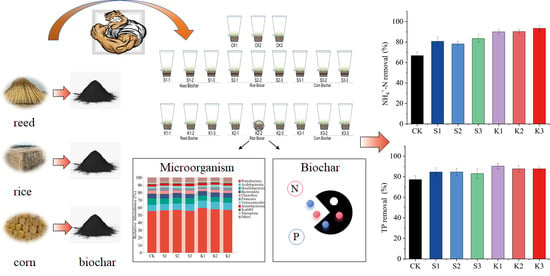
Added Biochars Promoted Nitrogen and Phosphorus Removal from Ecological Ditches at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Operation and Sampling
2.3. Data Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties of Water
3.2. N and P Removal by Each Group
3.3. Changes in Plant Physicochemical Properties
3.4. Changes in Physical and Chemical Properties of Sediments
3.5. Microbial Community Analysis
3.5.1. Diversity Analysis
3.5.2. Bacterial Community Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nsenga Kumwimba, M.; Meng, F.; Iseyemi, O.; Moore, M.T.; Zhu, B.; Tao, W.; Liang, T.J.; Ilunga, L. Removal of non-point source pollutants from domestic sewage and agricultural runoff by vegetated drainage ditches (VDDs): Design, mechanism, management strategies, and future directions. Sci. Total Environ. 2018, 639, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Ding, H.; Fu, H.; Liu, J.; Chen, Y.; Dai, T.; Lou, Q.; Zhong, X.; Fan, H.; et al. Low-dose biochar added to sediment improves water quality and promotes the growth of submerged macrophytes. Sci. Total Environ. 2020, 742, 140602. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, S.; Mi, M.; Zhuang, Y.; Zhang, L. What makes ditches and ponds more efficient in nitrogen control? Agric. Ecosyst. Environ. 2021, 314, 107409. [Google Scholar] [CrossRef]
- Kumwimba, M.N.; Zhu, B.; Suanon, F.; Muyembe, D.K.; Dzakpasu, M. Long-term impact of primary domestic sewage on metal/loid accumulation in drainage ditch sediments, plants and water: Implications for phytoremediation and restoration. Sci. Total Environ. 2017, 581–582, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Hu, Z.; Hou, C.; Liu, H.; Ngo, H.H.; Guo, W.; Lu, S.; Zhang, J. New insights for enhancing the performance of constructed wetlands at low temperatures. Bioresour. Technol. 2020, 301, 122722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, H.; Wang, W.; Hu, Z.; Yin, X.; Ngo, H.H.; Guo, W.; Fan, J. Enhancement of surface flow constructed wetlands performance at low temperature through seasonal plant collocation. Bioresour. Technol. 2017, 224, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, F.; Huang, Z.; Xiao, R.; Zhu, H.; Wu, J. Are vegetated drainage ditches effective for nitrogen removal under cold temperatures? Bioresour. Technol. 2020, 301, 122744. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Yin, X. Improving low-temperature performance of surface flow constructed wetlands using Potamogeton crispus L. plant. Bioresour. Technol. 2016, 218, 1257–1260. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, S.; Han, B.; Hua, Z.; Fu, D.; Li, P. Impacts of water flow on epiphytic microbes and nutrients removal in constructed wetlands dominated by Vallisneria natans with decreasing temperature. Bioresour. Technol. 2020, 318, 124058. [Google Scholar] [CrossRef]
- Cui, N.; Zhang, X.; Cai, M.; Chen, G.; Zhou, L.; Zou, G. Does rice straw addition and/or Vallisneria natans (Lour.) planting contribute to enhancement in nitrate nitrogen and phosphorus removal in constructed wetlands under low temperature? Bioresour. Technol. 2022, 350, 126896. [Google Scholar] [CrossRef]
- Nifong, R.L.; Taylor, J.M.; Moore, M.T. Mulch-Derived Organic Carbon Stimulates High Denitrification Fluxes from Agricultural Ditch Sediments. J. Environ. Qual. 2019, 48, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H. Biochar and Water Quality. J. Environ. Qual. 2019, 48, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.-L.; Li, M.; Li, Y.; Zhang, L.; Xu, X.; Wu, H.; Liang, S.; Su, C.; Zhang, J. The performance and mechanism of biochar-enhanced constructed wetland for wastewater treatment. J. Water Process Eng. 2022, 45, 102522. [Google Scholar] [CrossRef]
- Cheng, Q.; Cheng, H.; Wu, Z.; Pu, X.; Lu, L.; Wang, J.; Zhao, J.; Zheng, A. Biochar amendment and Calamagrostis angustifolia planting affect sources and production pathways of N2O in agricultural ditch systems. Environ. Sci. Process Impacts 2019, 21, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Cheng, H.; Lu, L.; Pu, X.; Wu, Z.; Sun, H. Fate of nitrogen in overlying water with biochar addition to sediment in planted ditches. Environ. Sci. Process Impacts 2018, 20, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, X.; Liu, G.; Huang, J.; Zhu, H.; Qiu, S.; Fu, X.; Wu, Y.; Bing, H. Novel ecological ditch system for nutrient removal from farmland drainage in plain area: Performance and mechanism. J. Environ. Manag. 2022, 318, 115638. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, Z.; Hu, T.; He, S.; Liu, Y.; Jiang, J.; Zhao, Q.; Wei, L. Performance and mechanisms of biochar-based materials additive in constructed wetlands for enhancing wastewater treatment efficiency: A review. Chem. Eng. J. 2023, 471, 144772. [Google Scholar] [CrossRef]
- Guo, F.; Luo, Y.; Nie, M.; Zheng, F.; Zhang, G.; Chen, Y. A comprehensive evaluation of biochar for enhancing nitrogen removal from secondary effluent in constructed wetlands. Chem. Eng. J. 2023, 478, 147469. [Google Scholar] [CrossRef]
- Zheng, F.; Fang, J.; Guo, F.; Yang, X.; Liu, T.; Chen, M.; Nie, M.; Chen, Y. Biochar based constructed wetland for secondary effluent treatment: Waste resource utilization. Chem. Eng. J. 2022, 432, 134377. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, W.; Lai, D.Y.F.; Yin, G.; Ren, D.; Guo, Z.; Zheng, Y.; Wang, J. Interaction of reed litter and biochar presences on performances of constructed wetlands. Water Res. 2024, 254, 121387. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L.; Teng, H. Study on treatment of city tail water by constructed wetland with corn straw biochar substrate. Environ. Technol. Innov. 2022, 28, 102855. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Wang, X.; Khan, M.B.; Lu, M.; Khan, K.Y.; Song, Y.; He, Z.; Yang, X.; Yan, B.; et al. Biochar from constructed wetland biomass waste: A review of its potential and challenges. Chemosphere 2022, 287, 132259. [Google Scholar] [CrossRef]
- Liang, J.F.; Li, Q.W.; Gao, J.Q.; Feng, J.G.; Zhang, X.Y.; Wu, Y.Q.; Yu, F.H. Biochar rhizosphere addition promoted Phragmites australis growth and changed soil properties in the Yellow River Delta. Sci. Total Environ. 2021, 761, 143291. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Wu, Y.; Chen, Y.; Zeng, G.; Zhang, J.; Li, H. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour. Technol. 2017, 243, 347–355. [Google Scholar] [CrossRef]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Koiv-Vainik, M.; Mander, U.; Truu, M. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Q.; Huang, L.; Liu, M.; Wang, N.; Chen, Y. Insight into the mechanisms of biochar addition on pollutant removal enhancement and nitrous oxide emission reduction in subsurface flow constructed wetlands: Microbial community structure, functional genes and enzyme activity. Bioresour. Technol. 2020, 307, 123249. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Huang, S.; Hu, H.; Lu, Y.; Wang, Y.; Yuan, J.; Gong, Z.; Wu, J.; Zhang, Y. Transformation of N and S pollutants and characterization of microbial communities in constructed wetlands with Vallisneria natans. J. Water Process Eng. 2021, 42, 102186. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, H.; Liu, Y.; Fu, Z.; Chen, G.; Zou, G.; Zhou, S. Reducing N losses through surface runoff from rice-wheat rotation by improving fertilizer management. Environ. Sci. Pollut. Res. Int. 2017, 24, 4841–4850. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, X.; Chang, J.S.; Guo, H.; Han, S.; Lee, D.J. Remediation of the black-odor water body by aquatic plants with plant growth-promoting Rhizobacteria: Lab and pilot tests. Environ. Res. 2023, 223, 115462. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Wang, D.; Wu, Z. Polypyrrole-Modified Tin Disulfide Nanoflower-Based Quartz Crystal Microbalance Sensor for Humidity Sensing. IEEE Sens. J. 2019, 19, 9166–9171. [Google Scholar] [CrossRef]
- Fu, W.; Song, G.; Wang, Y.; Wang, Q.; Duan, P.; Liu, C.; Zhang, X.; Rao, Z. Advances in Research Into and Applications of Heterotrophic Nitrifying and Aerobic Denitrifying Microorganisms. Front. Environ. Sci. 2022, 10, 887093. [Google Scholar] [CrossRef]
- Powers, S.M.; Baulch, H.M.; Hampton, S.E.; Labou, S.G.; Lottig, N.R.; Stanley, E.H. Nitrification contributes to winter oxygen depletion in seasonally frozen forested lakes. Biogeochemistry 2017, 136, 119–129. [Google Scholar] [CrossRef]
- Yu, G.; Chen, H.; Chen, J.; Chen, S.; Long, Y.; Huang, J.; Wang, Y.; He, S. Enhanced nitrogen removal through aerobic denitrifying bacteria in horizontal subsurface flow constructed wetlands: Influencing factors and microbial community structure. Chem. Eng. J. 2024, 481, 148654. [Google Scholar] [CrossRef]
- Chang, H.; Yang, X.-y.; Liang, D.; Chen, Z.-q.; Liu, X. Enhanced removal of ammonium nitrogen from aqueous solutions using a novel biochar derived from millet shells through both static adsorption and dynamic column experiments. J. Water Process Eng. 2024, 58, 104848. [Google Scholar] [CrossRef]
- He, Z.; Cao, H.; Liang, J.; Hu, Q.; Zhang, Y.; Nan, X.; Li, Z. Effects of biochar particle size on sorption and desorption behavior of NH4+-N. Ind. Crops Prod. 2022, 189, 115837. [Google Scholar] [CrossRef]
- Wang, W.; Pan, X.; Shu, X.; Tan, X.; Zhao, B.; Zhang, Q. Direct evidence indicates that revegetation improves organic carbon limitation in sediment denitrification in a eutrophic headwater river. Ecol. Eng. 2024, 198, 107132. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Fu, Z.; Song, X.; Yang, L.; Liu, F. Application performance and nutrient stoichiometric variation of ecological ditch systems in treating non-point source pollutants from paddy fields. Agric. Ecosyst. Environ. 2020, 299, 106989. [Google Scholar] [CrossRef]
- Iseyemi, O.O.; Farris, J.L.; Moore, M.T.; Locke, M.A.; Choi, S. Phosphorus dynamics in agricultural drainage ditches: An influence of landscape properties. J. Soil Water Conserv. 2018, 73, 558–566. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, M.; Lu, X.; Wang, T. Nutrient removal performance from agricultural drainage by strengthening ecological ditches in hilly areas. Agric. Water Manag. 2024, 291, 108623. [Google Scholar] [CrossRef]
- Wang, S.; Teng, Y.; Cheng, F.; Lu, X. Application Potential of Constructed Wetlands on Different Operation Mode for Biologically Pre-Treatment of Rural Domestic Wastewater. Sustainability 2023, 15, 1799. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T.D. Removal of nutrients, organics and suspended solids in vegetated agricultural drainage ditch. Ecol. Eng. 2018, 118, 97–103. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, S.; Chen, D.; Liu, K.; Lu, J. Response of biofilms-leaves of two submerged macrophytes to high ammonium. Chemosphere 2018, 192, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wu, H.-P.; Hao, B.-B.; Liu, G.-H. Stoichiometric characteristics and responses of submerged macrophytes to eutrophication in lakes along the middle and lower reaches of the Yangtze River. Ecol. Eng. 2013, 54, 16–21. [Google Scholar] [CrossRef]
- Dai, M.; Xiao, Y.; Wang, T.; Xu, J.; Wang, Y. Influence of N:P Ratio of Water on Ecological Stoichiometry of Vallisneria natans and Hydrilla verticillata. Water 2022, 14, 1263. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhong, J.; Fu, H.; Tu, J.; Fan, H. Submerged Macrophytes Exhibit Different Phosphorus Stoichiometric Homeostasis. Front. Plant Sci. 2018, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gan, X.; Wang, Z.; Jiang, S.; Zheng, X.; Zhao, M.; Zhang, Y.; Fan, C.; Wu, S.; Du, L. Research status on remediation of eutrophic water by submerged macrophytes: A review. Process Saf. Environ. Prot. 2023, 169, 671–684. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, L.; Ma, S.; Wu, Y.; Ye, Q.; Chang, Y.; Ye, Y.; Chen, K. Response of a submerged macrophyte (Vallisneria natans) to water depth gradients and sediment nutrient concentrations. Sci. Total Environ. 2023, 912, 169154. [Google Scholar] [CrossRef]
- Yan, H.; Li, F.; Liu, G. Diminishing influence of negative relationship between species richness and evenness on the modeling of grassland α-diversity metrics. Front. Ecol. Evol. 2023, 11, 8739. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, S.; Zhang, C.; Zeng, G.; Tan, X.; Song, B.; Zhang, P.; Yang, H.; Li, M.; Chen, Q. Application of biochar for the remediation of polluted sediments. J. Hazard. Mater. 2021, 404, 124052. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, P.; Lang-Yona, N.; Xu, M.; Guo, C.; Gu, J.D. Global landfill leachate characteristics: Occurrences and abundances of environmental contaminants and the microbiome. J. Hazard. Mater. 2024, 461, 132446. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef] [PubMed]





| Original Value | CK | S1 | S2 | S3 | K1 | K2 | K3 | |
|---|---|---|---|---|---|---|---|---|
| N/P (leaf) | 5.95 ± 0.21 bc | 5.83 ± 0.76 c | 6.48 ± 0.36 abc | 6.8 ± 0.36 abc | 7.22 ± 1.26 abc | 6.87 ± 0.14 abc | 7.63 ± 1.65 a | 7.53 ± 1.25 ab |
| N/P (root) | 7.59 ± 0.27 b | 5.53 ± 0.49 a | 5.02 ± 0.8 a | 5.5 ± 0.54 a | 5.44 ± 0.46 a | 5.4 ± 0.55 a | 5.95 ± 0.67 a | 4.95 ± 0.39 a |
| C/P (leaf) | 96.48 ± 3.35 d | 46.41 ± 4.63 b | 53.41 ± 3.06 b | 54.69 ± 2.48 ab | 59.40 ± 10.49 ab | 59.88 ± 1.12 ab | 68.23 ± 15.47 a | 68.40 ± 12.5 a |
| C/P (root) | 135.13 ± 2.85 d | 74.55 ± 7.19 ab | 68.87 ± 14.33 b | 81.28 ± 7.55 ab | 80.26 ± 5.55 ab | 78.82 ± 5.89 ab | 90.76 ± 9.64 a | 76.47 ± 3.95 ab |
| C/N (leaf) | 16.22 ± 0.02 c | 8 ± 0.28 a | 8.25 ± 0.2 a | 8.05 ± 0.09 a | 8.22 ± 0.18 a | 8.71 ± 0.01 b | 8.92 ± 0.17 b | 9.06 ± 0.35 b |
| C/N (root) | 17.81 ± 0.27 d | 13.46 ± 0.24 c | 13.64 ± 0.93 bc | 14.8 ± 0.18 a | 14.77 ± 0.45 a | 14.63 ± 0.4 ab | 15.31 ± 1.03 a | 15.49 ± 0.46 a |
| Original Value | CK | S1 | S2 | S3 | K1 | K2 | K3 | |
|---|---|---|---|---|---|---|---|---|
| TN (mg/kg) | 3.12 ± 0.29 a | 3.55 ± 0.62 a | 3.58 ± 0.29 a | 3.46 ± 0.36 a | 3.76 ± 0.03 a | 3.47 ± 0.16 a | 3.15 ± 0.18 a | 3.52 ± 0.03 a |
| TP (mg/kg) | 7.62 ± 0.33 e | 11.92 ± 1.25 d | 15.2 ± 0.49 abc | 14 ± 0.88 c | 14.83 ± 0.83 bc | 16.51 ± 1.29 ab | 17.29 ± 0.79 a | 16.82 ± 0.07 ab |
| C/N | 8.56 ± 0.28 c | 8.46 ± 0.21 c | 8.32 ± 0.15 c | 8.25 ± 0.26 c | 8.19 ± 0.05 c | 10.91 ± 0.4 a | 9.67 ± 0.93 b | 10.15 ± 0.43 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.; Pang, S.; Bi, D.; Wang, S.; Cai, M.; Kong, L.; Shen, Z.; Zhang, Y. Added Biochars Promoted Nitrogen and Phosphorus Removal from Ecological Ditches at Low Temperature. Water 2024, 16, 1191. https://doi.org/10.3390/w16081191
Bai G, Pang S, Bi D, Wang S, Cai M, Kong L, Shen Z, Zhang Y. Added Biochars Promoted Nitrogen and Phosphorus Removal from Ecological Ditches at Low Temperature. Water. 2024; 16(8):1191. https://doi.org/10.3390/w16081191
Chicago/Turabian StyleBai, Guangsha, Si Pang, Dongsu Bi, Siqi Wang, Min Cai, Lingqi Kong, Zheng Shen, and Yalei Zhang. 2024. "Added Biochars Promoted Nitrogen and Phosphorus Removal from Ecological Ditches at Low Temperature" Water 16, no. 8: 1191. https://doi.org/10.3390/w16081191