Ecotoxicological Assays with the Calanoid Copepod Acartia tonsa: A Comparison between Mediterranean and Baltic Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
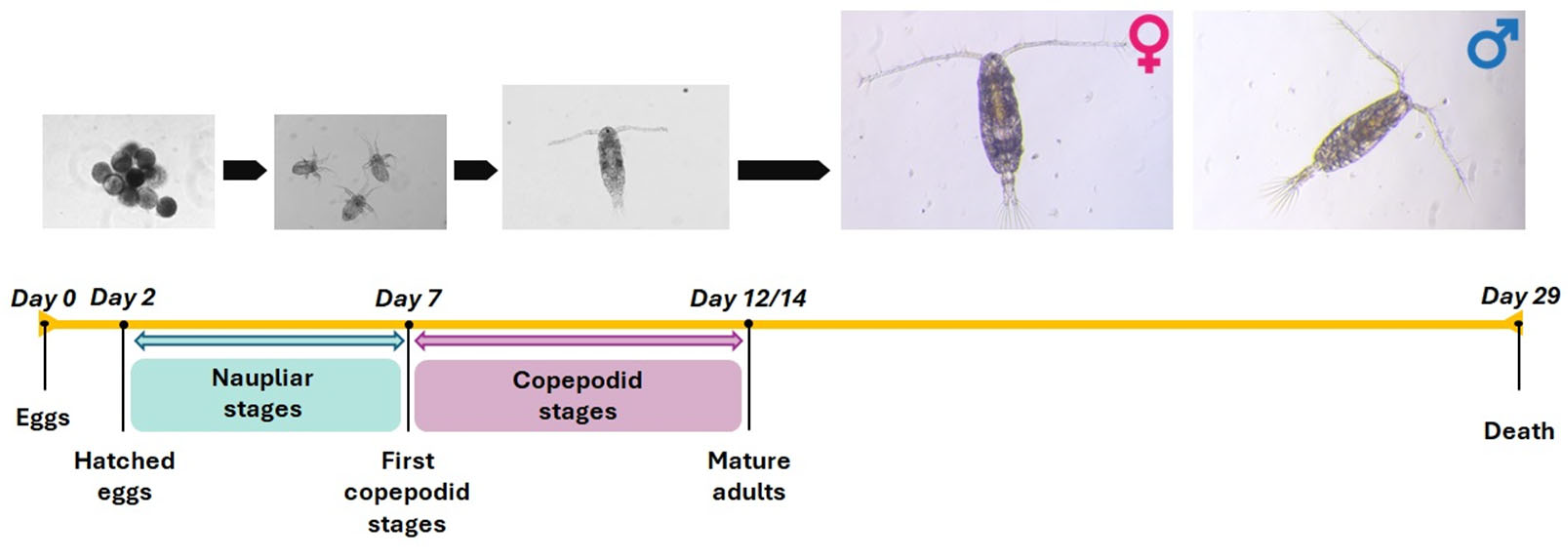
2.2. Copepod Culture
2.3. Life Cycle Assessment

2.4. Ecotoxicological Tests
2.5. Statistical Analyses
3. Results
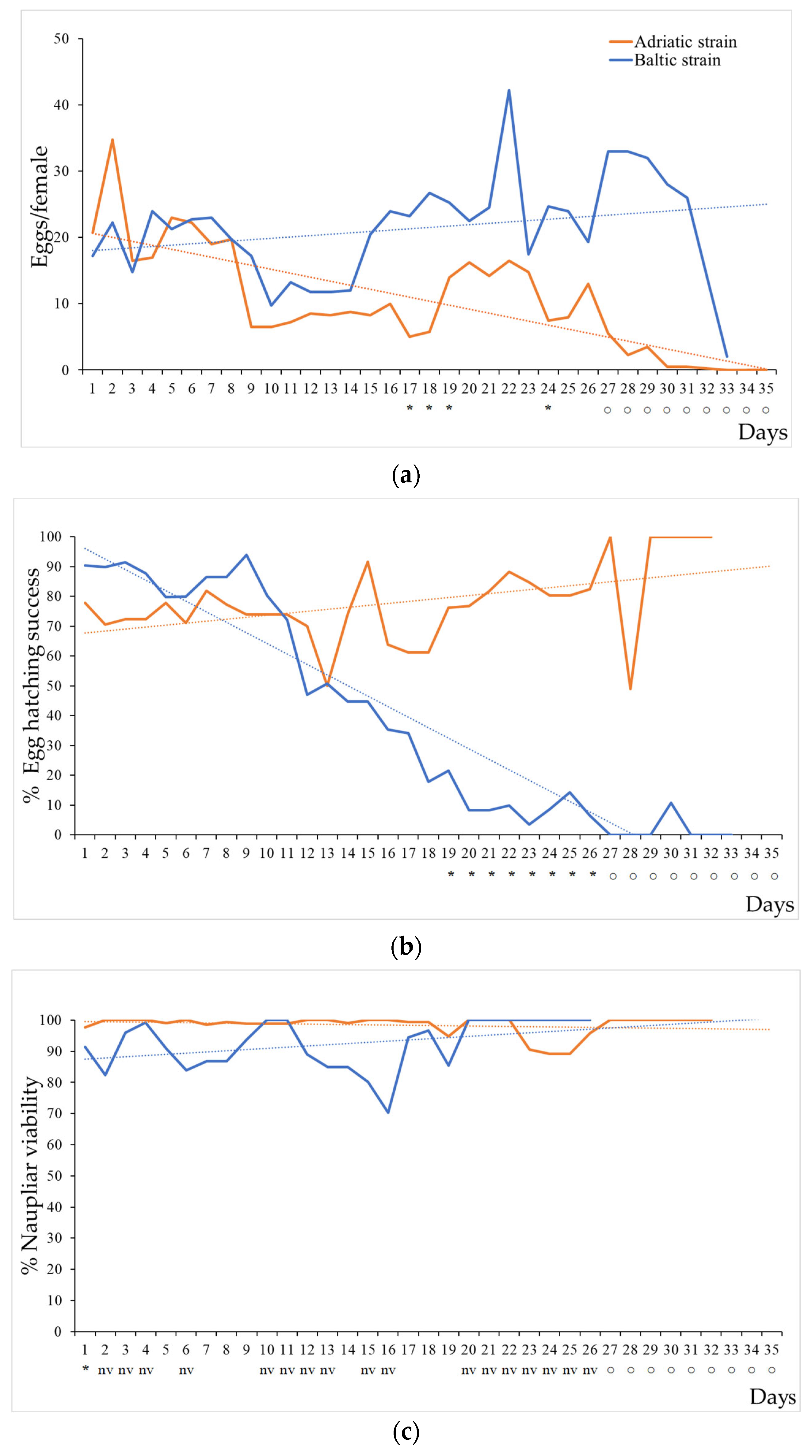
3.1. Life Cycle Assessment
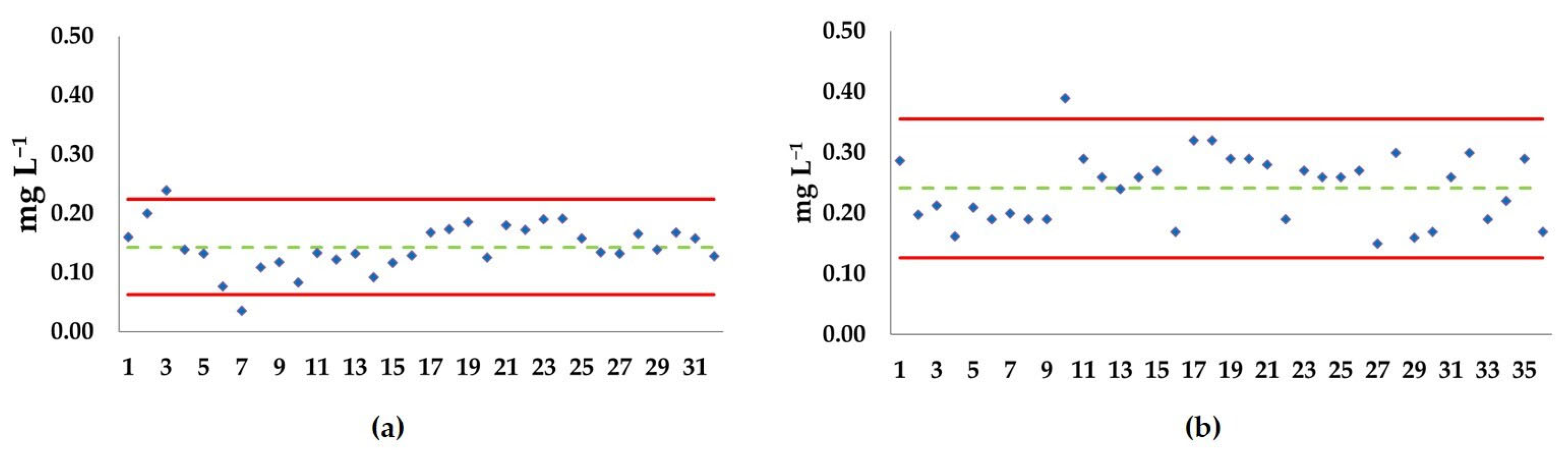
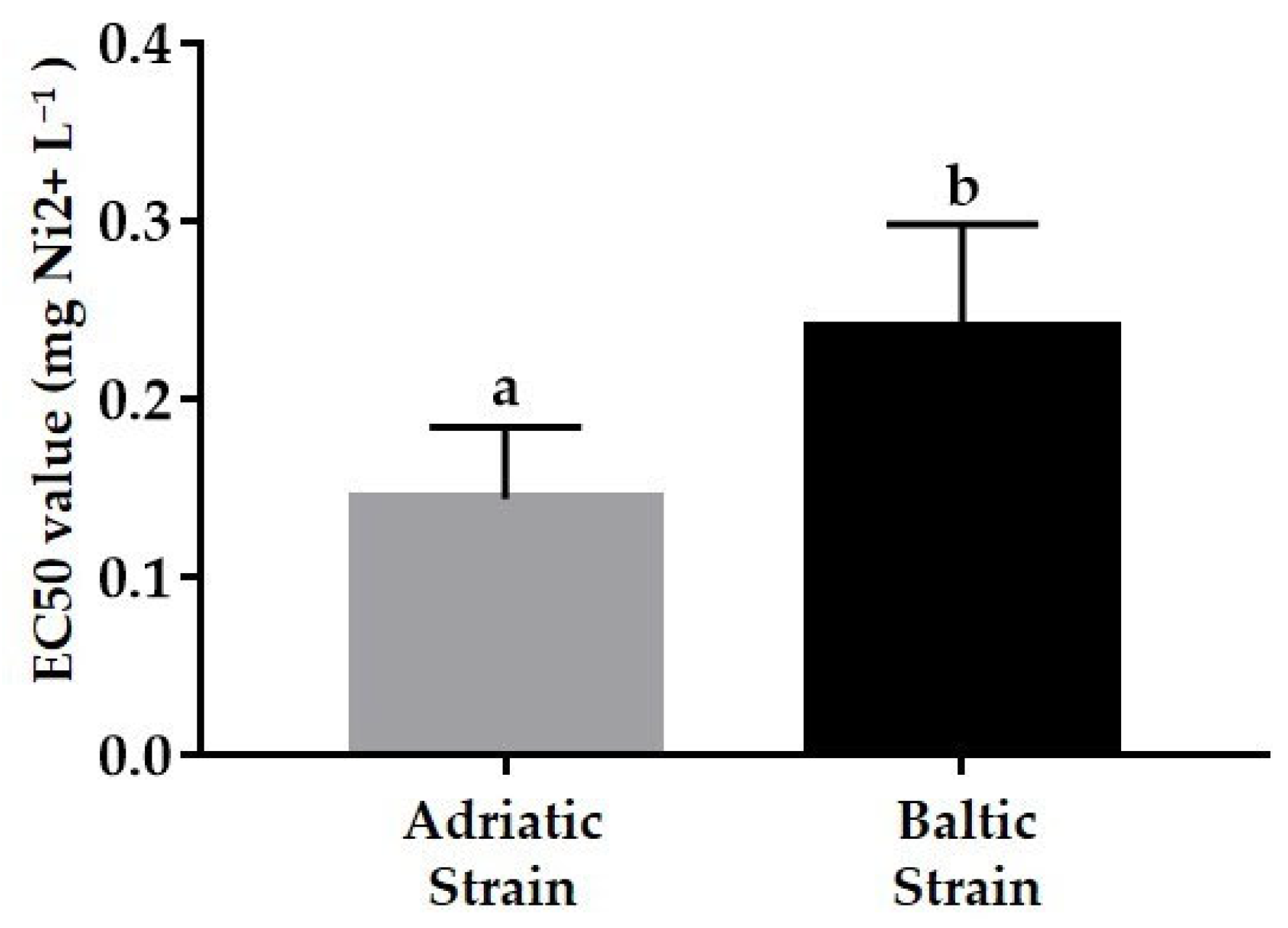
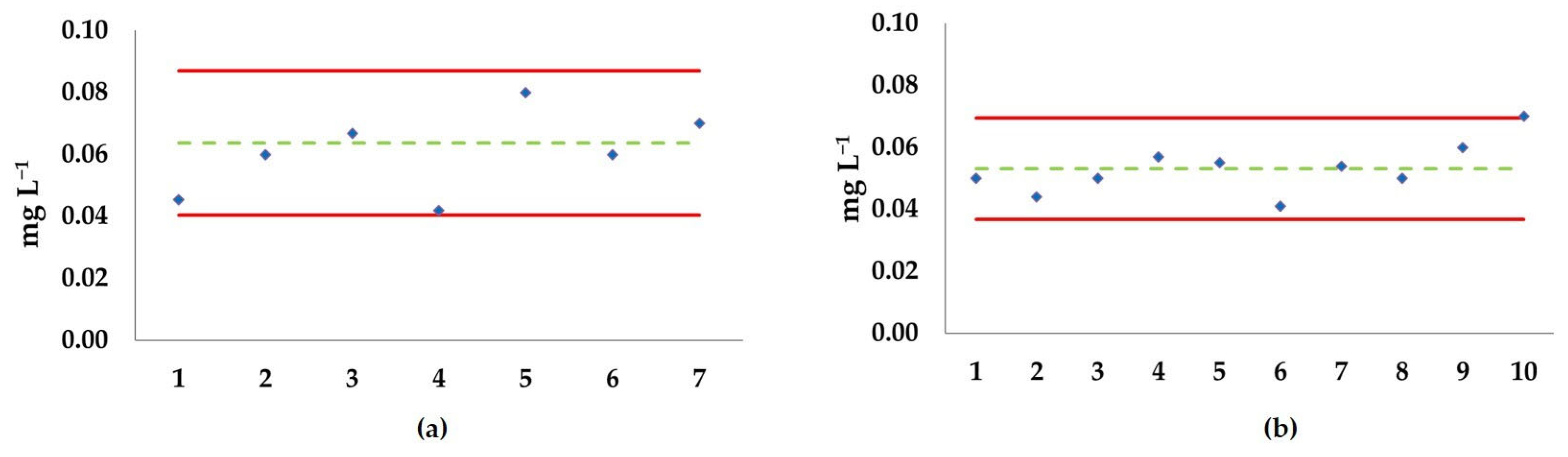
3.2. Ecotoxicological Tests
3.2.1. Acute Test
3.2.2. Semi-Chronic Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piva, F.; Ciaprini, F.; Onorati, F.; Benedetti, M.; Fattorini, D.; Ausili, A.; Regoli, F. Assessing sediment hazard through a Weight of Evidence approach with bioindicator organisms: A practical model to elaborate data from sediment chemistry, bioavailability, biomarkers and ecotoxicological bioassays. Chemosphere 2011, 83, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Ministerial Decree 173. Regolamento Recante Modalità e Criteri per L’autorizzazione All’immersione in Mare Dei Materiali di Escavo di Fondali Marini—Technical Attachment. Decreto del Ministero dell’Ambiente e della Tutela del Territorio e del Mare. 2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/09/06/16G00184/sg (accessed on 13 July 2023).
- Pandard, P.; Devillers, J.; Charissou, A.-M.; Poulsen, V.; Jourdain, M.-J.; Férard, J.-F.; Grand, C.; Bispo, A. Selecting a battery of bioassays for ecotoxicological characterization of wastes. Sci. Total Environ. 2006, 363, 114–125. [Google Scholar] [CrossRef] [PubMed]
- ISO 11348-3; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent bacteria test). Part 3: Method Using Freeze-Dried Bacteria. International Organization for Standardization: Genève, Switzerland, 2007.
- ISO 10253; Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum tricornutum. International Organization for Standardization: Genève, Switzerland, 2016.
- ASTM E1218; Standard Guide for Conducting Static Toxicity Tests with Microalgae. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ISO 17244; Water Quality—Determination of the Toxicity of Water Samples on the Embryo-Larval Development of Japanese Oyster (Crassostrea gigas) and Mussel (Mytilus edulis or Mytilus galloprovincialis). International Organization for Standardization: Genève, Switzerland, 2015.
- ASTM E724; Standard Guide for Conducting Static Short-Term Chronic Toxicity Tests Starting with Embryos of Four Species of Saltwater Bivalve Molluscs. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ISO 19820; Water Quality—Determination of the Acute Toxicity to the Marine Rotifer Brachionus plicatilis. International Organization for Standardization: Genève, Switzerland, 2016.
- USEPA 600-4-90-027F; Methods of Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. United States Environmental Protection Agency: Washington, DC, USA, 1993.
- Razouls, C.; Desreumaux, N.; Kouwenberg, J.; de Bovée, F. Biodiversity of Marine Planktonic Copepods (Morphology, Geographical Distribution and Biological Data). Sorbonne University, CNRS Centre National de la Recherche Scientifique: Paris, France, 2005–2024. Available online: http://copepodes.obs-banyuls.fr/en (accessed on 25 March 2024).
- Sarkisian, B.L.; Lemus, J.T.; Apeitos, A.; Blaylock, R.B.; Saillant, E.A. An intensive, large-scale batch culture system to produce the calanoid copepod, Acartia tonsa. Aquaculture 2019, 501, 272–278. [Google Scholar] [CrossRef]
- Parrish, K.K.; Wilson, D.F. Fecundity studies on Acartia tonsa (Copepoda: Calanoida) in standardized culture. Mar. Biol. 1978, 46, 65–81. [Google Scholar] [CrossRef]
- Hansen, B.W.; Buttino, I.; Cunha, M.E.; Drillet, G. Embryonic cold storage capability from seven strains of Acartia spp. isolated in different geographical areas. Aquaculture 2016, 457, 131–139. [Google Scholar] [CrossRef]
- Hoffmeyer, M.S. Decadal change in zooplankton seasonal succession in the Bahía Blanca Estuary, Argentina, following introduction of two zooplankton species. J. Plankton Res. 1994, 26, 181–189. [Google Scholar] [CrossRef]
- Durbin, E.G.; Durbin, A.G.; Smayda, T.J.; Verity, P.G. Food limitation of production by adult Acartia tonsa in Narragansett Bay, Rhode Island. Limnol. Oceanogr. 1983, 28, 1199–1213. [Google Scholar] [CrossRef]
- Gruszka, P. The River Odra estuary as a gateway for alien species immigration to the Baltic Sea basin. Acta Hydrochim. Hydrobiol. 1999, 27, 374–382. [Google Scholar] [CrossRef]
- Brylinski, J.M. Report on the presence of Acartia tonsa Dana (Copepoda) in the harbour of Dunkirk (France) and its geo-graphical distribution in Europe. J. Plankton Res. 1981, 3, 255–260. [Google Scholar] [CrossRef]
- Calliari, D.; Andersen Borg, M.C.; Thor, P.; Gorokhova, E.; Tiselius, P. Instantaneous salinity reductions affect the survival and feeding rates of the cooccurring copepods Acartia tonsa Dana and A. clausi Giesbrecht differently. J. Exp. Mar. Biol. Ecol. 2008, 362, 18–25. [Google Scholar] [CrossRef]
- ISO 14669; Water Quality—Determination of Acute Lethal Toxicity to Marine Copepods (Copepoda, Crustacea). International Organization for Standardization: Genève, Switzerland, 1999.
- UNICHIM M.U. 2365; Qualità dell’acqua: Determinazione Dell’inibizione Della Mobilità di Naupli di Acartia tonsa Dana (Crustacea: Copepoda) dopo 24 h e 48 h di Esposizione. Associazione per l‘Unificazione del Settore dell’Industria Chimica: Milan, Italy, 2012; p. 22.
- ISO 16778; Water Quality—Calanoid Copepod Early-Life Stage Test with Acartia tonsa. International Organization for Stand-ardization: Genève, Switzerland, 2015.
- UNICHIM M.U. 2366; Qualità Dell’acqua: Determinazione Dell’inibizione Della Mobilità di Naupli di Acartia tonsa Dana (Crustacea: Copepoda) Dopo 7 Giorni di Esposizione. Associazione per l‘Unificazione del Settore dell’Industria Chimica: Milan, Italy, 2012; p. 24.
- Carotenuto, Y.; Vitiello, V.; Gallo, A.; Libralato, G.; Trifuoggi, M.; Toscanesi, M.; Lofrano, G.; Esposito, F.; Buttino, I. Assessment of the relative sensitivity of the copepods Acartia tonsa and Acartia clausi exposed to sediment-derived elutriates from the Bagnoli-Coroglio industrial area. Mar. Environ. Res. 2020, 155, 104878. [Google Scholar] [CrossRef] [PubMed]
- Picone, M.; Bergamin, M.; Delaney, E.; Volpi Ghirardini, A.; Kusk, K.O. Testing lagoonal sediments with early life stages of the copepod Acartia tonsa (Dana): An approach to assess sediment toxicity in the Venice Lagoon. Ecotox. Environ. Safe 2018, 147, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Picone, M.; Distefano, G.G.; Marchetto, D.; Russo, M.; Vecchiato, M.; Gambaro, A.; Barbante, C.; Volpi Ghirardini, A. Fragrance materials (FMs) affect the larval development of the copepod Acartia tonsa: An emerging issue for marine ecosystems. Ecotox. Environ. Safe 2021, 215, 112146. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, F. Acartia spp. (Copepoda: Calanoida) as Model Organisms to Evaluate the Toxicity of Emerging Contaminants: An Ecotoxicogenomic Approach. Ph.D. Thesis, The Open University, Milton Keynes, UK, 2023. [Google Scholar] [CrossRef]
- Sørensen, L.; Størseth, T.R.; Altin, D.; Nordtug, T.; Faksness, L.-G.; Hansen, B.H. A simple protocol for estimating the acute toxicity of unresolved polar compounds from field weathered oils. Toxicol. Mech. Method. 2024, 34, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, V.; Zhou, C.; Scuderi, A.; Pellegrini, D.; Buttino, I. Cold storage of Acartia tonsa eggs: A practical use in ecotoxicological studies. Ecotoxicology 2016, 25, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ianora, A.; Wu, C.; Pellegrini, D.; Esposito, F.; Buttino, I. How to increase productivity of the copepod Acartia tonsa (Dana): Effects of population density and food concentration. Aquac. Res. 2015, 46, 2982–2990. [Google Scholar] [CrossRef]
- Gorbi, G.; Invidia, M.; Savorelli, F.; Faraponova, O.; Giacco, E.; Cigar, M.; Buttino, I.; Leoni, T.; Prato, E.; Lacchetti, I.; et al. Standardized methods for acute and semichronic toxicity tests with the copepod Acartia tonsa. Environ. Toxicol. Chem. 2012, 31, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, F.; Vitiello, V.; Pellegrini, D.; Carotenuto, Y.; Buttino, I. Historical control data in ecotoxicology: Eight years of tests with the copepod Acartia tonsa. Environ. Pollut. 2021, 284, 117468. [Google Scholar] [CrossRef] [PubMed]
- Leandro, S.M.; Queiroga, H.; Rodriguez-Grana, L.; Tiselius, P. Temperature dependent development and somatic growth in two allopatric populations of Acartia clausi (Copepoda: Calanoida). Mar. Ecol. Prog. Ser. 2006, 322, 189–197. [Google Scholar] [CrossRef]
- Caudill, C.C.; Bucklin, A. Molecular phylogeography and evolutionary history of the estuarine copepod, Acartia tonsa, on the Northwest Atlantic coast. Hydrobiologia 2004, 511, 91–102. [Google Scholar] [CrossRef]
- Hill, R.S. Genetic Diversity and Structure of Calanoid Copepods: Molecular Evolutionary Patterns in Coastal Estuaries (Acartia tonsa) and the Open Ocean (Calanus spp.). Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2004. Available online: https://scholars.unh.edu/dissertation/246 (accessed on 13 July 2023).
- Chen, F.; Marcus, N.H. Subitaneous, diapause, and delayed-hatching eggs of planktonic copepods from the northern Gulf of Mexico: Morphology and hatching success. Mar. Biol. 1997, 127, 587–597. [Google Scholar] [CrossRef]
- Drillet, G.; Goetze, E.; Jepsen, P.M.; Højgaard, J.K.; Hansen, B.W. Strain-specific vital rates in four Acartia tonsa cultures, I: Strain origin, genetic differentiation and egg survivorship. Aquaculture 2008, 280, 109–116. [Google Scholar] [CrossRef]
- Drillet, G.; Jepsen, P.M.; Højgaard, J.K.; Jørgensen, N.O.G.; Hansen, B.W. Strainspecific vital rates in four Acartia tonsa cultures, II: Life history traits and biochemical contents of eggs and adults. Aquaculture 2008, 279, 47–54. [Google Scholar] [CrossRef]
- Kiørboe, T.; Ceballos, S.; Thygesen, U.H. Interrelations between senescence, life-history traits, and behavior in planktonic copepods. Ecology 2015, 96, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Holste, L.; Peck, M.A. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): A laboratory investigation. Mar. Biol. 2006, 148, 1061–1070. [Google Scholar] [CrossRef]
- Jepsen, P.M.; Andersen, N.; Holm, T.; Jørgensen, A.T.; Højgaard, J.K.; Hansen, B.W. Effects of adult stocking density on egg production and viability in cultures of the calanoid copepod Acartia tonsa (Dana). Aquac. Res. 2007, 38, 764–772. [Google Scholar] [CrossRef]
- Rodríguez-Graña, L.; Calliari, D.; Tiselius, P.; Hansen, B.W.; Sköld, H.N. Gender-specific ageing and non-Mendelian inher-itance of oxidative damage in marine copepods. Mar. Ecol. Prog. Ser. 2010, 401, 1–13. [Google Scholar] [CrossRef]
- Khosrovyan, A.; Rodríguez-Romero, A.; Salamanca, M.J.; Del Valls, T.A.; Riba, I.; Serrano, F. Comparative performances of eggs and embryos of sea urchin (Paracentrotus lividus) in toxicity bioassays used for assessment of marine sediment quality. Mar. Pollut. Bull. 2013, 70, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Romero, A.; Khosrovyan, A.; Del Valls, T.A.; Obispo, R.; Serrano, F.; Conradi, M.; Riba, I. Several benthic species can be used interchangeably in integrated sediment quality assessment. Ecotox. Environ. Safe 2013, 92, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Morroni, L.; Valentini, A.; Vitiello, V.; Renzi, M.; Nuccio, C.; Pellegrini, D. Comparison of different ecotoxicological batteries with WOE approach for the environmental quality evaluation of harbour sediments. Aquat. Toxicol. 2021, 237, 105905. [Google Scholar] [CrossRef] [PubMed]
- Voloshko, L.N.; Gavrilova, O.V.; Moustakas, M.B. Response of cyanobacteria strains to stress induced by heavy metal ions. Nova Hedwig. Beih. 2001, 123, 487–498. [Google Scholar]
- Reis, M.; Kraberg, A.C.; Erler, K.; Luckas, B. Ecotoxicology of different strains of Lingulodinium polyedrum from the Portugese coast. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; pp. 323–325. [Google Scholar]
- Soucek, D.J.; Mount, D.R.; Dickinson, A.; Hockett, R.; McEwen, A.R. Contrasting effects of chloride on growth, reproduction, and toxicant sensitivity in two genetically distinct strains of Hyalella azteca. Environ. Toxicol. Chem. 2015, 34, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation N. 1907 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/ EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EC, 93/67/EEC, 93/105/EC and 2001/21/EC, The European Parliament and the Council of the European Union. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1907-20140410 (accessed on 2 April 2023).
- EC Regulation N. 1272 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EEC, and Amending Regulation 1907/2006. European Commission, ed. 2008. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:353:0001:1355:EN:PDF (accessed on 13 July 2023).
- Barata, C.; Baird, D.J.; Mitchell, S.E.; Soares, A.M.V.M. Among- and within-population variability in tolerance to cadmium stress in natural populations of Daphnia magna: Implications for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Altin, D.; Rørvik, S.F.; Øverjordet, I.B.; Olsen, A.J.; Nordtug, T. Comparative study on acute effects of water accommodated fractions of an artificially weathered crude oil on Calanus finmarchicus and Calanus glacialis (Crustacea: Copepoda). Sci. Total Environ. 2011, 409, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Carotenuto, Y.; Miralto, A.; Procaccini, G.; Ianora, A. Copepod population-specific response to a toxic diatom diet. PLoS ONE 2012, 7, e47262. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.J.; Barata, C. Genetic variation in the response of Daphnia to toxic substances: Implications for risk assessment. In Genetic and Ecotoxicology; Forbes, V.E., Ed.; Taylor & Francis: Philadelphia, PA, USA, 1998; pp. 207–220. [Google Scholar] [CrossRef]
- Lovett Doust, L.; Lovett Doust, J.; Schmidt, M. In praise of plants as biomonitors send in the clones. Funct. Ecol. 1993, 7, 754–758. [Google Scholar]
- Picado, A.; Chankova, S.; Fernandes, A.; Simões, F.; Leverett, D.; Johnson, I.; Hernan, R.; Pires, A.M.; Matos, J. Genetic varia-bility in Daphnia magna and ecotoxicological evaluation. Ecotoxicol. Environ. Safe 2007, 67, 406–410. [Google Scholar] [CrossRef]

| Adriatic Strain | Baltic Strain | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Couple 1 | Couple 2 | Couple 3 | Couple 4 | Day | Couple 1 | Couple 2 | Couple 3 | Couple 4 | ||||||||
| M1 | F1 | M2 | F2 | M3 | F3 | M4 | F4 | M1 | F1 | M2 | F2 | M3 | F3 | M4 | F4 | ||
| 1 | 1 | ||||||||||||||||
| 2 | 2 | ||||||||||||||||
| 3 | 3 | ||||||||||||||||
| 4 | 4 | ||||||||||||||||
| 5 | ○ | 5 | |||||||||||||||
| 6 | 6 | ||||||||||||||||
| 7 | ○ | 7 | |||||||||||||||
| 8 | ○ | ○ | 8 | ||||||||||||||
| 9 | 9 | ||||||||||||||||
| 10 | 10 | ○ | |||||||||||||||
| 11 | 11 | ||||||||||||||||
| 12 | ○ | ○ | 12 | ○ | ○ | ||||||||||||
| 13 | ○ | ○ | ○ | 13 | |||||||||||||
| 14 | ○ | 14 | |||||||||||||||
| 15 | ○ | 15 | |||||||||||||||
| 16 | 16 | ||||||||||||||||
| 17 | 17 | ||||||||||||||||
| 18 | ○ | ○ | 18 | ||||||||||||||
| 19 | 19 | ○ | ○ | ||||||||||||||
| 20 | 20 | ||||||||||||||||
| 21 | 21 | ||||||||||||||||
| 22 | 22 | ○ | |||||||||||||||
| 23 | 23 | ● | ● | ||||||||||||||
| 24 | 24 | ||||||||||||||||
| 25 | 25 | ● | |||||||||||||||
| 26 | 26 | ||||||||||||||||
| 27 | ○ | ● | 27 | ||||||||||||||
| 28 | 28 | ||||||||||||||||
| 29 | ● | 29 | ● | ● | |||||||||||||
| 30 | 30 | ||||||||||||||||
| 31 | 31 | ||||||||||||||||
| 32 | ● | ● | ● | 32 | |||||||||||||
| 33 | ● | 33 | ● | ||||||||||||||
| 34 | 34 | ||||||||||||||||
| 35 | ● | ● | 35 | ||||||||||||||
| Acartia tonsa | ||
|---|---|---|
| Mean Value | Adriatic Strain | Baltic Strain |
| Egg production female−1 day−1 | 10.4 ± 8.1 a | 21.3 ± 7.8 b |
| % egg-hatching success | 77.9 ± 13.1 a | 39.5 ± 36.0 b |
| % naupliar viability | 98.3 ± 3.1 a | 92.2 ± 8.1 b |
| Adult survival (days) | 35 (F) 32 (M) | 33 (F) 29 (M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitiello, V.; Oliva, M.; Renzi, M.; Cuccaro, A.; Fumagalli, G.; Anselmi, S.; Bentivoglio, T.; Matarazzi, I.; Sanna, V.; Pellegrini, D.; et al. Ecotoxicological Assays with the Calanoid Copepod Acartia tonsa: A Comparison between Mediterranean and Baltic Strains. Water 2024, 16, 1171. https://doi.org/10.3390/w16081171
Vitiello V, Oliva M, Renzi M, Cuccaro A, Fumagalli G, Anselmi S, Bentivoglio T, Matarazzi I, Sanna V, Pellegrini D, et al. Ecotoxicological Assays with the Calanoid Copepod Acartia tonsa: A Comparison between Mediterranean and Baltic Strains. Water. 2024; 16(8):1171. https://doi.org/10.3390/w16081171
Chicago/Turabian StyleVitiello, Valentina, Matteo Oliva, Monia Renzi, Alessia Cuccaro, Giorgia Fumagalli, Serena Anselmi, Tecla Bentivoglio, Iliana Matarazzi, Valeria Sanna, David Pellegrini, and et al. 2024. "Ecotoxicological Assays with the Calanoid Copepod Acartia tonsa: A Comparison between Mediterranean and Baltic Strains" Water 16, no. 8: 1171. https://doi.org/10.3390/w16081171





