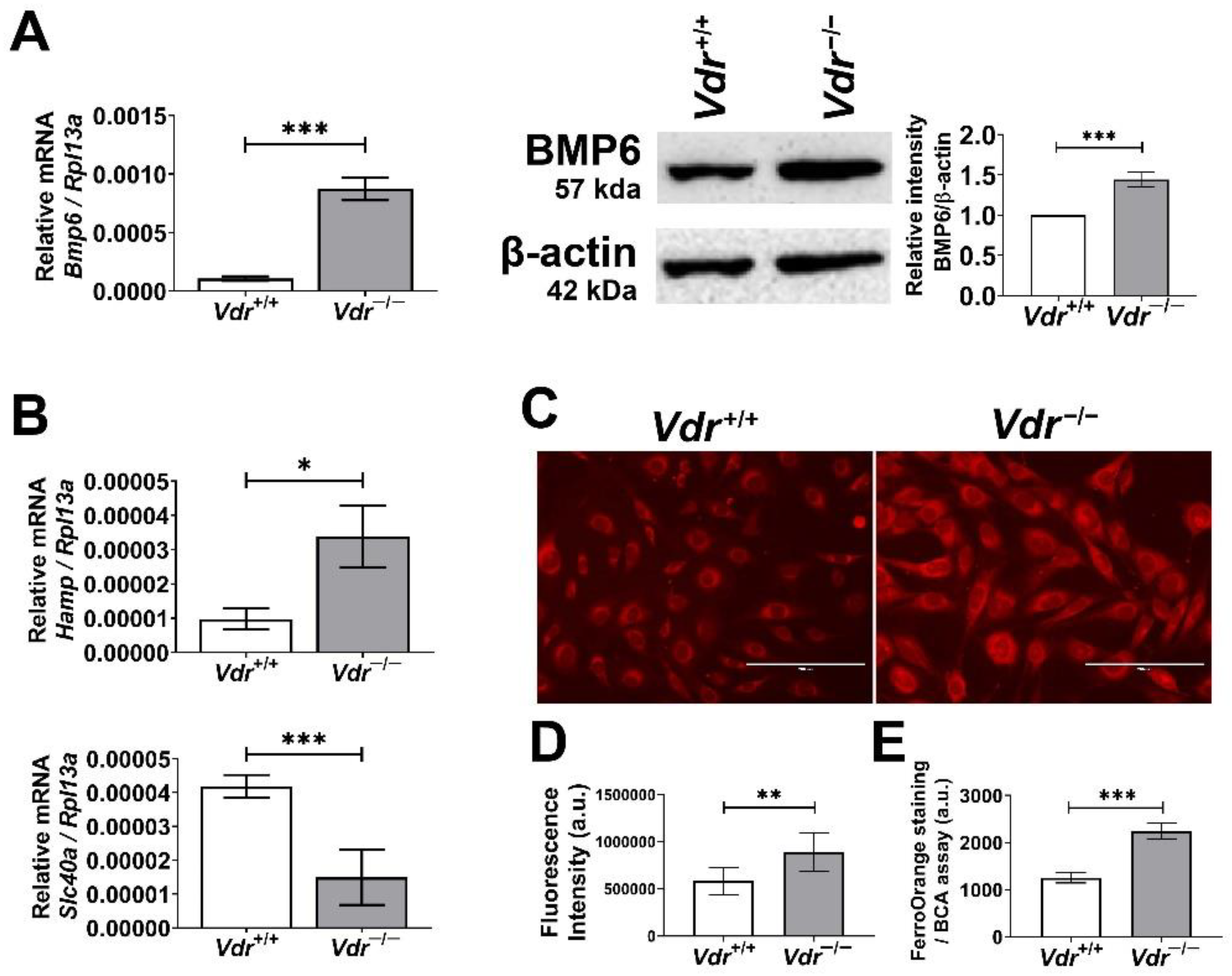
3.7. Increased Expression of BMP6 and Its Down-Stream Target Gene Hamp in Vdr−/− Retinal EC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin d and regulation of vascular cell function. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef] [Green Version]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin d is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Tay, H.M.; Yeap, W.H.; Dalan, R.; Wong, S.C.; Hou, H.W. Increased monocyte-platelet aggregates and monocyte-endothelial adhesion in healthy individuals with vitamin d deficiency. FASEB J. 2020, 34, 11133–11142. [Google Scholar] [CrossRef]
- Guo, Y.X.; He, L.Y.; Zhang, M.; Wang, F.; Liu, F.; Peng, W.X. 1,25-dihydroxyvitamin d3 regulates expression of lrp1 and rage in vitro and in vivo, enhancing aβ1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience 2016, 322, 28–38. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuki, S.; Nezu, Y.; Koitabashi, Y.; Murata, S.; Terasaki, T. 1α,25-dihydroxyvitamin d3 enhances cerebral clearance of human amyloid-β peptide(1-40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS 2011, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ichiyama, T.; Ohsaki, A.; Hasegawa, S.; Shiraishi, M.; Furukawa, S. Anti-inflammatory effect of 1α,25-dihydroxyvitamin d3 in human coronary arterial endothelial cells: Implication for the treatment of kawasaki disease. J. Steroid Biochem. Mol. Biol. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Gonzalez-Pardo, V.; D’Elia, N.; Verstuyf, A.; Boland, R.; Russo de Boland, A. Nfκb pathway is down-regulated by 1α,25(oh)(2)-vitamin d(3) in endothelial cells transformed by kaposi sarcoma-associated herpes virus g protein coupled receptor. Steroids 2012, 77, 1025–1032. [Google Scholar] [CrossRef]
- Cimmino, G.; Morello, A.; Conte, S.; Pellegrino, G.; Marra, L.; Golino, P.; Cirillo, P. Vitamin d inhibits tissue factor and cams expression in oxidized low-density lipoproteins-treated human endothelial cells by modulating nf-κb pathway. Eur. J. Pharmacol. 2020, 885, 173422. [Google Scholar] [CrossRef]
- Lai, C.C.; Juang, W.C.; Sun, G.C.; Tseng, Y.K.; Jhong, R.C.; Tseng, C.J.; Wong, T.Y.; Cheng, P.W. Vitamin d attenuates loss of endothelial biomarker expression in cardio-endothelial cells. Int. J. Mol. Sci. 2020, 21, 2196. [Google Scholar] [CrossRef]
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin d prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin d receptor-mediated nf-kb signaling pathways. PLoS ONE 2015, 10, e0122821. [Google Scholar] [CrossRef] [Green Version]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin d protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2013, 99, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Haas, M.J.; Jafri, M.; Wehmeier, K.R.; Onstead-Haas, L.M.; Mooradian, A.D. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin d in endothelial cells. Free Radic. Biol. Med. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Jamali, N.; Wang, S.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Vitamin d receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(oh)2d3. PLoS ONE 2017, 12, e0190131. [Google Scholar] [CrossRef] [Green Version]
- Jamali, N.; Song, Y.-S.; Sorenson, C.M.; Sheibani, N. 1,25(oh)2d3 regulates the proangiogenic activity of pericyte through vdr-mediated modulation of vegf production and signaling of vegf and pdgf receptors. FASEB BioAvances 2019, 1, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A role for vegf as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef]
- Martínez-Miguel, P.; Valdivielso, J.M.; Medrano-Andrés, D.; Román-García, P.; Cano-Peñalver, J.L.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D.; López-Ongil, S. The active form of vitamin d, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1085–E1096. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Watts, S.W.; Ng, M.; Chen, S.; Glenn, D.J.; Gardner, D.G. Elimination of vitamin d receptor in vascular endothelial cells alters vascular function. Hypertension 2014, 64, 1290–1298. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Gu, B.; Gu, Y.; Groome, L.J.; Sun, J.; Wang, Y. Activation of vitamin d receptor promotes vegf and cuzn-sod expression in endothelial cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Ai, S.; He, Z.; Ding, R.; Wu, F.; Huang, Z.; Wang, J.; Huang, S.; Dai, X.; Zhang, J.; Chen, J.; et al. Reduced vitamin d receptor on circulating endothelial progenitor cells: A new risk factor of coronary artery diseases. J. Atheroscler. Thromb. 2018, 25, 410–421. [Google Scholar] [CrossRef]
- Bozic, M.; Álvarez, Á.; de Pablo, C.; Sanchez-Niño, M.-D.; Ortiz, A.; Dolcet, X.; Encinas, M.; Fernandez, E.; Valdivielso, J.M. Impaired vitamin d signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: Implications for atherosclerosis development. PLoS ONE 2015, 10, e0136863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baecker, V. Imagej macro tool sets for biological image analysis. In Proceedings of the ImageJ User and Developer Conference, Luxembourg, 24–26 October 2012. Centre de Recherche Public Henri Tudor. [Google Scholar]
- Zhu, Y.; Sun, Y.; Xie, L.; Jin, K.; Sheibani, N.; Greenberg, D.A. Hypoxic induction of endoglin via mitogen-activated protein kinases in mouse brain microvascular endothelial cells. Stroke 2003, 34, 2483–2488. [Google Scholar] [CrossRef]
- Giannotta, M.; Trani, M.; Dejana, E. Ve-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampietro, C.; Taddei, A.; Corada, M.; Sarra-Ferraris, G.M.; Alcalay, M.; Cavallaro, U.; Orsenigo, F.; Lampugnani, M.G.; Dejana, E. Overlapping and divergent signaling pathways of n-cadherin and ve-cadherin in endothelial cells. Blood 2012, 119, 2159–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiers, J.L.; Peng, X.; Fanning, A.S.; DeMali, K.A. Zo-1 recruitment to α-catenin–a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J. Cell Sci 2013, 126, 3904–3915. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.; Han, G.; Seshadri, M.; Gillard, B.M.; Yu, W.D.; Foster, B.A.; Trump, D.L.; Johnson, C.S. Role of vitamin d receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009, 69, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [Green Version]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Yen, M.-C.; Liao, S.-H.; Hsu, Y.-L.; Lai, C.-S.; Chang, K.-P.; Hsu, Y.-L. Secreted protein acidic and rich in cysteine (sparc) enhances cell proliferation, migration, and epithelial mesenchymal transition, and sparc expression is associated with tumor grade in head and neck cancer. Int J. Mol. Sci 2017, 18, 1556. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.W.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, S.G.; Fischer, A.J.; Martin, S.M.; Schafer, F.Q.; Buettner, G.R. Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic. Biol. Med. 2006, 40, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, L.-M.; Sánchez-Duffhues, G.; Ten Dijke, P.; Yu, P.B. Bone morphogenetic protein 6 and oxidized low-density lipoprotein synergistically recruit osteogenic differentiation in endothelial cells. Cardiovasc. Res. 2015, 108, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Benn, A.; Bredow, C.; Casanova, I.; Vukičević, S.; Knaus, P. Ve-cadherin facilitates bmp-induced endothelial cell permeability and signaling. J. Cell Sci. 2016, 129, 206–218. [Google Scholar] [CrossRef] [Green Version]
- Canali, S.; Zumbrennen-Bullough, K.B.; Core, A.B.; Wang, C.-Y.; Nairz, M.; Bouley, R.; Swirski, F.K.; Babitt, J.L. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 2016, blood-2016-2006-721571. [Google Scholar] [CrossRef] [Green Version]
- Picard, E.; Daruich, A.; Youale, J.; Courtois, Y.; Behar-Cohen, F. From rust to quantum biology: The role of iron in retina physiopathology. Cells 2020, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-L.; Senecal, T.; Ghosh, M.C.; Ollivierre-Wilson, H.; Tu, T.; Rouault, T.A. Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood 2011, 118, 2868–2877. [Google Scholar] [CrossRef] [Green Version]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin d. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Pike, J.W.; Meyer, M.B.; Lee, S.M.; Onal, M.; Benkusky, N.A. The vitamin d receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin d and human health: Lessons from vitamin d receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, O.; Hanel, A.; Carlberg, C. Key vitamin d target genes with functions in the immune system. Nutrients 2020, 12, 1140. [Google Scholar] [CrossRef] [Green Version]
- Cardús, A.; Parisi, E.; Gallego, C.; Aldea, M.; Fernández, E.; Valdivielso, J.M. 1,25-dihydroxyvitamin d3 stimulates vascular smooth muscle cell proliferation through a vegf-mediated pathway. Kidney Int. 2006, 69, 1377–1384. [Google Scholar] [CrossRef] [Green Version]
- Reins, R.Y.; McDermott, A.M. Vitamin d: Implications for ocular disease and therapeutic potential. Exp. Eye Res. 2015, 134, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Nebbioso, M.; Buomprisco, G.; Pascarella, A.; Pescosolido, N. Modulatory effects of 1,25-dihydroxyvitamin d3 on eye disorders: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 57, 559–565. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, P.; Guan, H.; Huang, Z.; He, X.; Wan, X.; Xiao, H.; Li, Y. Vitamin d and its receptor regulate lipopolysaccharide-induced transforming growth factor-β, angiotensinogen expression and podocytes apoptosis through the nuclear factor-κb pathway. J. Diabetes Investig 2016, 7, 680–688. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gu, Y.; Lewis, D.F.; Cooper, D.B.; McCathran, C.E.; Wang, Y. Downregulation of vitamin d receptor and mir-126-3p expression contributes to increased endothelial inflammatory response in preeclampsia. Am. J. Reprod. Immunol. 2019, 82, e13172. [Google Scholar] [CrossRef]
- Demyanets, S.; Stojkovic, S.; Huber, K.; Wojta, J. The paradigm change of il-33 in vascular biology. Int. J. Mol. Sci. 2021, 22, 13288. [Google Scholar] [CrossRef]
- Cao, K.; Liao, X.; Lu, J.; Yao, S.; Wu, F.; Zhu, X.; Shi, D.; Wen, S.; Liu, L.; Zhou, H. Il-33/st2 plays a critical role in endothelial cell activation and microglia-mediated neuroinflammation modulation. J. Neuroinflamm. 2018, 15, 136. [Google Scholar] [CrossRef] [Green Version]
- Demyanets, S.; Konya, V.; Kastl, S.P.; Kaun, C.; Rauscher, S.; Niessner, A.; Pentz, R.; Pfaffenberger, S.; Rychli, K.; Lemberger, C.E.; et al. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arter. Thromb. Vasc. Biol. 2011, 31, 2080–2089. [Google Scholar] [CrossRef]
- Aoki, S.; Hayakawa, M.; Ozaki, H.; Takezako, N.; Obata, H.; Ibaraki, N.; Tsuru, T.; Tominaga, S.; Yanagisawa, K. St2 gene expression is proliferation-dependent and its ligand, il-33, induces inflammatory reaction in endothelial cells. Mol. Cell. Biochem. 2010, 335, 75–81. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, H.J.; Min, J.K.; Pyun, B.J.; Maeng, Y.S.; Park, H.; Kim, J.; Kim, Y.M.; Kwon, Y.G. Interleukin-33 induces angiogenesis and vascular permeability through st2/traf6-mediated endothelial nitric oxide production. Blood 2009, 114, 3117–3126. [Google Scholar] [CrossRef]
- Jang, T.Y.; Kim, Y.H. Interleukin-33 and mast cells bridge innate and adaptive immunity: From the allergologist’s perspective. Int. Neurourol. J. 2015, 19, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Saluja, R.; Khan, M.; Church, M.K.; Maurer, M. The role of il-33 and mast cells in allergy and inflammation. Clin. Transl. Allergy 2015, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Umebashi, K.; Tokito, A.; Yamamoto, M.; Jougasaki, M. Interleukin-33 induces interleukin-8 expression via jnk/c-jun/ap-1 pathway in human umbilical vein endothelial cells. PLoS ONE 2018, 13, e0191659. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Tian, J.; Zheng, W.; Fan, Q.; Wu, X.; Tang, Y.; Liu, T.; Yin, H. Interleukin-33 protects mice against hindlimb ischemic injury by enhancing endothelial angiogenesis. Int. Immunopharmacol. 2022, 109, 108850. [Google Scholar] [CrossRef]
- Sharma, D.; Bisen, S.; Kaur, G.; Van Buren, E.C.; Rao, G.N.; Singh, N.K. Il-33 enhances jagged1 mediated notch1 intracellular domain (nicd) deubiquitination and pathological angiogenesis in proliferative retinopathy. Commun. Biol. 2022, 5, 479. [Google Scholar] [CrossRef]
- Stojkovic, S.; Kaun, C.; Basilio, J.; Rauscher, S.; Hell, L.; Krychtiuk, K.A.; Bonstingl, C.; de Martin, R.; Gröger, M.; Ay, C.; et al. Tissue factor is induced by interleukin-33 in human endothelial cells: A new link between coagulation and inflammation. Sci. Rep. 2016, 6, 25171. [Google Scholar] [CrossRef] [Green Version]
- Someya, H.; Ito, M.; Nishio, Y.; Sato, T.; Harimoto, K.; Takeuchi, M. Osteopontin-induced vascular hyperpermeability through tight junction disruption in diabetic retina. Exp. Eye Res. 2022, 220, 109094. [Google Scholar] [CrossRef]
- Rivera, L.B.; Bradshaw, A.D.; Brekken, R.A. The regulatory function of sparc in vascular biology. Cell. Mol. Life Sci. 2011, 68, 3165–3173. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, M.; Xiang, X.; Liu, K.; Xu, X. Glucose affects cell viability, migration, angiogenesis and cellular adhesion of human retinal capillary endothelial cells via sparc. Exp. Ther. Med. 2019, 17, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Alkabie, S.; Basivireddy, J.; Zhou, L.; Roskams, J.; Rieckmann, P.; Quandt, J.A. Sparc expression by cerebral microvascular endothelial cells in vitro and its influence on blood-brain barrier properties. J. Neuroinflamm. 2016, 13, 225. [Google Scholar] [CrossRef] [Green Version]
- Masli, S.; Sheibani, N.; Cursiefen, C.; Zieske, J. Matricellular protein thrombospondins: Influence on ocular angiogenesis, wound healing and immuneregulation. Curr. Eye Res. 2014, 39, 759–774. [Google Scholar] [CrossRef] [Green Version]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Albarrán-Juárez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and g(q)/g(11) promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel piezo1 controls blood pressure by mediating flow-induced atp release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Pastor, C.; Rubio-Moscardo, F.; Vogel-González, M.; Serra, S.A.; Afthinos, A.; Mrkonjic, S.; Destaing, O.; Abenza, J.F.; Fernández-Fernández, J.M.; Trepat, X.; et al. Piezo2 channel regulates rhoa and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. USA 2018, 115, 1925–1930. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Cai, Z.; Sen, P.; van Uden, D.; van de Kamp, E.; Thuillet, R.; Tu, L.; Guignabert, C.; Boomars, K.; Van der Heiden, K.; et al. Loss of lung microvascular endothelial piezo2 expression impairs no synthesis, induces endmt, and is associated with pulmonary hypertension. Am. J. Physiol Heart Circ. Physiol 2022, 323, H958–H974. [Google Scholar] [CrossRef]
- Andriopoulos Jr, B.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y. Bmp6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 2009, 41, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, B.H.; Shu, W.; Song, Y.; Simpson, E.M.; Lakhal-Littleton, S.; Dunaief, J.L. Ferroportin-mediated iron export from vascular endothelial cells in retina and brain. Exp. Eye Res. 2019, 187, 107728. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Baumann, B.H.; Song, Y.; Liu, Y.; Wu, X.; Dunaief, J.L. Iron accumulates in retinal vascular endothelial cells but has minimal retinal penetration after ip iron dextran injection in mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4378–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashok, A.; Chaudhary, S.; McDonald, D.; Kritikos, A.; Bhargava, D.; Singh, N. Local synthesis of hepcidin in the anterior segment of the eye: A novel observation with physiological and pathological implications. Exp. Eye Res. 2020, 190, 107890. [Google Scholar] [CrossRef]
- Kawabata, T. Iron-induced oxidative stress in human diseases. Cells 2022, 11, 2152. [Google Scholar] [CrossRef]





| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| Vdr | GGCTTCCACTTCAACGCTATG | TGCTCCGCCTGAAGAAACC |
| Mcp-1 | GTCTGTGCTGACCCCAAGAAG | TGGTTCCGATCCAGGTTTTTA |
| Il-6 | CAACCACGGCCTTCCCTACT | TTGGGAGTGGTATCCTCTGTGA |
| Il-33 | GGTGAACATGAGTCCCATCA | CGTCACCCCTTTGAAGCTC |
| St2 | GGACCATCAAGTGGAGGGAA | GCACTGGCATTTGGTACCTC |
| Cxcl1 | ACAGGGGCGCCTATCGCCAA | CGGTTTGGGTGCAGTGGGGC |
| Cxcl2 | CCCTTGGACATTTTATGTCTTCC | GACACGAAAAGGCATGACAA |
| Icam1 | GCCATAAAACTCAAGGGACAA | GGCTGAGGGTAAATGCTGTC |
| Vcam1 | TCGCGGTCTTGGGAGCCTCA | TGACTCGCAGCCCGTAGTGC |
| Ager | GTCACAGAAACCGGCGAT | TACTACTCCCAGGCCTCCC |
| F3 | AAGTGCTTCTCGACCACAGA | TGGGACAGAGAGGACCTTTG |
| Fas | TGCTTGCTGGCTCACAGTTA | TATCAGTTTCACGAACCCGC |
| Vegf | GGAGAGCAGAAGTCCCATGA | ACTCCAGGGCTTCATCGTTA |
| Stat3 | ACCAACATCCTGGTGTCTCC | CACTACCTGGGTCGGCTTC |
| Plau | CGATTCTGGAGGACCGCTTA | GACACCGGGCTTGTTTTTCT |
| Nos2 | GGCAGCCTGTGAGACCTTTG | CATTGGAAGTGAAGCGTTTCG |
| Piezo1 | TCCTCTTCCTCATCGCCATC | AGGGTGACGGTGACATCAAT |
| Piezo2 | CCCATTTCTGACTGAGCTGC | ATGTGCGCGTAAATGTCCTC |
| Bmp6 | GTGACACCGCAGCACAAC | TCGTAAGGGCCGTCTCTG |
| Hamp | GCATCTTCTGCTGTAAATGCTG | TGGCTCTAGGCTATGTTTTGC |
| Slc40a | ATGTGAACAAGAGCCCACCT | CCCATCCATCTCGGAAAGT |
| Rpl13a | TCTCAAGGTTGTTCGGCTGAA | GCCAGACGCCCCAGGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-S.; Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells. Cells 2023, 12, 335. https://doi.org/10.3390/cells12020335
Song Y-S, Jamali N, Sorenson CM, Sheibani N. Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells. Cells. 2023; 12(2):335. https://doi.org/10.3390/cells12020335
Chicago/Turabian StyleSong, Yong-Seok, Nasim Jamali, Christine M. Sorenson, and Nader Sheibani. 2023. "Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells" Cells 12, no. 2: 335. https://doi.org/10.3390/cells12020335





