An Exploratory Study to Determine Whether BRCA1 and BRCA2 Mutation Carriers Have Higher Risk of Cardiac Toxicity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
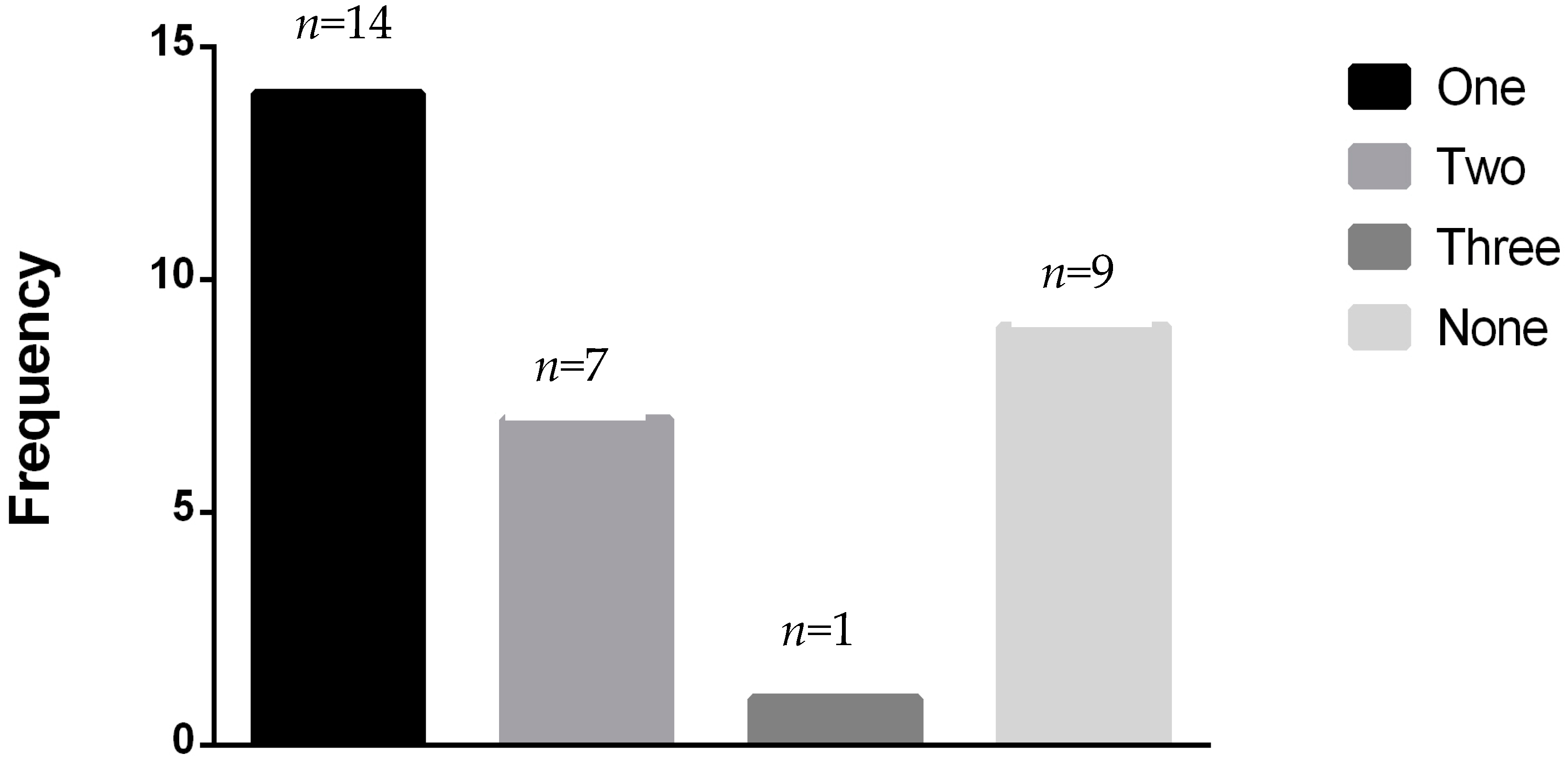
3.1. Population Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Claus, E.B.; Schildkraut, J.M.; Thompson, W.D.; Risch, N.J. The genetic attributable risk of breast and ovarian cancer. Cancer 1996, 77, 2318–2324. [Google Scholar] [CrossRef]
- Struewing, J.P.; Hartge, P.; Wacholder, S.; Baker, S.M.; Berlin, M.; McAdams, M.; Timmerman, M.M.; Tucker, M.A. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 1997, 336, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Chatterjee, N.; Hartge, P.; Tucker, M.; Brody, L.; Struewing, J.P.; Wacholder, S. Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS ONE 2009, 4, e4812. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.; Easton, D.F.; Stratton, M.; Narod, S.; Goldgar, D.; Devilee, P.; Bishop, D.T.; Weber, B.; Lenoir, G.; Chang-Claude, J.; et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1998, 62, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [PubMed]
- Minow, R.A.; Benjamin, R.S.; Lee, E.T.; Gottlieb, J.A. Adriamycin cardiomyopathy—Risk factors. Cancer 1977, 39, 1397–1402. [Google Scholar] [CrossRef]
- Barac, A.; Lynce, F.; Smith, K.L.; Mete, M.; Shara, N.M.; Asch, F.M.; Nardacci, M.P.; Wray, L.; Herbolsheimer, P.; Nunes, R.A.; et al. Cardiac function in BRCA1/2 mutation carriers with history of breast cancer treated with anthracyclines. Breast. Cancer Res. Treat. 2016, 155, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.G.; Moynahan, M.E. BRCA gene structure and function in tumor suppression: A repair-centric perspective. Cancer J. 2010, 16, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.C.; Singh, K.K.; Lovren, F.; Pan, Y.; Leong-Poi, H.; Erret, L.; Verma, S. BRCA1 as an essential regulator of cardiac function. Nat. Commun. 2011, 20. [Google Scholar] [CrossRef]
- Singh, K.K.; Shukla, P.C.; Quan, A.; Desjardins, J.F.; Lovren, F.; Pan, Y.; Garg, V.; Gosal, S.; Garg, A.; Szmitko, P.E.; et al. BRCA2 protein deficiency exaggerates doxorubicin-induced cardiomyocyte apoptosis and cardiac failure. J. Biol. Chem. 2012, 287, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Sato, H.; Inoue, K.; Tsunoda, T.; Sakata, Y.; Mizuno, H.; Lin, T.H.; Miyamoto, Y.; Aoki, A.; Onouchi, Y.; et al. SNPs in BRAP associated with risk of myocardial infarction in Asian populations. Nat. Genet. 2009, 41, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Nozynski, J.K.; Konecka-Mrowka, D.; Zakliczynski, M.; Zembala-Nozynska, E.; Lange, D.; Zembala, M. BRCA1 Reflects Myocardial Adverse Remodeling in Idiopathic Dilated Cardiomyopathy. Transplant. Proc. 2016, 48, 1746–1750. [Google Scholar]
- Bordeleau, L.; Lipscombe, L.; Lubinski, J.; Ghadirian, P.; Foulkes, W.D.; Neuhausen, S.; Ainsworth, P.; Pollak, M.; Sun, P.; Narod, S.A. Hereditary Breast Cancer Clinical Study Group. Diabetes and breast cancer among women with BRCA1 and BRCA2 mutations. Cancer 2011, 117, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- van Westerop, L.L.; Arts-de Jong, M.; Hoogerbrugge, N.; de Hullu, J.A.; Maas, A.H. Cardiovascular risk of BRCA1/2 mutation carriers: A review. Maturitas 2016, 91, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, L.L.; Crow, R.S.; Shahar, E.; Hutchinson, R.G.; Rautaharju, P.M.; Folsom, A.R. Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am. J. Cardiol. 1998, 81, 453–459. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Barnes, M.E.; Bailey, K.R.; Cha, S.S.; Gersh, B.J.; Seward, J.B.; Tsang, T.S. Mortality trends in patients diagnosed with first atrial fibrillation: A 21-year community-based study. J. Am. Coll. Cardiol. 2007, 49, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients (%) (n = 401) | |
|---|---|
| BRCA status BRCA1 BRCA2 BRCA1 and BRCA2 | 232 (57.9) 159 (39.7) 10 (2.5) |
| Hypertension | 97 (24.2) |
| Diabetes | 18 (4.5) |
| Hyperlipidemia | 207 (51.6) |
| Tobacco use | 37 (9.2) |
| Age group 20–39 years 40–59 years 60–79 years No answer | 28 (7.0) 264 (65.8) 76 (19.0) 33 (8.2) |
| Patient Group | Heart Failure, No. of Patients (%) | No Heart Failure | Total | 95% Confidence Interval (%) | p Value Compared to 2% |
|---|---|---|---|---|---|
| BRCA1 | 17 (7.3%) | 215 | 232 | 4.3–11.5 | <0.0001 |
| BRCA2 | 13 (8.2%) | 146 | 159 | 4.4–13.6 | <0.0001 |
| BRCA1 and BRCA2 | 1 (10%) | 9 | 10 | 0.3–44.5 | 0.37 |
| Total | 31 (7.7%) | 370 | 401 | 5.3–10.8 | <0.0001 |
| Number of Patients | % HF on Anthracycline | % HF Chemotherapy Naïve | ||||
|---|---|---|---|---|---|---|
| Received Anthracycline | Chemotherapy Naïve | HF on Anthracycline | HF Chemotherapy Naïve | |||
| BRCA1 | 106 | 126 | 6 | 11 | 5.7 | 8.7 |
| BRCA2 | 70 | 89 | 6 | 7 | 8.6 | 7.9 |
| Both | 7 | 3 | 1 | 0 | 14.2 | 0 |
| Total | 183 | 218 | 13 | 18 | 7.1 | 8.3 |
| Patient Group | Irregular Rhythm, n | No Irregular Rhythm or Unknown, n | Total, n | Irregular Rhythm, % (95% CI) |
|---|---|---|---|---|
| BRCA1 | 21 | 211 | 232 | 9.1 (5.7–13.5) |
| BRCA2 | 13 | 146 | 159 | 8.2 (4.4–13.6) |
| BRCA1 and BRCA2 | 3 | 7 | 10 | 30.0 (6.7–65.3) |
| Total | 37 | 364 | 401 | 9.2 (6.6–12.5) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajjad, M.; Fradley, M.; Sun, W.; Kim, J.; Zhao, X.; Pal, T.; Ismail-Khan, R. An Exploratory Study to Determine Whether BRCA1 and BRCA2 Mutation Carriers Have Higher Risk of Cardiac Toxicity. Genes 2017, 8, 59. https://doi.org/10.3390/genes8020059
Sajjad M, Fradley M, Sun W, Kim J, Zhao X, Pal T, Ismail-Khan R. An Exploratory Study to Determine Whether BRCA1 and BRCA2 Mutation Carriers Have Higher Risk of Cardiac Toxicity. Genes. 2017; 8(2):59. https://doi.org/10.3390/genes8020059
Chicago/Turabian StyleSajjad, Monique, Michael Fradley, Weihong Sun, Jongphil Kim, Xiuhua Zhao, Tuya Pal, and Roohi Ismail-Khan. 2017. "An Exploratory Study to Determine Whether BRCA1 and BRCA2 Mutation Carriers Have Higher Risk of Cardiac Toxicity" Genes 8, no. 2: 59. https://doi.org/10.3390/genes8020059





