Recent Achievements in the Heterogeneity of Mammalian and Human Retinal Pigment Epithelium: In Search of a Stem Cell
Abstract
:1. Introduction
2. Morphological Heterogeneity of the Retinal Pigment Epithelium
3. Morphometric Studies of the Adult Human Retinal Pigment Epithelium
4. Molecular and Functional Heterogeneity of the Retinal Pigment Epithelium
4.1. Differences of the Human Macular and Peripheral Retinal Pigment Epithelium
| Human Macular RPE | ||
| Marker Genes | Functions | References |
| ID3 | Macular RPE marker. A transcription factor that prevents epithelial–mesenchymal transition (EMT) in epithelial cells [61]. | [2] |
| CTGF | Connective tissue growth factor. Angiogenesis, cell adhesion, and cell migration Promotes EMT. | [2,62] |
| FST | Follistatin neutralizes the effect of activin and other members of the TGF-β superfamily and is involved in the processes of neurogenesis. | [2] |
| KCNMA1 | This gene encodes the alpha subunit of the calcium-activated BK channel and is involved neuronal excitability. | [2] |
| ADIRF | A factor involved in the development of the nervous system. | [2] |
| IRX3 | A transcription factor involved in cell growth and adhesion to the substrate. | [2] |
| KLF2 | A transcription factor involved in the organization of the extracellular matrix. | [2] |
| FOXP1 | A transcription factor involved in the development of the visual system. | [2,63] |
| EGFR | Receptor. Autophagy, phagocytosis, and proliferation of RPE cells. | [2] |
| NR1 | Receptor. Angiogenesis and development of the nervous system. | [2] |
| CXCL14 | A chemokine that regulates the migration of immune cells and increases angiogenesis. | [21] |
| WFDC1 | Protease inhibitor. | [2] |
| c-KIT reduced | A cell cycle gene. | [25] |
| GSTM1 reduced | A risk factor for age-related macular degeneration. | [25] |
| ALD6 reduced | ALD6 is an important enzyme in the synthesis of retinoic acid. | [25] |
| Human Peripheral RPE | ||
| Marker Genes | Functions | References |
| CRYAB | Crystallin Alpha B. Participates in the cellular response to stimuli and the cellular response to heat stress. Peripheral RPE marker. | [2] |
| IGFBP5 | Participates in the regulation of migration and the proliferation of smooth muscle cells, as well as in the binding of fibronectin and insulin-like growth factor I. | [2] |
| RARRES | Retinoic acid receptor. Participates in cell differentiation and the negative regulation of cell proliferation. | [2] |
| FBXO32 | F-Box protein 32 that mediates ubiquitination and subsequent proteasomal degradation of target proteins. | [2] |
| CHCHD10 | A mitochondrial protein involved in peroxisomal lipid metabolism. | [2] |
| EGFR ligands | Autophagy, phagocytosis, and proliferation of RPE cells. | [2] |
| NFIB | Participates in the organization of the basement membrane and in the transmembrane transport of sodium ions. | [2] |
| TFPI2 | Participates in RPE proliferation in vitro. | [22] |
| IGFBP5 | Participates in reducing neovascularization. | [22] |
| TYRP1 | Melanin synthesis [64]. RPE subpopulation Pr 2-1 marker. | [2] |
| RLBP1 | Associated with the visual cycle [65]. RPE subpopulation Pr 2-1 marker. | [2] |
4.2. Molecular Heterogeneity of Mammalian Retinal Pigment Epithelium Cells
| Human RPE Subpopulations [2] | GO | Description | Mouse RPE Clusters [67] | Mouse RPE [26] |
|---|---|---|---|---|
| M 1 | GO:0034109 | homotypic cell–cell adhesion | C1 | |
| GO:0031589 | cell–substrate adhesion | C1 | ||
| GO:0030198 | extracellular matrix organization | C1, C2 | C2 | |
| GO:0045229 | external encapsulating structure organization | C1, C2 | ||
| GO:0034446 | substrate adhesion-dependent cell spreading | C1 | ||
| GO:0010810 | regulation of cell–substrate adhesion | C1 | C2 | |
| GO:0034329 | cell junction assembly | C5 | ||
| GO:0150115 | cell–substrate junction organization | C5 | ||
| M 2 | GO:0006457 | protein folding | C4 | |
| GO:0050821 | protein stabilization | C4 | ||
| Pr 1 | GO:0001667 | ameboidal-type cell migration | C1, C5, C3 | |
| GO:0010038 | response to metal ion | C1, C5 | ||
| GO:0050673 | epithelial cell proliferation | C1, C5 | ||
| GO:0050678;GO:0050673 | regulation of epithelial cell proliferation | C1, C5 | C2 | |
| GO:0071294 | cellular response to zinc ion | C1, C5, C2 | C1 | |
| GO:2001026 | regulation of endothelial cell chemotaxis | C5 | ||
| GO:0035767 | endothelial cell chemotaxis | C5 | ||
| Pr 2-1 | GO:2001057 | reactive nitrogen species metabolic process | C1 | |
| GO:0098869 | cellular oxidant detoxification | C2 | ||
| GO:0007601 | visual perception | C4, C6 | C1 | |
| GO:0001523 | retinoid metabolic process | C6 | C1 | |
| Pr 2-2 | GO:0030301 | cholesterol transport | C3, C6 | |
| Pr 3 | GO:0042542 | response to hydrogen peroxide | C5 | |
| GO:0030336 | negative regulation of cell migration | C1 | C2 | |
| GO:0001952 | regulation of cell–matrix adhesion | C5 | ||
| Pr 4 | GO:0062012 | regulation of small molecule metabolic process | C5 | |
| GO:0071248 | cellular response to metal ion | C5 | ||
| Pr 5 | GO:0031667 | response to nutrient levels | C3, C5 | |
| GO:0043270 | positive regulation of ion transport | C1 | ||
| Pr 6 | GO:0008203 | cholesterol metabolic process | C3 | |
| Pr 7 | GO:0006575 | cellular modified amino acid metabolic process | C1 |
5. Heterogeneity of Retinal Pigment Epithelium Derived from Induced Pluripotent Stem Cells
6. Proliferation and Plasticity of Retinal Pigment Epithelium Cells
6.1. Proliferation of Retinal Pigment Epithelium Cells
6.2. Expression of Stem Cell Markers and Differentiation Potential of the RPE Cells
6.3. Differentiation of Stem/Progenitor Cells from the Retinal Pigment Epithelium into Muscle and Adipo-, Osteo-, and Chondrogenic Cells
6.4. Differentiation of Stem/Progenitor Cells from the Retinal Pigment Epithelium along the Neuronal Pathway
6.5. Differentiation of Stem/Progenitor Cells from the Retinal Pigment Epithelium into Retinal Photoreceptor Cells
6.6. Differentiation of Stem/Progenitor Cells from the Retinal Pigment Epithelium along the RPE Pathway (Redifferentiation)
6.7. The Potential of Retinal Pigment Epithelium Cell Subpopulations for Transplantation
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortolan, D.; Sharma, R.; Volkov, A.; Maminishkis, A.; Hotaling, N.A.; Huryn, L.A.; Cukras, C.; Di Marco, S.; Bisti, S.; Bharti, K. Single-Cell–Resolution Map of Human Retinal Pigment Epithelium Helps Discover Subpopulations with Differential Disease Sensitivity. Proc. Natl. Acad. Sci. USA 2022, 119, e2117553119. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liao, X.; Li, N.; Zhou, H.; Li, H.; Zhang, Q.; Hu, K.; Yang, P.; Hou, S. A Single-Cell Transcriptome Atlas of the Human Retinal Pigment Epithelium. Front. Cell Dev. Biol. 2021, 9, 3534. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The Cell Biology of the Retinal Pigment Epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef] [PubMed]
- Sparrrow, J.R.; Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Markitantova, Y.; Simirskii, V. Endogenous and Exogenous Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells: An Updated Antioxidant Perspective. Int. J. Mol. Sci. 2023, 24, 10776. [Google Scholar] [CrossRef]
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative Vitreoretinopathy after Eye Injuries: An Overexpression of Growth Factors and Cytokines Leading to a Retinal Keloid. Mediat. Inflamm. 2013, 2013, 269787. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 1976. [Google Scholar] [CrossRef]
- Ach, T.; Huisingh, C.; McGwin, G.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Theodore Smith, R.; Sloan, K.R.; et al. Quantitative Autofluorescence and Cell Density Maps of the Human Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Kapphahn, R.J.; Stahl, M.R.; Olsen, T.W.; Ferrington, D.A. Dissecting Regulators of Aging and Age-Related Macular Degeneration in the Retinal Pigment Epithelium. Oxid. Med. Cell. Longev. 2022, 2022, 6009787. [Google Scholar] [CrossRef]
- Gu, X.; Neric, N.J.; Crabb, J.S.; Crabb, J.W.; Bhattacharya, S.K.; Rayborn, M.E.; Hollyfield, J.G.; Bonilha, V.L. Age-Related Changes in the Retinal Pigment Epithelium (RPE). PLoS ONE 2012, 7, e38673. [Google Scholar] [CrossRef]
- Strunnikova, N.V.; Maminishkis, A.; Barb, J.J.; Wang, F.; Zhi, C.; Sergeev, Y.; Chen, W.; Edwards, A.O.; Stambolian, D.; Abecasis, G.; et al. Transcriptome Analysis and Molecular Signature of Human Retinal Pigment Epithelium. Hum. Mol. Genet. 2010, 19, 2468–2486. [Google Scholar] [CrossRef] [PubMed]
- Aldiri, I.; Xu, B.; Wang, L.; Chen, X.; Hiler, D.; Griffiths, L.; Valentine, M.; Shirinifard, A.; Thiagarajan, S.; Sablauer, A.; et al. The Dynamic Epigenetic Landscape of the Retina During Development, Reprogramming, and Tumorigenesis. Neuron 2017, 94, 550–568.e10. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lee, R.; Lim, S.Y.; Zhong, Z.; Wang, J.; Liu, Y.; Fan, G. Global Transcriptional and Epigenetic Reconfiguration during Chemical Reprogramming of Human Retinal Pigment Epithelial Cells into Photoreceptor-like Cells. Cells 2022, 11, 3146. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Ratnapriya, R.; Brooks, M.J.; Chaitankar, V.; Wilken, M.S.; Zhang, C.; Starostik, M.R.; Gieser, L.; La Torre, A.; Nishio, M.; et al. Molecular Anatomy of the Developing Human Retina. Dev. Cell 2017, 43, 763–779.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 2017, 20, 858–873.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jia, C.; Kazmierkiewicz, K.L.; Bowman, A.S.; Tian, L.; Liu, Y.; Gupta, N.A.; Gudiseva, H.V.; Yee, S.S.; Kim, M.; et al. Comprehensive Analysis of Gene Expression in Human Retina and Supporting Tissues. Hum. Mol. Genet. 2014, 23, 4001. [Google Scholar] [CrossRef] [PubMed]
- Rheaume, B.A.; Jereen, A.; Bolisetty, M.; Sajid, M.S.; Yang, Y.; Renna, K.; Sun, L.; Robson, P.; Trakhtenberg, E.F. Single Cell Transcriptome Profiling of Retinal Ganglion Cells Identifies Cellular Subtypes. Nat. Commun. 2018, 9, 2759. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, K.; Lapan, S.W.; Whitney, I.E.; Tran, N.M.; Macosko, E.Z.; Kowalczyk, M.; Adiconis, X.; Levin, J.Z.; Nemesh, J.; Goldman, M.; et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 2016, 166, 1308–1323.e30. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.P.; Mullin, N.K.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-Cell RNA Sequencing in Vision Research: Insights into Human Retinal Health and Disease. Prog. Retin. Eye Res. 2021, 83, 100934. [Google Scholar] [CrossRef]
- Voigt, A.P.; Mulfaul, K.; Mullin, N.K.; Flamme-Wiese, M.J.; Giacalone, J.C.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-Cell Transcriptomics of the Human Retinal Pigment Epithelium and Choroid in Health and Macular Degeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 24100–24107. [Google Scholar] [CrossRef]
- Voigt, A.P.; Whitmore, S.S.; Flamme-Wiese, M.J.; Riker, M.J.; Wiley, L.A.; Tucker, B.A.; Stone, E.M.; Mullins, R.F.; Scheetz, T.E. Molecular Characterization of Foveal versus Peripheral Human Retina by Single-Cell RNA Sequencing. Exp. Eye Res. 2019, 184, 234. [Google Scholar] [CrossRef]
- Whitmore, S.S.; Wagner, A.H.; DeLuca, A.P.; Drack, A.V.; Stone, E.M.; Tucker, B.A.; Zeng, S.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E. Transcriptomic Analysis across Nasal, Temporal, and Macular Regions of Human Neural Retina and RPE/Choroid by RNA-Seq. Exp. Eye Res. 2014, 129, 93. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Peng, Y.R.; van Zyl, T.; Regev, A.; Shekhar, K.; Juric, D.; Sanes, J.R. Cell Atlas of The Human Fovea and Peripheral Retina. Sci. Rep. 2020, 10, 9802. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tian, J.; Handa, J.T. Similarity of MRNA Phenotypes of Morphologically Normal Macular and Peripheral Retinal Pigment Epithelial Cells in Older Human Eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.Y.; Chae, J.B.; Park, C.W.; Kim, C.; Ryu, J.H.; Jang, J.; Kim, N.; Chung, H. Single-Cell Transcriptome of the Mouse Retinal Pigment Epithelium in Response to a Low-Dose of Doxorubicin. Commun. Biol. 2022, 5, 722. [Google Scholar] [CrossRef]
- Shen, H.; Ding, C.; Yuan, S.; Pan, T.; Li, D.; Li, H.; Huang, B.; Liu, Q. Vitamin C- and Valproic Acid-Induced Fetal RPE Stem-like Cells Recover Retinal Degeneration via Regulating SOX2. Mol. Ther. 2020, 28, 1645–1657. [Google Scholar] [CrossRef]
- Starnes, A.C.; Huisingh, C.; McGwin, G.; Sloan, K.R.; Ablonczy, Z.; Smith, R.T.; Curcio, C.A.; Ach, T. Multi-Nucleate Retinal Pigment Epithelium Cells of the Human Macula Exhibit a Characteristic and Highly Specific Distribution. Vis. Neurosci. 2016, 33, e001. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human Retinal Pigment Epithelium: In Vivo Cell Morphometry, Multispectral Autofluorescence, and Relationship to Cone Mosaic. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yu, H.; Summers, V.R.; Donaldson, K.J.; Ferdous, S.; Shelton, D.; Zhang, N.; Chrenek, M.A.; Jiang, Y.; Grossniklaus, H.E.; et al. Morphometric Analysis of Retinal Pigment Epithelial Cells From C57BL/6J Mice During Aging. Investig. Ophthalmol. Vis. Sci. 2021, 62, 32. [Google Scholar] [CrossRef]
- Burke, J.M.; Hjelmeland, L.M. Mosaicism of the Retinal Pigment Epithelium: Seeing the Small Picture. Mol. Interv. 2005, 5, 241–249. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Rashid, A.; Chrenek, M.A.; Zhang, Q.; Bruce, B.B.; Klein, M.; Boatright, J.H.; Jiang, Y.; Grossniklaus, H.E.; Nickerson, J.M. Analysis of RPE Morphometry in Human Eyes. Mol. Vis. 2016, 22, 898–916. [Google Scholar]
- Weirer, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal Pigment Epithelial Lipofuscin and Melanin and Choroidal Melanin in Human Eyes. Investig. Ophthalmol. Vis. Sci. 1986, 27, 145–152. [Google Scholar]
- Kim, J.; Lee, Y.J.; Won, J.Y. Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 12298. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K.; Wu, G.; Ebenstein, D.; Garsd, A.; Weiter, J.J. Cell Loss in the Aging Retina. Relationship to Lipofuscin Accumulation and Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1691–1699. [Google Scholar]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal Pigment Epithelial Cell Count, Distribution, and Correlations in Normal Human Eyes. Am. J. Ophthalmol. 1996, 121, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Priore, L.V.D.; Kuo, Y.-H.; Tezel, T.H. Age-Related Changes in Human RPE Cell Density and Apoptosis Proportion In Situ. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3312–3318. [Google Scholar]
- Ahnelt, P.K. The Photoreceptor Mosaic. Eye 1998, 12 Pt 3b, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Gong, L.; Zhu, X.; Qi, R.; Zou, M.; Chen, B.; Liu, W.; Huang, S.; Liu, Y.; Li, D.W.C. Multinucleated Retinal Pigment Epithelial Cells Adapt to Vision and Exhibit Increased DNA Damage Response. Cells 2022, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.; Hollyfield, J.G. Aging of the Human Retina. Differential Loss of Neurons and Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Watzke, R.C.; Soldevilla, J.D.; Trune, D.R. Morphometric Analysis of Human Retinal Pigment Epithelium: Correlation with Age and Location. Curr. Eye Res. 1993, 12, 133–142. [Google Scholar] [CrossRef]
- Fleming, P.A.; Harman, A.M.; Beazley, L.D. Development and Ageing of the RPE in a Marsupial, the Quokka. Exp. Eye Res. 1996, 62, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Ts’o, M.O.M.; Friedman, E. The Retinal Pigment Epithelium: III. Growth and Development. Arch. Ophthalmol. 1968, 80, 214–216. [Google Scholar] [CrossRef]
- van Soest, S.S.; de Wit, G.M.J.; Essing, A.H.W.; ten Brink, J.B.; Kamphuis, W.; de Jong, P.T.V.M.; Bergen, A.A.B. Comparison of Human Retinal Pigment Epithelium Gene Expression in Macula and Periphery Highlights Potential Topographic Differences in Bruch’s Membrane. Mol. Vis. 2007, 13, 1608–1617. [Google Scholar] [PubMed]
- Tian, L.; Kazmierkiewicz, K.L.; Bowman, A.S.; Li, M.; Curcio, C.A.; Stambolian, D.E. Transcriptome of the Human Retina, Retinal Pigmented Epithelium and Choroid. Genomics 2015, 105, 253–264. [Google Scholar] [CrossRef]
- Harman, A.M.; Fleming, P.A.; Hoskins, R.V.; Moore, S.R. Development and Aging of Cell Topography in the Human Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2016–2026. [Google Scholar]
- Hwang, B.; Lee, J.H.; Bang, D. Single-Cell RNA Sequencing Technologies and Bioinformatics Pipelines. Exp. Mol. Med. 2018, 50, 96. [Google Scholar] [CrossRef]
- Liang, Q.; Dharmat, R.; Owen, L.; Shakoor, A.; Li, Y.; Kim, S.; Vitale, A.; Kim, I.; Morgan, D.; Liang, S.; et al. Single-Nuclei RNA-Seq on Human Retinal Tissue Provides Improved Transcriptome Profiling. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Lukowski, S.W.; Lo, C.Y.; Sharov, A.A.; Nguyen, Q.; Fang, L.; Hung, S.S.; Zhu, L.; Zhang, T.; Grünert, U.; Nguyen, T.; et al. A Single-cell Transcriptome Atlas of the Adult Human Retina. EMBO J. 2019, 38, e100811. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Choi, J.; Li, J.; Ferdous, S.; Liang, Q.; Moffitt, J.R.; Chen, R. Spatial Organization of the Mouse Retina at Single Cell Resolution. bioRxiv 2022. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, X.; Wang, J.; Owen, L.; Shakoor, A.; Lillvis, J.L.; Zhang, C.; Farkas, M.; Kim, I.K.; Li, Y.; et al. A Multi-Omics Atlas of the Human Retina at Single-Cell Resolution. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Hu, B.; Mao, Y.; Chen, Y.; Yan, L.; Yong, J.; Dong, J.; Wei, Y.; Wang, W.; et al. Dissecting the Transcriptome Landscape of the Human Fetal Neural Retina and Retinal Pigment Epithelium by Single-Cell RNA-Seq Analysis. PLoS Biol. 2019, 17, e3000365. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shiau, F.; Yi, W.; Lu, S.; Wu, Q.; Pearson, J.D.; Kallman, A.; Zhong, S.; Hoang, T.; Zuo, Z.; et al. Single-Cell Analysis of Human Retina Identifies Evolutionarily Conserved and Species-Specific Mechanisms Controlling Development. Dev. Cell 2020, 53, 473. [Google Scholar] [CrossRef]
- Boulton, M.; Moriarty, P.; Jarvis-Evans, J.; Marcyniuk, B. Regional Variation and Age-Related Changes of Lysosomal Enzymes in the Human Retinal Pigment Epithelium. Br. J. Ophthalmol. 1994, 78, 125. [Google Scholar] [CrossRef] [PubMed]
- Rickman, C.B.; Ebright, J.N.; Zavodni, Z.J.; Yu, L.; Wang, T.; Daiger, S.P.; Wistow, G.; Boon, K.; Hauser, M.A. Defining the Human Macula Transcriptome and Candidate Retinal Disease Genes Using EyeSAGE. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulos, I.; Shahabi, G.; Colman, A.; Jeffery, G. Mature Peripheral RPE Cells Have an Intrinsic Capacity to Proliferate; A Potential Regulatory Mechanism for Age-Related Cell Loss. PLoS ONE 2011, 6, e18921. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Liu, W.; Wang, Y.; Xu, R.; Zeng, S.; Zhang, R.; Zhu, S.; Gillies, M.C.; Zhu, L.; et al. Metabolic Features of Mouse and Human Retinas: Rods versus Cones, Macula versus Periphery, Retina versus RPE. iScience 2020, 23, 101672. [Google Scholar] [CrossRef]
- Skeie, J.M.; Mahajan, V.B. Proteomic Landscape of the Human Choroid-Retinal Pigment Epithelial Complex. JAMA Ophthalmol. 2014, 132, 1271–1281. [Google Scholar] [CrossRef]
- Radeke, M.J.; Peterson, K.E.; Johnson, L.V.; Anderson, D.H. Disease Susceptibility of the Human Macula: Differential Gene Transcription in the Retinal Pigmented Epithelium/Choroid. Exp. Eye Res. 2007, 85, 366–380. [Google Scholar] [CrossRef]
- Kowanetz, M.; Valcourt, U.; Bergström, R.; Heldin, C.-H.; Moustakas, A. Id2 and Id3 Define the Potency of Cell Proliferation and Differentiation Responses to Transforming Growth Factor Beta and Bone Morphogenetic Protein. Mol. Cell Biol. 2004, 24, 4241–4254. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, T.; Wu, T.; Ye, W.; Wang, Y.; Dou, G.; Du, H.; Hui, Y.; Guo, C. Connective Tissue Growth Factor Promotes Retinal Pigment Epithelium Mesenchymal Transition via the PI3K/AKT Signaling Pathway. Mol. Med. Rep. 2021, 23, 389. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, S.; Lindmayer, C.; Hans, F.P.; Hoefer, I.; Helbing, T.; Pasterkamp, G.; Bode, C.; de Kleijn, D.; Moser, M. FoxP1 Stimulates Angiogenesis by Repressing the Inhibitory Guidance Protein Semaphorin 5B in Endothelial Cells. PLoS ONE 2013, 8, 70873. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Chatterjee, B.; Bhowmick, S.; Sagar, S.; Roy, S.S. Beclin 1 Controls Pigmentation by Changing the Nuclear Localization of Melanogenic Factor MITF. Biochem. Biophys. Res. Commun. 2020, 528, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Li, S.; Li, H.; Chen, Y.; Ma, X.; Wang, J.; Lu, F.; Qu, J.; Hou, L. Microphthalmia-Associated Transcription Factor Regulates the Visual Cycle Genes Rlbp1 and Rdh5 in the Retinal Pigment Epithelium. Sci. Rep. 2016, 6, 21208. [Google Scholar] [CrossRef]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.A.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-Cell Regulatory Network Inference and Clustering. Nat. Methods 2017, 14, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.S.; Krebs, M.P.; Bolisetty, M.T.; Charette, J.R.; Naggert, J.K.; Robson, P.; Nishina, P.M.; Carter, G.W. Single-Cell RNA Sequencing Reveals Molecular Features of Heterogeneity in the Murine Retinal Pigment Epithelium. Int. J. Mol. Sci. 2022, 23, 10419. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The Major Cell Populations of the Mouse Retina. J. Neurosci. 1998, 18, 8936. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y. The Challenge of Modeling Macular Degeneration in Mice. Trends Genet. 2007, 23, 225–231. [Google Scholar] [CrossRef]
- Markitantova, Y.V.; Simirskii, V.N. Conservatism and Variability of the Antioxidant Defense System in the Retinal Pigment Epithelium of Vertebrates. J. Evol. Biochem. Physiol. 2023, 59, 655–675. [Google Scholar] [CrossRef]
- Farjood, F.; Manos, J.D.; Wang, Y.; Williams, A.L.; Zhao, C.; Borden, S.; Alam, N.; Prusky, G.; Temple, S.; Stern, J.H.; et al. Identifying Biomarkers of Retinal Pigment Epithelial Cell Stem Cell-Derived RPE Cell Heterogeneity and Transplantation Efficacy. bioRxiv 2022. [Google Scholar] [CrossRef]
- Brandl, C. Generation of Functional Retinal Pigment Epithelium from Human Induced Pluripotent Stem Cells. Methods Mol. Biol. 2019, 1834, 87–94. [Google Scholar] [CrossRef]
- Foltz, L.P.; Clegg, D.O. Rapid, Directed Differentiation of Retinal Pigment Epithelial Cells from Human Embryonic or Induced Pluripotent Stem Cells. J. Vis. Exp. 2017, 2017, e56274. [Google Scholar] [CrossRef]
- Osakada, F.; Jin, Z.B.; Hirami, Y.; Ikeda, H.; Danjyo, T.; Watanabe, K.; Sasai, Y.; Takahashi, M. In Vitro Differentiation of Retinal Cells from Human Pluripotent Stem Cells by Small-Molecule Induction. J. Cell Sci. 2009, 122, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Sheets Aiming for Clinical Application. Stem. Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Lidgerwood, G.E.; Lim, S.Y.; Crombie, D.E.; Ali, R.; Gill, K.P.; Hernández, D.; Kie, J.; Conquest, A.; Waugh, H.S.; Wong, R.C.B.; et al. Defined Medium Conditions for the Induction and Expansion of Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Stem. Cell Rev. Rep. 2016, 12, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Mirshahi, R.; Shoae-Hassani, A.; Naseripour, M. Human-Induced Pluripotent Stem Cells-Derived Retinal Pigmented Epithelium, a New Horizon for Cells-Based Therapies for Age-Related Macular Degeneration. Stem. Cell Res. Ther. 2022, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Bennis, A.; Jacobs, J.G.; Catsburg, L.A.E.; ten Brink, J.B.; Koster, C.; Schlingemann, R.O.; van Meurs, J.; Gorgels, T.G.M.F.; Moerland, P.D.; Heine, V.M.; et al. Stem Cell Derived Retinal Pigment Epithelium: The Role of Pigmentation as Maturation Marker and Gene Expression Profile Comparison with Human Endogenous Retinal Pigment Epithelium. Stem. Cell Rev. Rep. 2017, 13, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Kokkinaki, M.; Sahibzada, N.; Golestaneh, N. Human Induced Pluripotent Stem-Derived Retinal Pigment Epithelium (RPE) Cells Exhibit Ion Transport, Membrane Potential, Polarized Vascular Endothelial Growth Factor Secretion, and Gene Expression Pattern Similar to Native RPE. Stem. Cells 2011, 29, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Lidgerwood, G.E.; Senabouth, A.; Smith-Anttila, C.J.A.; Gnanasambandapillai, V.; Kaczorowski, D.C.; Amann-Zalcenstein, D.; Fletcher, E.L.; Naik, S.H.; Hewitt, A.W.; Powell, J.E.; et al. Transcriptomic Profiling of Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelium over Time. Genom. Proteom. Bioinform. 2021, 19, 223–242. [Google Scholar] [CrossRef]
- Senabouth, A.; Daniszewski, M.; Lidgerwood, G.E.; Liang, H.H.; Hernández, D.; Mirzaei, M.; Zhang, R.; Han, X.; Neavin, D.; Rooney, L.; et al. Transcriptomic and Proteomic Retinal Pigment Epithelium Signatures of Age-Related Macular Degeneration. bioRxiv 2021. [Google Scholar] [CrossRef]
- Fuhrmann, S. Eye Morphogenesis and Patterning of the Optic Vesicle. Curr. Top Dev. Biol. 2010, 93, 61–84. [Google Scholar] [CrossRef]
- Fuhrmann, S.; Zou, C.; Levine, E.M. Retinal Pigment Epithelium Development, Plasticity, and Tissue Homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Kam, J.H.; Vugler, A.; Semo, M.; Jeffery, G. Mature Retinal Pigment Epithelium Cells Are Retained in the Cell Cycle and Proliferate in Vivo. Mol. Vis. 2008, 14, 1784–1791. [Google Scholar] [PubMed]
- Rapaport, D.H.; Rakic, P.; Yasamura, D.; LaVail, M.M. Genesis of the Retinal Pigment Epithelium in the Macaque Monkey. J. Comp. Neurol. 1995, 363, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Temple, S. Retinal Pigment Epithelial Cell Proliferation. Exp. Biol. Med. 2015, 240, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Vorotelyak, E.; Vasiliev, A.; Terskikh, V. The Problem of Stem Cell Definition. Cell Tissue Biol. 2020, 14, 169–177. [Google Scholar] [CrossRef]
- Sharma, P.; Ramachandran, R. Retina Regeneration: Lessons from Vertebrates. Oxf. Open Neurosci. 2022, 1, kvac012. [Google Scholar] [CrossRef]
- Grigoryan, E.N.; Markitantova, Y.V. Cellular and Molecular Preconditions for Retinal Pigment Epithelium (RPE) Natural Reprogramming during Retinal Regeneration in Urodela. Biomedicines 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Markitantova, Y.V.; Simirskii, V.N. Role of the Redox System in Initiation of a Regenerative Response of Neural Eye Tissues in Vertebrates. Russ. J. Dev. Biol. 2020, 51, 16–30. [Google Scholar] [CrossRef]
- Chiba, C.; Mitashov, V. Cellular and Molecular Events in the Adult Newt Retinal Regeneration. In Strategies for Retinal Tissue Repair and Regeneration in Vertebrates: From Fish to Human; Chiba, C., Ed.; Research Signpost: Trivandrum, India, 2008; pp. 15–33. ISBN 978-81-308-0200-8. [Google Scholar]
- Islam, M.R.; Nakamura, K.; Casco-Robles, M.M.; Kunahong, A.; Inami, W.; Toyama, F.; Maruo, F.; Chiba, C. The Newt Reprograms Mature RPE Cells into a Unique Multipotent State for Retinal Regeneration. Sci. Rep. 2014, 4, 6043. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, E.N.; Markitantova, Y.V. Molecular Strategies for Transdifferentiation of Retinal Pigment Epithelial Cells in Amphibians and Mammals In Vivo. Russ. J. Dev. Biol. 2021, 52, 220–243. [Google Scholar] [CrossRef]
- Luz-Madrigal, A.; Grajales-Esquivel, E.; McCorkle, A.; DiLorenzo, A.M.; Barbosa-Sabanero, K.; Tsonis, P.A.; Del Rio-Tsonis, K. Reprogramming of the Chick Retinal Pigmented Epithelium after Retinal Injury. BMC Biol. 2014, 12, 28. [Google Scholar] [CrossRef]
- Blenkinsop, T.A.; Saini, J.S.; Maminishkis, A.; Bharti, K.; Wan, Q.; Banzon, T.; Lotfi, M.; Davis, J.; Singh, D.; Rizzolo, L.J.; et al. Human Adult Retinal Pigment Epithelial Stem Cell–Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7085. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, X.; Chen, Y.; Liu, J.Y.; Lu, H.; Wang, W.; Lu, X.; Dean, K.C.; Gao, L.; Kaplan, H.J.; et al. Sphere-Induced Reprogramming of RPE Cells into Dual-Potential RPE Stem-like Cells. EBioMedicine 2020, 52, 102618. [Google Scholar] [CrossRef]
- Davis, R.J.; Alam, N.M.; Zhao, C.; Müller, C.; Saini, J.S.; Blenkinsop, T.A.; Mazzoni, F.; Campbell, M.; Borden, S.M.; Charniga, C.J.; et al. The Developmental Stage of Adult Human Stem Cell-Derived Retinal Pigment Epithelium Cells Influences Transplant Efficacy for Vision Rescue. Stem Cell Rep. 2017, 9, 42. [Google Scholar] [CrossRef]
- Liu, Z.; Harshad Parikh, B.; Shu Woon Tan, Q.; Soo Lin Wong, D.; Haur Ong, K.; Yu, W.; Seah, I.; Holder, G.E.; Hunziker, W.; Tan, G.S.; et al. Article Surgical Transplantation of Human RPE Stem Cell-Derived RPE Monolayers into Non-Human Primates with Immunosuppression. Stem Cell Rep. 2021, 16, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Stanzel, B.V.; Liu, Z.; Somboonthanakij, S.; Wongsawad, W.; Brinken, R.; Eter, N.; Corneo, B.; Holz, F.G.; Temple, S.; Stern, J.H.; et al. Human RPE Stem Cells Grown into Polarized RPE Monolayers on a Polyester Matrix Are Maintained after Grafting into Rabbit Subretinal Space. Stem Cell Rep. 2014, 2, 64–77. [Google Scholar] [CrossRef]
- Salero, E.; Blenkinsop, T.A.; Corneo, B.; Harris, A.; Rabin, D.; Stern, J.H.; Temple, S. Adult Human RPE Can Be Activated into a Multipotent Stem Cell That Produces Mesenchymal Derivatives. Cell Stem Cell 2012, 10, 88–95. [Google Scholar] [CrossRef]
- Frayer, W.C. Reactivity of the Retinal Pigment Epithelium: An Experimental and Histopathologic Study. Trans. Am. Ophthalmol. Soc. 1966, 64, 586. [Google Scholar]
- Tso, M.O.; Fine, B.S. Repair and Late Degeneration of the Primate Foveola after Injury by Argon Laser. Investig. Ophthalmol. Vis. Sci. 1979, 18, 447–461. [Google Scholar]
- Achilleos, A.; Trainor, P.A. Neural Crest Stem Cells: Discovery, Properties and Potential for Therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE Lipofuscin and Its Role in Retinal Pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef]
- Milyushina, L.A.; Kuznetsova, A.V.; Grigoryan, E.N.; Aleksandrova, M.A. Phenotypic Plasticity of Retinal Pigment Epithelial Cells from Adult Human Eye in Vitro. Bull. Exp. Biol. Med. 2011, 151, 506–511. [Google Scholar] [CrossRef]
- Milyushina, L.A.; Verdiev, B.I.; Kuznetsova, A.V.; Aleksandrova, M.A. Expression of Multipotent and Retinal Markers in Pigment Epithelium of Adult Human in Vitro. Bull. Exp. Biol. Med. 2012, 153, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Firsova, N.V.; Markitantova, Y.V.; Smirnova, Y.A.; Panova, I.G.; Sukhikh, G.T.; Zinovieva, R.D.; Mitashov, V.I. Identification of the OCT4-Pg1 Retrogene and NANOG Gene Expression in the Human Fetal Eye. Biol. Bull. 2008, 35, 108–112. [Google Scholar] [CrossRef]
- Kuznetsova, A.V.; Kurinov, A.M.; Rzhanova, L.A.; Aleksandrova, M.A. Mechanisms of Dedifferentiation of Adult Human Retinal Pigment Epithelial Cells in Vitro. Morphological and Molecular Genetic Analysis. Cell Tissue Biol. 2019, 13, 107–119. [Google Scholar] [CrossRef]
- Kuznetsova, A.V.; Rzhanova, L.A.; Kurinov, A.M.; Aleksandrova, M.A. The Effect of Basic Fibroblast Growth Factor on Signaling Pathways in Adult Human Retinal Pigment Epithelial Cells. Cell Tissue Biol. 2019, 13, 292–304. [Google Scholar] [CrossRef]
- Milyushina, L.A.; Poltavtseva, R.A.; Marei, M.V.; Podgornyi, O.V.; Sukhikh, G.T.; Aleksandrova, M.A. In Vitro Phenotypic Modifi Cation of Pigmented Epithelium Cells from Human Eye at Early Stages of Development. Bull. Exp. Biol. Med. 2009, 148, 113–119. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Seemungal, R.J.; Ivanov, D. The Epigenetic Basis for the Impaired Ability of Adult Murine Retinal Pigment Epithelium Cells to Regenerate Retinal Tissue. Sci. Rep. 2019, 9, 3860. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Lypka, K.R.; Ivanov, D. The Potential Role of Epigenetic Mechanisms in the Development of Retinitis Pigmentosa and Related Photoreceptor Dystrophies. Front. Genet. 2022, 13, 827274. [Google Scholar] [CrossRef] [PubMed]
- Boles, N.C.; Fernandes, M.; Swigut, T.; Srinivasan, R.; Schiff, L.; Rada-Iglesias, A.; Wang, Q.; Saini, J.S.; Kiehl, T.; Stern, J.H.; et al. Epigenomic and Transcriptomic Changes During Human RPE EMT in a Stem Cell Model of Epiretinal Membrane Pathogenesis and Prevention by Nicotinamide. Stem Cell Rep. 2020, 14, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; McArdle, B.; Schiff, L.; Blenkinsop, T.A. Stem Cell–Derived Retinal Pigment Epithelial Layer Model from Adult Human Globes Donated for Corneal Transplants. Curr. Protoc. Stem Cell Biol. 2018, 45, e53. [Google Scholar] [CrossRef] [PubMed]
- Shamsnajafabadi, H.; Soheili, Z.S.; Samiee, S.; Ahmadieh, H.; Pirmardan, E.R.; Haghighi, M. Neural Differentiation of Human Retinal Pigment Epithelial Cells on Alginate/Gelatin Substrate. Mol. Vis. 2022, 28, 412. [Google Scholar] [PubMed]
- Liu, Y.; Ye, F.; Li, Q.; Tamiya, S.; Darling, D.S.; Kaplan, H.J.; Dean, D.C. Zeb1 Represses Mitf and Regulates Pigment Synthesis, Cell Proliferation and Epithelial Morphology. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5080. [Google Scholar] [CrossRef] [PubMed]
- Akrami, H.; Soheili, Z.S.; Khalooghi, K.; Ahmadieh, H.; Rezaie-Kanavi, M.; Samiei, S.; Davari, M.; Ghaderi, S.; Sanie-Jahromi, F. Retinal pigment epithelium culture;a potential source of retinal stem cells. J. Ophthalmic. Vis. Res. 2009, 4, 134–141. [Google Scholar] [PubMed]
- Carr, A.J.; Vugler, A.A.; Yu, L.; Semo, M.; Coffey, P.; Moss, S.E.; Greenwood, J. The expression of retinal cell markers in human retinal pigment epithelial cells and their augmentation by the synthetic retinoid fenretinide. Mol. Vis. 2011, 17, 1701–1715. [Google Scholar] [PubMed]
- Fu, X.; Huu, V.A.N.; Duan, Y.; Kermany, D.S.; Valentim, C.C.S.; Zhang, R.; Zhu, J.; Zhang, C.L.; Sun, X.; Zhang, K. Clinical Applications of Retinal Gene Therapies. Precis. Clin. Med. 2018, 1, 5–20. [Google Scholar] [CrossRef]
- Jiang, D.J.; Xu, C.L.; Tsang, S.H. Revolution in Gene Medicine Therapy and Genome Surgery. Genes 2018, 9, 575. [Google Scholar] [CrossRef]
- Otteson, D.C. Talkin’ about My (Re)Generation: The Who of Intrinsic Retinal Stem Cells. Neuroscience 2017, 346, 447–449. [Google Scholar] [CrossRef]
- Léveillard, T.; Klipfel, L. Mechanisms Underlying the Visual Benefit of Cell Transplantation for the Treatment of Retinal Degenerations. Int. J. Mol. Sci. 2019, 20, 557. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A Bioengineered Retinal Pigment Epithelial Monolayer for Advanced, Dry Age-Related Macular Degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Y. Application of Stem Cell-Derived Retinal Pigmented Epithelium in Retinal Degenerative Diseases: Present and Future. Int. J. Ophthalmol. 2018, 11, 150–159. [Google Scholar] [PubMed]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic Stem Cell Trials for Macular Degeneration: A Preliminary Report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Chichagova, V.; Hallam, D.; Collin, J.; Zerti, D.; Dorgau, B.; Felemban, M.; Lako, M.; Steel, D.H. Cellular Regeneration Strategies for Macular Degeneration: Past, Present and Future. Eye 2018, 32, 946–971. [Google Scholar] [CrossRef] [PubMed]
- Artero-Castro, A.; Popelka, S.; Jendelova, P.; Motlik, J.; Ardan, T.; Rodriguez Jimenez, F.J.; Erceg, S. The Identification of Small Molecules That Stimulate Retinal Pigment Epithelial Cells: Potential Novel Therapeutic Options for Treating Retinopathies. Expert Opin. Drug Discov. 2019, 14, 169–177. [Google Scholar] [CrossRef]
- Öner, A. Stem Cell Treatment in Retinal Diseases: Recent Developments. Turk. J. Ophthalmol. 2018, 48, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Wong, R.C.-B. Neuroregeneration Using in Vivo Cellular Reprogramming. Neural Regen. Res. 2017, 12, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Fortress, A.M.; Miyagishima, K.J.; Reed, A.A.; Temple, S.; Clegg, D.O.; Tucker, B.A.; Blenkinsop, T.A.; Harb, G.; Greenwell, T.N.; Ludwig, T.E.; et al. Stem Cell Sources and Characterization in the Development of Cell-Based Products for Treating Retinal Disease: An NEI Town Hall Report. Stem Cell Res. Ther. 2023, 14, 1–13. [Google Scholar] [CrossRef]
- Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.R.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N.; Ahmadieh, H.; Baharvand, H. Intravitreal Injection of Bone Marrow Mesenchymal Stem Cells in Patients with Advanced Retinitis Pigmentosa; a Safety Study. J. Ophthalmic Vis. Res. 2017, 12, 58–64. [Google Scholar] [CrossRef]
- Markitantova, Y.; Simirskii, V. Inherited Eye Diseases with Retinal Manifestations through the Eyes of Homeobox Genes. Int. J. Mol. Sci. 2020, 21, 1602. [Google Scholar] [CrossRef]
- Sachewsky, N.; Xu, W.; Fuehrmann, T.; van der Kooy, D.; Morshead, C.M. Lineage Tracing Reveals the Hierarchical Relationship between Neural Stem Cell Populations in the Mouse Forebrain. Sci. Rep. 2019, 9, 17730. [Google Scholar] [CrossRef]
- Reyes-Aguirre, L.I.; Lamas, M. Oct4 Methylation-Mediated Silencing As an Epigenetic Barrier Preventing Müller Glia Dedifferentiation in a Murine Model of Retinal Injury. Front. Neurosci. 2016, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, E.N. Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye. Cells 2022, 11, 3755. [Google Scholar] [CrossRef]
- Eminli, S.; Utikal, J.; Arnold, K.; Jaenisch, R.; Hochedlinger, K. Reprogramming of Neural Progenitor Cells into Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2 Expression. Stem Cells 2008, 26, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Rzhanova, L.A.; Kuznetsova, A.V.; Aleksandrova, M.A. Reprogramming of Differentiated Mammalian and Human Retinal Pigment Epithelium: Current Achievements and Prospects. Russ. J. Dev. Biol. 2020, 51, 212–230. [Google Scholar] [CrossRef]
- Goldman, J.A.; Poss, K.D. Gene Regulatory Programmes of Tissue Regeneration. Nat. Rev. Genet. 2020, 21, 511–525. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease. Life 2022, 12, 382. [Google Scholar] [CrossRef]
- Petrik, D.; Jörgensen, S.; Eftychidis, V.; Siebzehnrubl, F.A. Singular Adult Neural Stem Cells Do Not Exist. Cells 2022, 11, 722. [Google Scholar] [CrossRef]
- Nagai, H.; Kalnins, V.I. Normally Occurring Loss of Single Cells and Repair of Resulting Defects in Retinal Pigment Epithelium In Situ. Exp. Eye Res. 1996, 62, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bystrykh, L.V.; Verovskaya, E.; Zwart, E.; Broekhuis, M.; De Haan, G. Counting Stem Cells: Methodological Constraints. Nat. Methods 2012, 9, 567–574. [Google Scholar] [CrossRef] [PubMed]
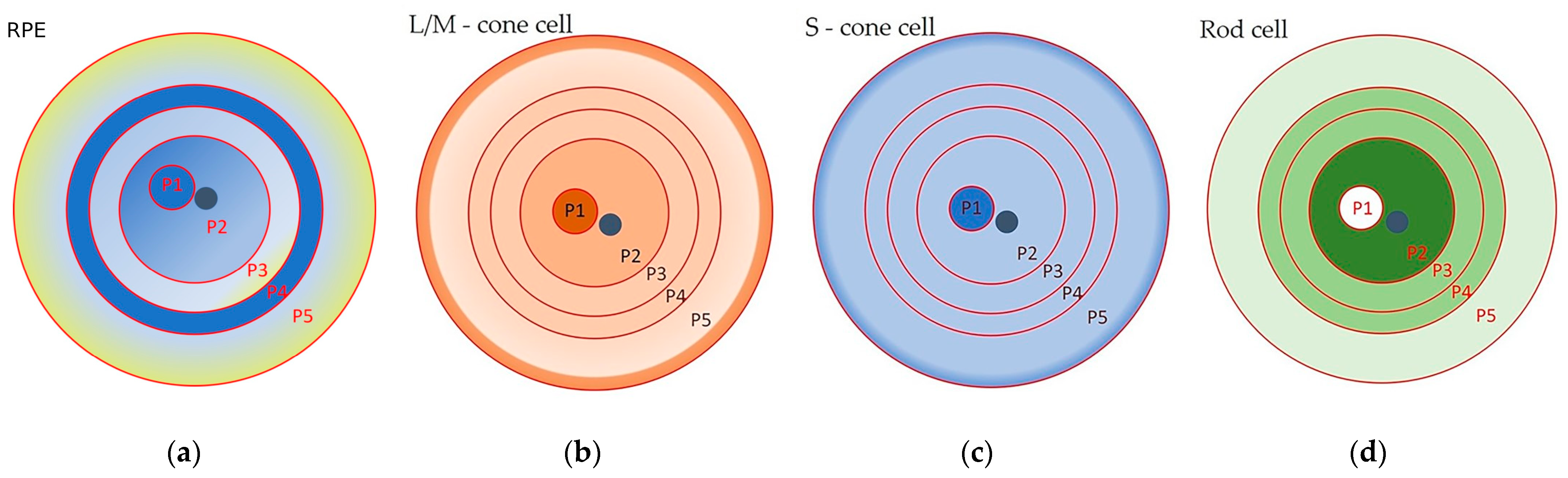
| Topographic Zone | Concentric Zones | Distance from the Optic Nerve, mm | Average Area, µm2 | Aspect Ratio | Hexagonality | Number of Neighboring Cells | Rod/Cone to RPE Ratio |
|---|---|---|---|---|---|---|---|
| Central (macular) RPE | P1 | a 3 mm wide spot | 147.24 ± 15.36 | 1.15 ± 0.04 | 9.31 ± 0.11 | 5.55 ± 0.35 | 0.002 |
| P2 | 0–10 | 201.74 ± 17.45 | 1.18 ± 0.02 | 9.25 ± 0.05 | 5.47 ± 0.43 | 0.129 (0.068 for the perifovea) | |
| Peripheral RPE | P3 | 10–14 | 231.21 ± 18.08 | 1.23 ± 0.03 | 9.12 ± 0.08 | 5.46 ± 0,63 | 0.087 |
| P4 | 14–17 | 176.76 ± 18.68 | 1.27 ± 0.04 | 9.00 ± 0.12 | 5.64 ± 0.25 | 0.096 | |
| P5 | 17–24 | 331.87 ± 27.23 | 1.33 ± 0.03 | 8.79 ± 0.11 | 5.04 ± 0.46 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzhanova, L.A.; Markitantova, Y.V.; Aleksandrova, M.A. Recent Achievements in the Heterogeneity of Mammalian and Human Retinal Pigment Epithelium: In Search of a Stem Cell. Cells 2024, 13, 281. https://doi.org/10.3390/cells13030281
Rzhanova LA, Markitantova YV, Aleksandrova MA. Recent Achievements in the Heterogeneity of Mammalian and Human Retinal Pigment Epithelium: In Search of a Stem Cell. Cells. 2024; 13(3):281. https://doi.org/10.3390/cells13030281
Chicago/Turabian StyleRzhanova, Lyubov A., Yuliya V. Markitantova, and Maria A. Aleksandrova. 2024. "Recent Achievements in the Heterogeneity of Mammalian and Human Retinal Pigment Epithelium: In Search of a Stem Cell" Cells 13, no. 3: 281. https://doi.org/10.3390/cells13030281






