2.2.1. Major Operational Parameters and Verification Method for Each Barrier
The performance of the AWTP is considered here. The plant consisted of a seven barrier process designed to meet the stringent LRV requirements for the small scale water recycling plant.
The seven barriers selected for the AWTP were:
Ozonation, employing a strong oxidation effect of ozone on pathogen inactivation, bio-degradability improvement of organic matter, degradation of chemicals of concern (CoC), a lower chemical consumption associated with cleaning of the downstream ceramic micro-filtration (MF) and improved backwash efficiency [
4,
5].
Ceramic microfiltration membranes (MF), providing a size exclusion barrier to pathogens and suspended solids and direct ozone compatibility to lower chemical consumption for membrane cleaning. Ceramic MF has much better robustness and long-term integrity than polymeric membranes, excluding the need for pinning of broken fibres.
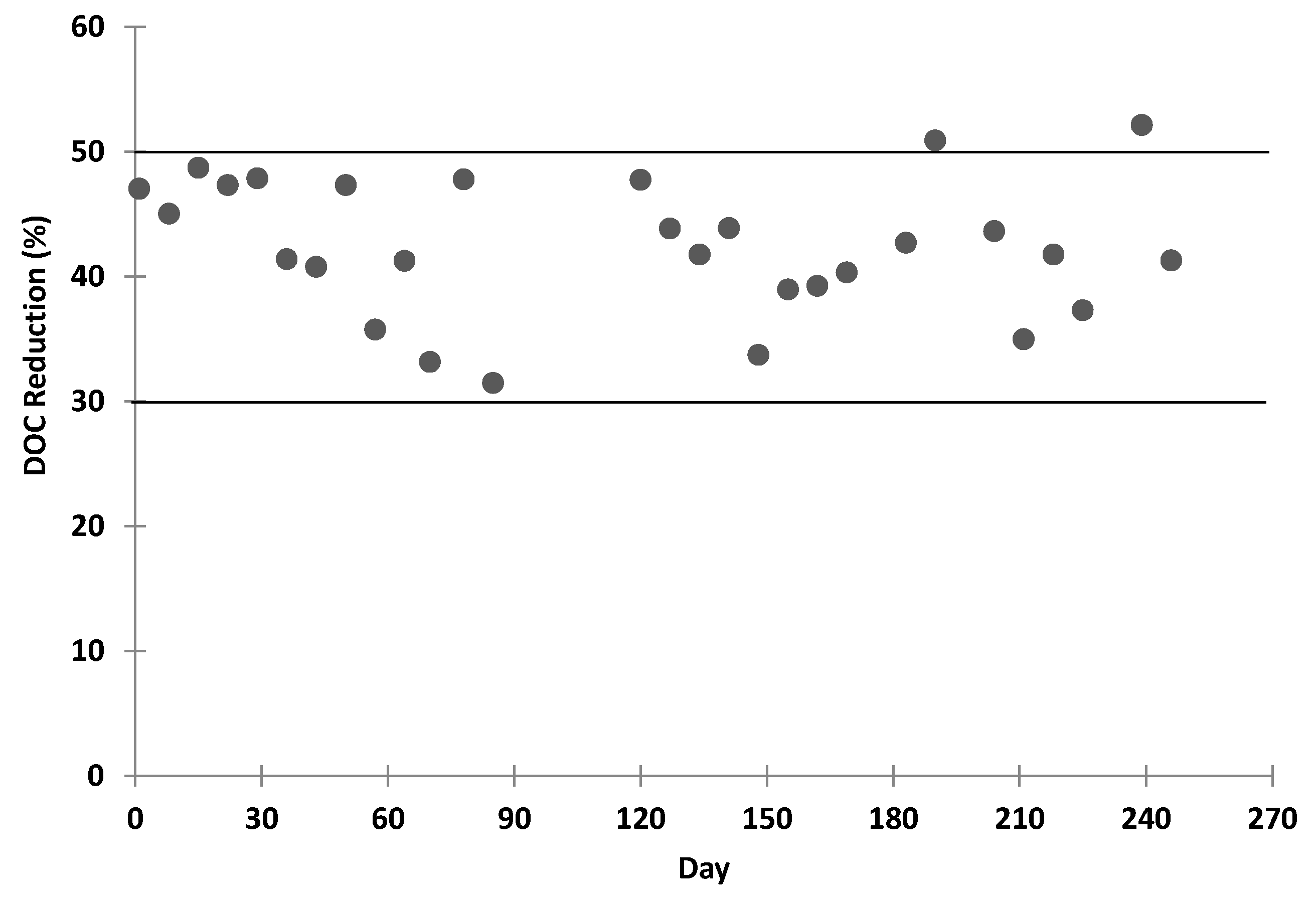
Biologically activated carbon (BAC) filter, biologically removes DOC to lower the organic fouling potential of the reverse osmosis (RO) feed so as to extend the RO element replacement period and reduce chemical consumption for clean-in-place (CIP) and also removes additional trace organic compounds from the RO brine that will be discharged to the Antarctic Ocean. However, the BAC will also increase the concentration of particulates in the BAC effluent and increases the frequency of cartridge filter replacement upstream of the RO array.
Reverse osmosis (RO) uses size exclusion to remove metals, pathogens and chemicals of concern (CoCs). No anti-scalant was used and only acids and alkalis used for RO membrane cleaning.
Ultraviolet radiation (UV), employs ultraviolet C (UVC) radiation to deactivate pathogens, especially protozoa.
Calcite contactor dissolves calcium carbonate into the RO permeate to increase water stability thereby reducing product water corrosivity, and
Chlorination via hypochlorite to provide an additional pathogen inactivation and chlorine residual to suppress pathogen regrowth in the distribution system.
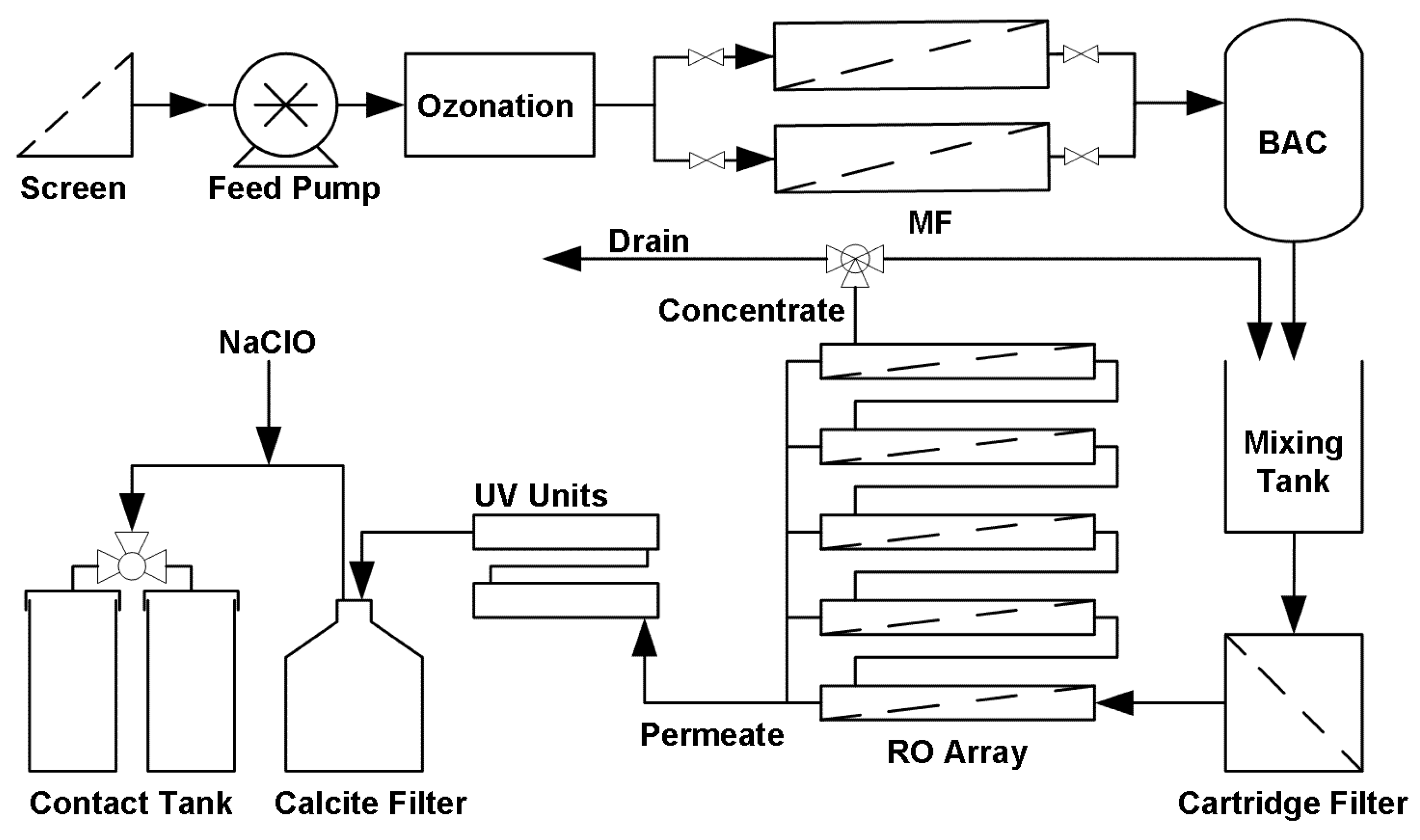
A schematic diagram of the pilot plant is shown in
Figure 1. Ozone production was via a Wedeco OCS-GSO 10 system with a nominal ozone production rate of 30 g/h. The unit was set at 19–20 mg per litre wastewater (≈24 g/h). The ozone system included a 480 L contact tank with an internal tank, a circulating Venturi dosing system operating at a circulation flowrate of 2 m
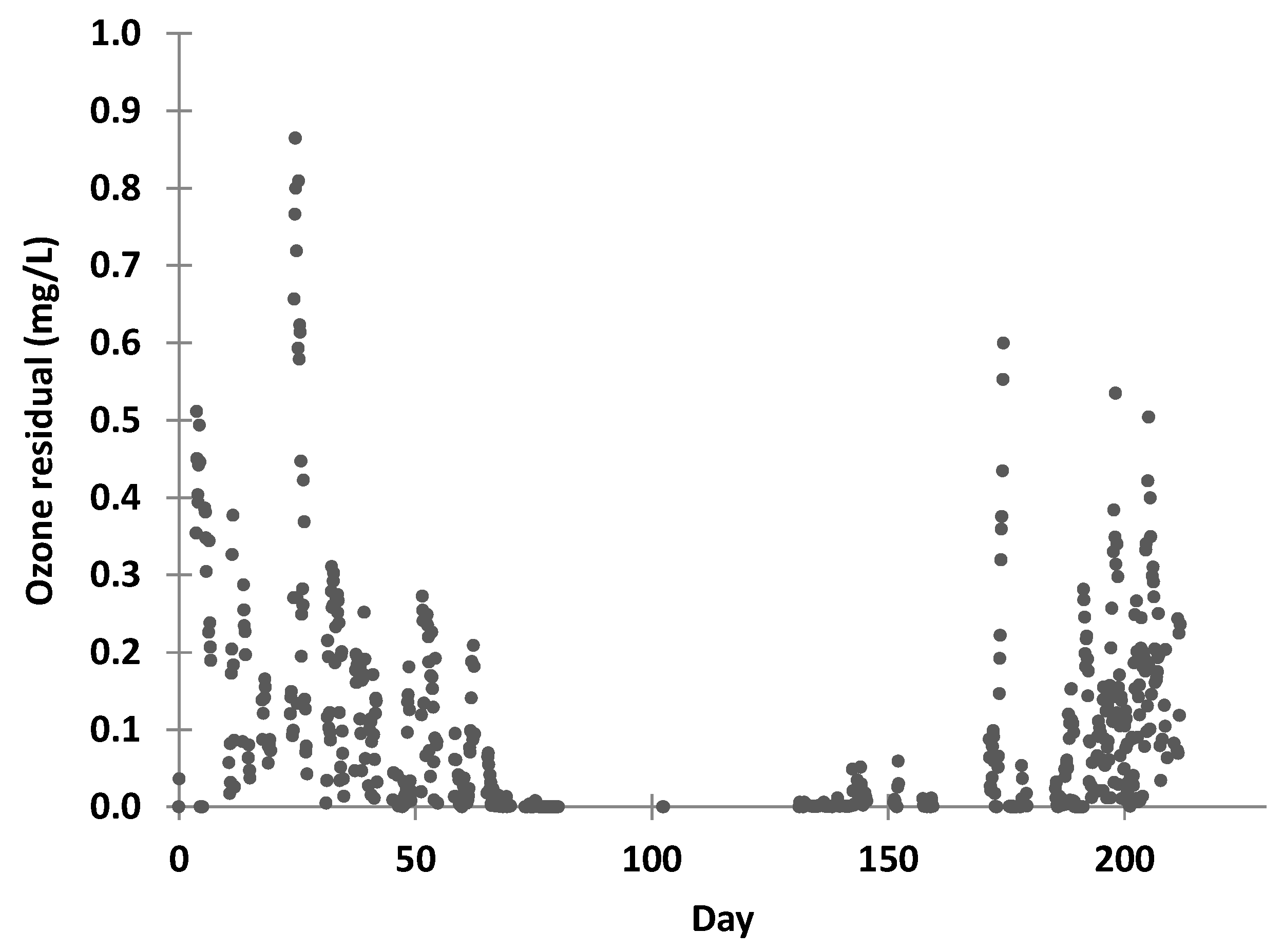
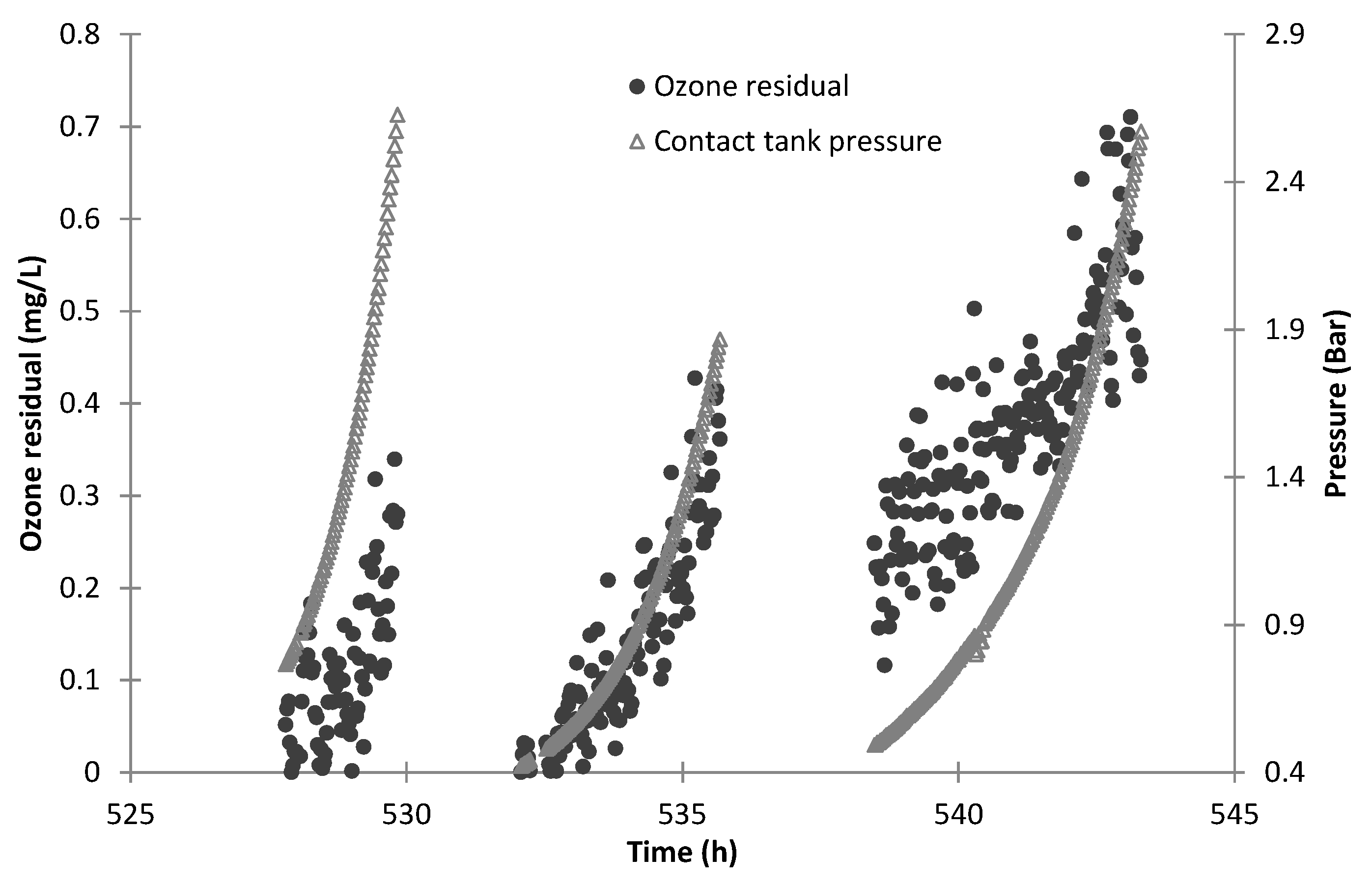
3/min, a pressure swing absorption oxygen generator and an ozone generator. The ozone system started approximately 10 to 15 min earlier than the feed pump to build up the ozone concentration in the ozone contact tank. The ozone mass transfer efficiency to the feed water was estimated by measuring the difference between the gas flow rate from the ozone destroyer between operation in dry mode (ozone generator not operating) and operational mode. The residual ozone concentration was measured by an in-line ozone sensor positioned in the discharge line from the ozone system.
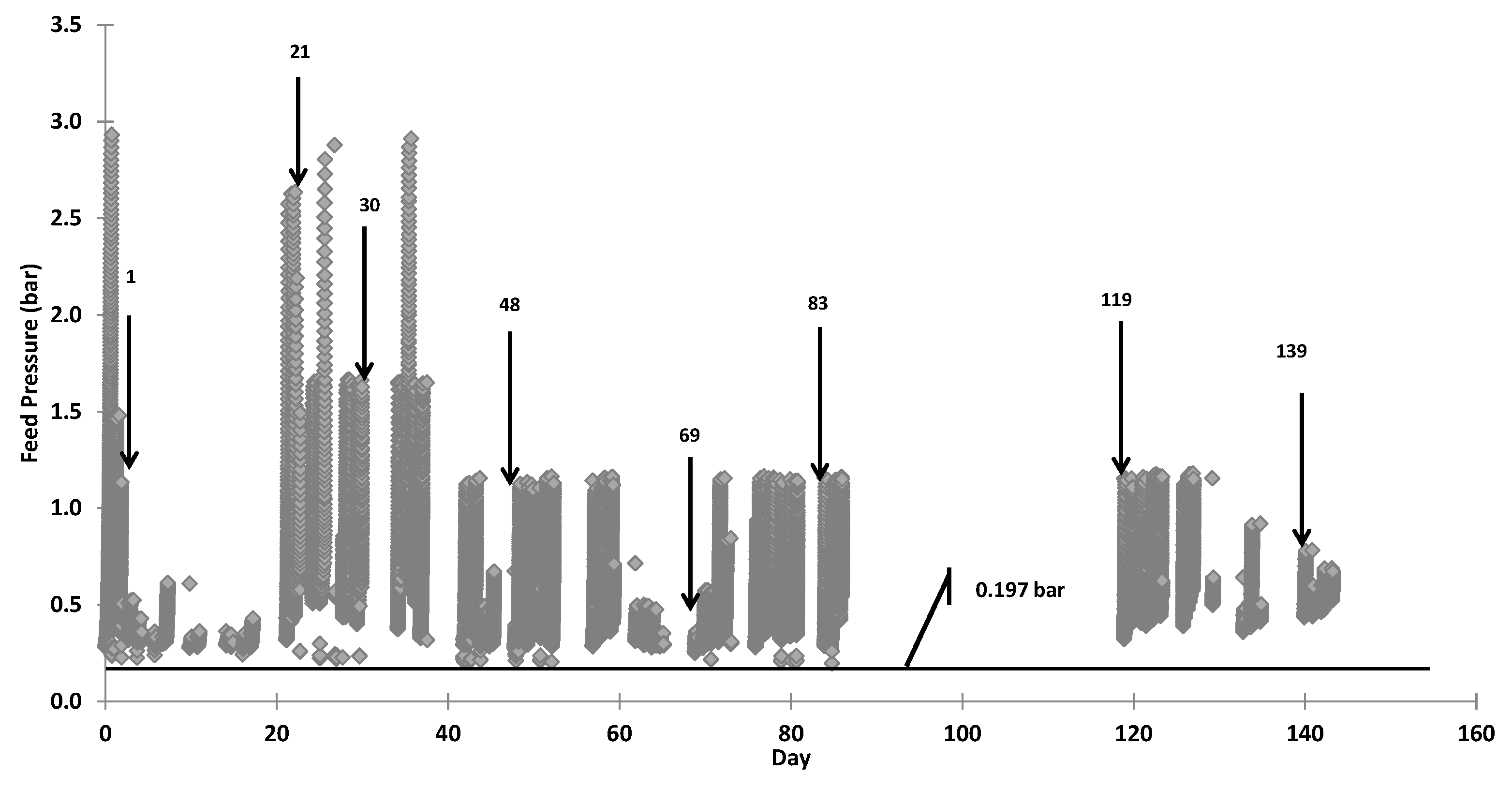
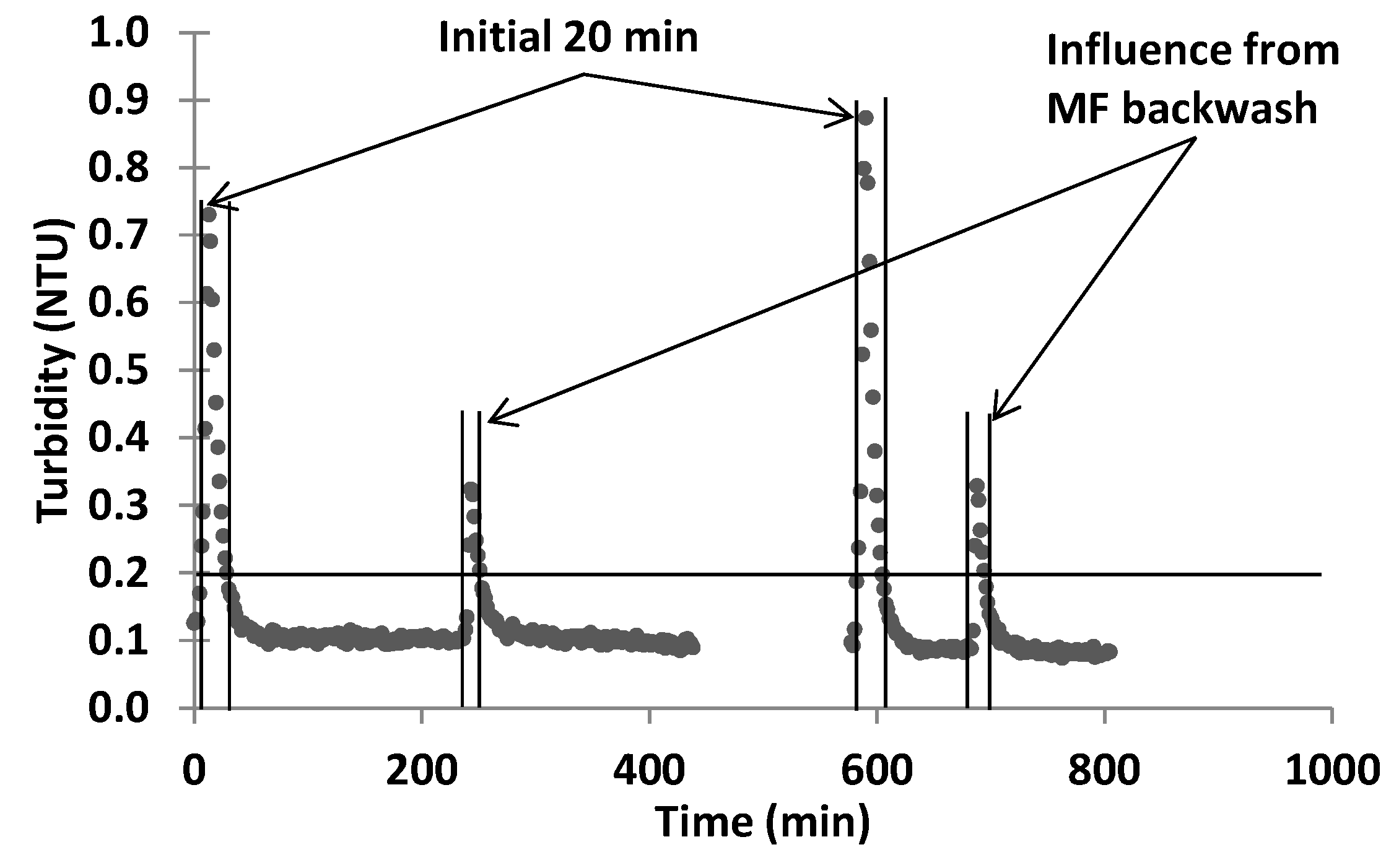
The MF barrier comprised two 0.1 µm Metawater® (Chiyoda-ku, Tokyo, Japan) ceramic membranes operated alternatively (duty/standby) in dead end mode. The area of each membrane was 25 m2. The operating flux was approximately 50 Lm−2·h−1. A pressure decay test (PDT) was used to ensure membrane integrity after each online period and the filtrate turbidity was used to trigger the integrity test during operation. To minimise labour requirements, clean-in-place (CIP) of the ceramic MF membranes with manual chemical addition to the CIP tank was not practiced. However, 100 mg/L NaClO solution was used for chemically enhanced backwash (CEB) instead of 50 mg/L as recommended by the manufacturer. The backwash pressure was also reduced to 1.6 bar from the manufacturer recommendation of 4 bar, which minimised hydraulic shock and vibration within the treatment system during backwash. The MF backwash was returned to an upstream trickling filter at SPWWTP and will also be returned to start of the wastewater treatment process at Davis Station.
The BAC barrier used Acticarb® (El Segundo, CA, USA) BAC GA1000N activated carbon with an Empty Bed Contact Time (EBCT) of 20 min. Monitoring of the BAC filtrate was performed by an in-line turbidity meter located in the BAC filtrate line. Turbidity was used to detect high turbidity filtrate flowing from the BAC into the RO mixing tank. The BAC backwash was activated when the head-loss increased to 25 mbar (on-line sensor) or the filtrate turbidity reached 1.5 NTU. Reticulated Hobart tap water (flowrate 3.3 L/s) was used to backwash the BAC. BAC backwash was returned to SPWWTP and at Davis station the BAC backwash will be returned to the start of the wastewater treatment plant. As the BAC was operated intermittently because of the batch operation of the treatment plant, air was intermittently fed to the BAC filter.
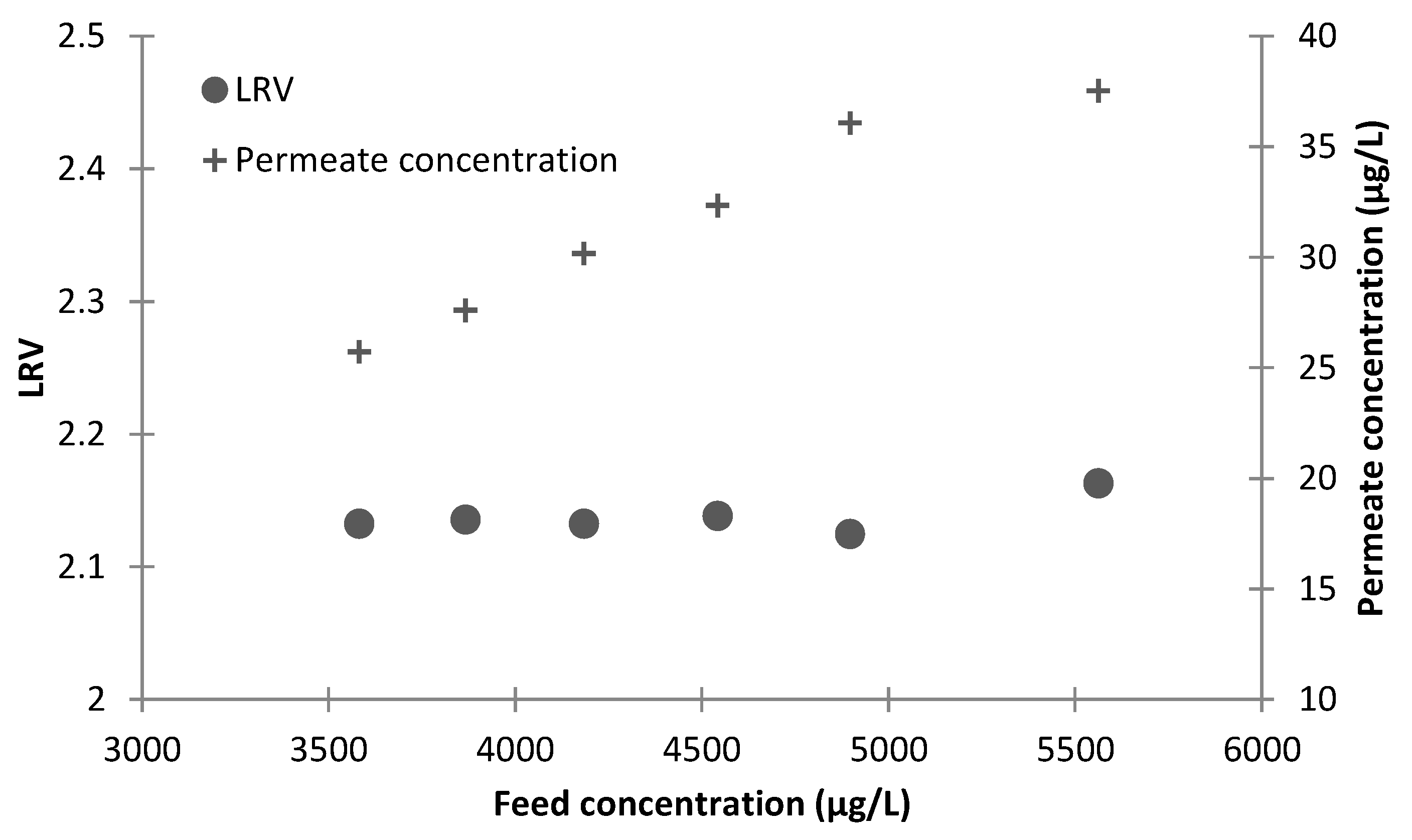
Five FILMTEC BW30-4040 RO elements were used in the RO array, and the designed transmembrane pressure and permeate flow were 9.4 bar and 14 L/min, respectively. The RO system incorporated a recycle stream to increase the overall recovery to 70%, with a single pass recovery of approximately 50%. The membrane integrity was monitored by both conductivity and PDTs. The PDT was conducted based on the method described by Zhang et al. [
6]. The RO PDT was used to achieve the required LRV for protozoa and a protozoa LRV of 2 across the RO membranes was claimed. For this system, the initial pressure used for the PDT was 85 kPa (transmembrane pressure of 45 kPa and a static head pressure of 40 kPa) and a pressure decay rate below 3.7 kPa/min indicated a protozoa LRV of 2 could be claimed for the RO system. The RO permeate would fill a head tank first before supply to the product pipeline. The RO permeate in the head tank was used to flush the concentrate side of the RO elements when the plant switched to standby mode to dilute the concentrate and reduce fouling and osmotic pressure. To reduce the chemical inventory and storage capacity, only 90 L of 600 mg/L NaOH and 90 L of 550 mg/L HCl solutions were used for the RO CIP and no anti-scalant was used. The RO integrity was challenged using rhodamine WT dye by adding the dye into the mixing tank and measuring the dye concentration in the feed and combined permeate. A membrane autopsy was performed once the RO membranes fouled. The RO housing was cut open, samples of fouled membrane dried at 40 °C and the sample weight determined before the samples were ultrasonically cleaned in water and again dried at 40 °C before weighing. The dried sample weights before and after cleaning were used to determine the dry foulant load. A sample of the foulants was also removed from the membrane, dried at 80 °C overnight in a pre-weighed crucible, before the crucible was placed in a preheated (565 °C) muffle furnace for 2 h. The crucible and dried foulant were cooled and weighed before being re-loaded into the Muffle furnace (565 °C) overnight. The crucible and residual foulant were then cooled and weighed. Organic matter was designated as the difference between weights and inorganic matter was designated as the residue left after heating.
Two UV units (Wedeco Spectron 6) were used in series to achieve a minimum UV-C dose of >189 mJ/cm2, as required for 4 LRV of virus. Each UV lamp was able to achieve an UV-C output of 25 W maximum, and the two units were operated to ensure water quality was maintained if one failed during service. The 10% residence time for CT value calculation, T10 (10% of the feed passes through the contactor) for the ozone contact tank and UV units were measured using the step dose method (USEPA, 1991) with rhodamine WT dye.
The calcite contactor (Puretec®, Box Hill, Victoria, Australia) had an EBCT value of 5 min to achieve a Ca2+ concentration no less than 20 mg/L. The need to replenish the calcite contactor with calcite was monitored using filtrate pH.
The designed free chlorine dose was 0.9 mg/L with a residual no less than 0.7 mg/L after 30 min of contact time. Both doses were monitored by online chlorine meters. All CCP related instrumentation, except for the pressure transmitters and flowrate meters, were verified weekly.
2.2.2. Plant Tests and Monitoring
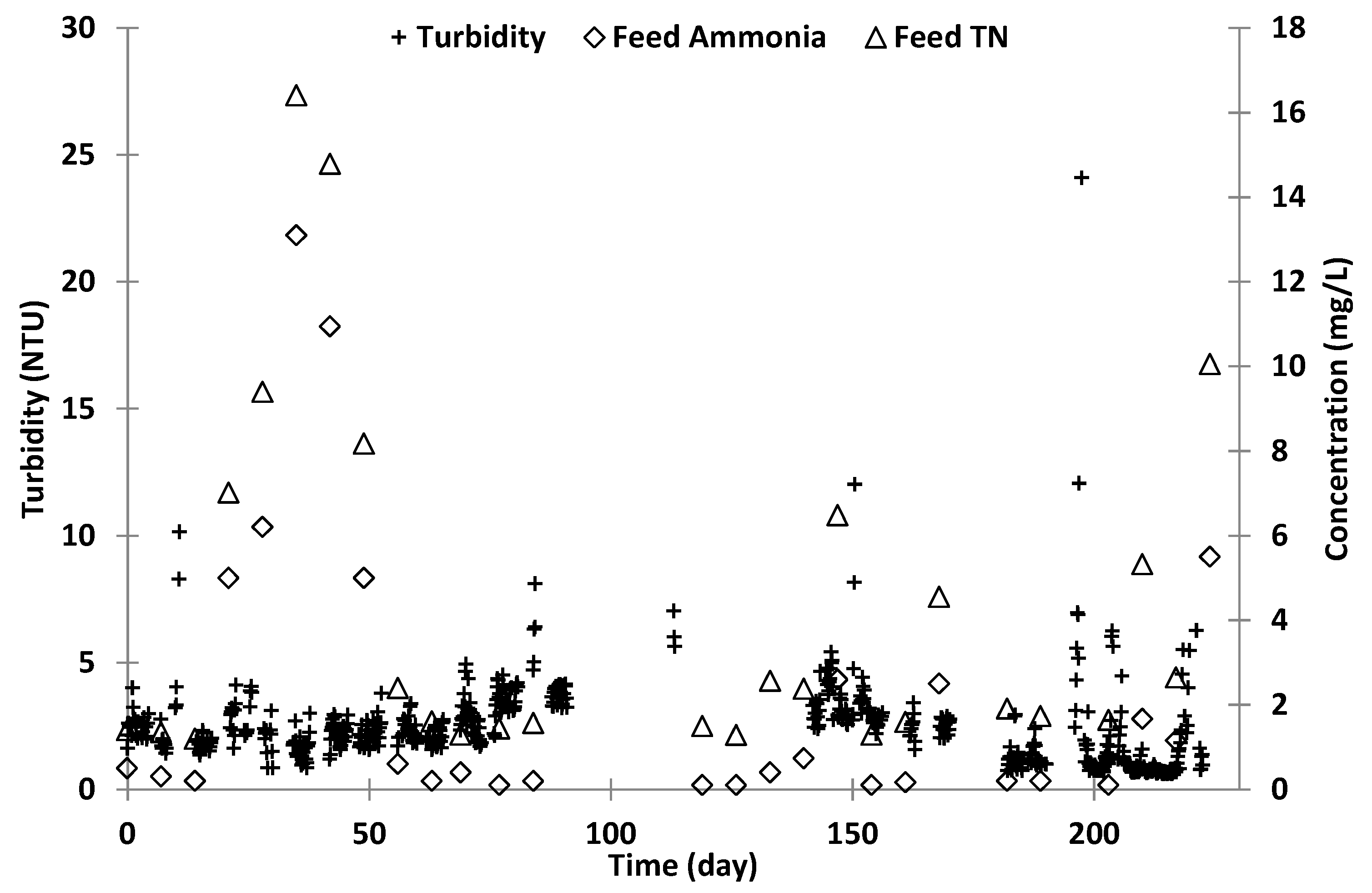
The AWTP was designed to achieve a water recovery of 70% at a constant feed rate of 20 L/min (1200 L/h) and could be remotely operated. The AWTP was designed to operate intermittently for a maximum of 21 h/day and had the capacity to reduce to 4 h operation every two days. It was designed to start and stop automatically and to enter standby mode when the feed tank to the treatment plant fell below a low level set point and to re-start once the tank exceeded a high level set point. The feed water quality was monitored to ensure it met the assumed feed water quality of the design, and target and alarm levels set for feedwater quality are shown in
Table 1.
The plant was commissioned for 2 months to establish remote operation, verify the detection of critical control point values, and confirm automatic start/stop operation. It also established criteria for the calibration of sensors, and the frequency required to re-fill chemical tanks. Assessment was also made of the level and types of interventions (level of technical expertise) required to re-start operations after critical faults. The plant typically operated for five days per week, while intermittent operation was controlled by levels in a ‘virtual’ tank so actual production and standby times were similar to what might be expected at Davis Station (i.e., 6–7 h operation and 4 h standby). A formal hand-over process was not conducted at the end of the commissioning period, and an ongoing process of fault improvement continued.
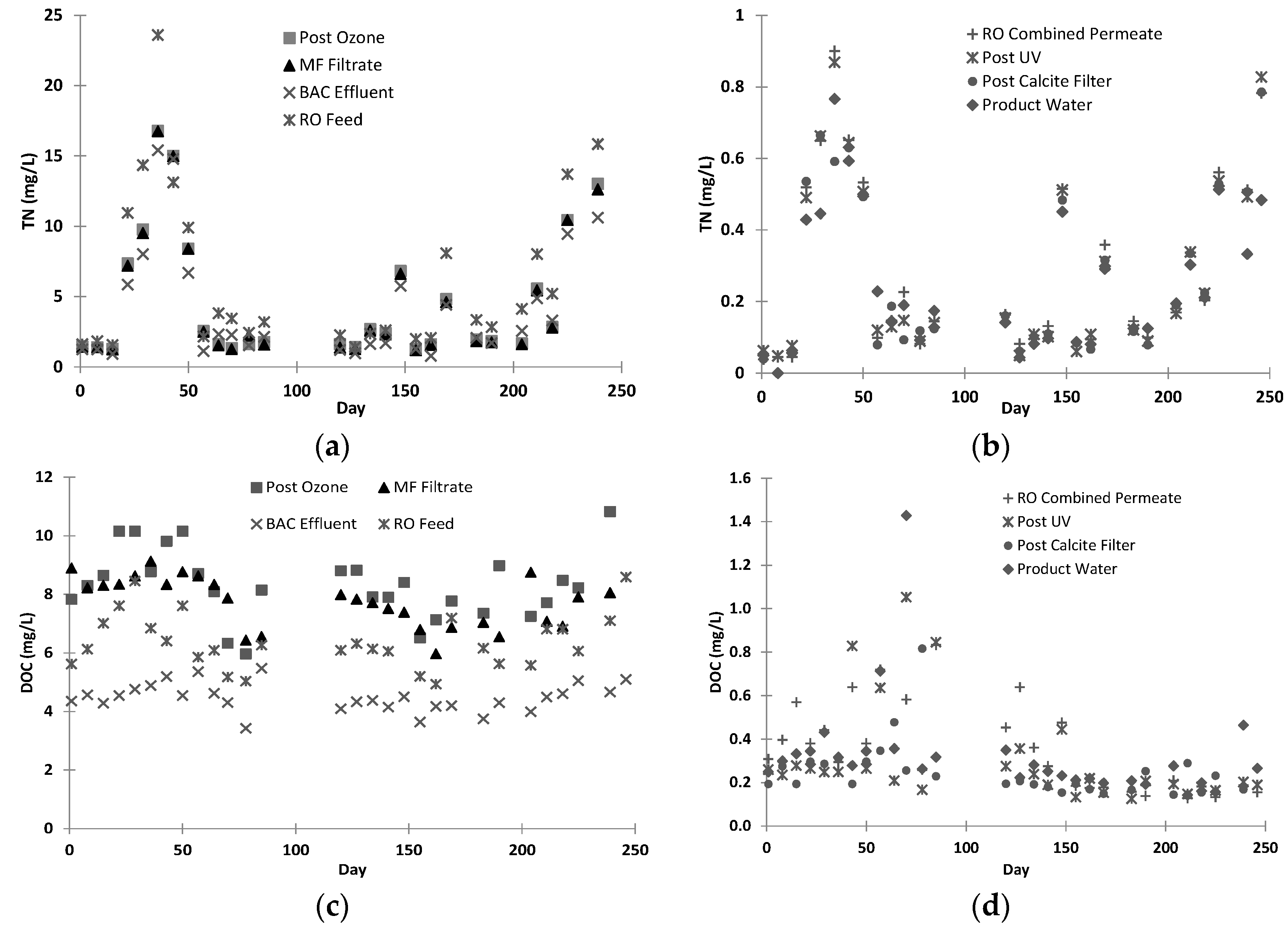
Two samples were taken for each barrier weekly for analysing dissolved organic carbon (DOC) (measured by a Shimadzu (Chiyoda-ku, Tokyo, Japan), TOC_V with TNM-1 unit), total nitrogen (TN measured by Shimadzu (Chiyoda-ku, Tokyo, Japan), TOC_V with TNM-1 unit), total phosphate (TP, Shimadzu (Chiyoda-ku, Tokyo, Japan), ICP2000), calcium (Shimadzu (Chiyoda-ku, Tokyo, Japan), ICP2000) and other metals (Shimadzu (Chiyoda-ku, Tokyo, Japan), ICP2000) for comparison with the Australian Drinking Water Guideline (ADWG).
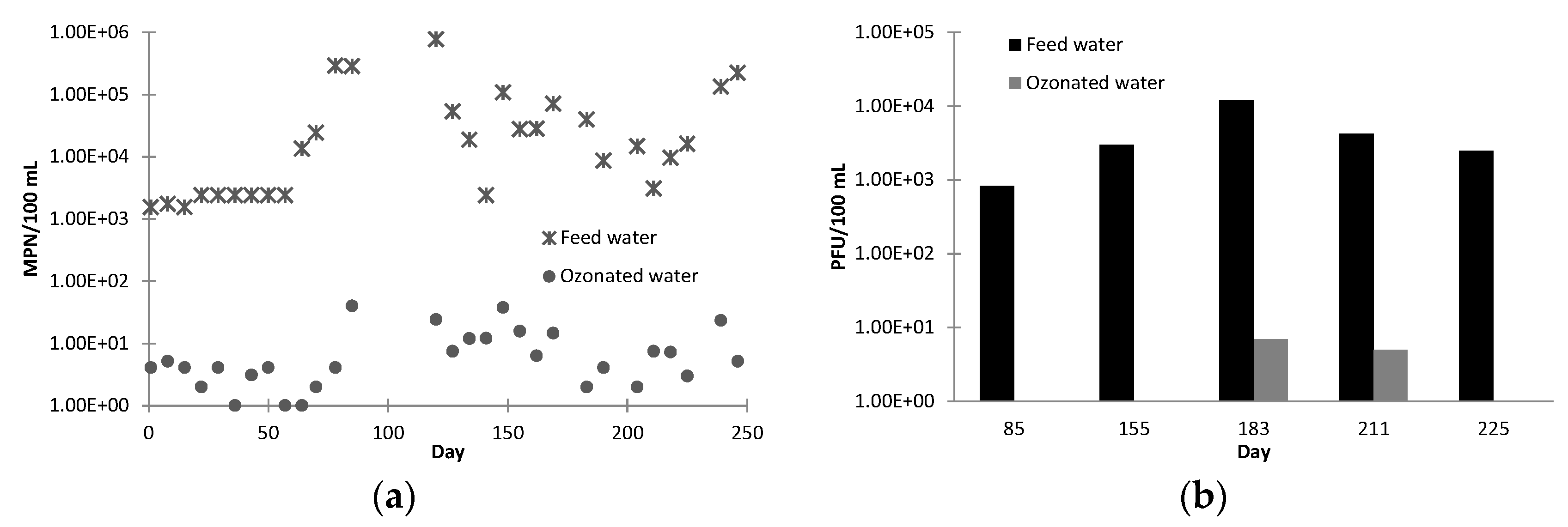
E. coli and total coliforms were tested weekly by plate counting for samples of plant feed, ozone effluent, ceramic MF filtrate, BAC filtrate, RO permeate and product water.
Somatic coliphage as a surrogate for virus in the plant feed, ozonation effluent, ceramic MF filtrate, BAC filtrate and product water were analysed five times during the operation period. Biodegradable dissolved organic carbon (BDOC) of feed, ozonation effluent, ceramic MF filtrate and BAC filtrate were analysed three times during the trial by the Joret method, and were performed by Research Laboratory Services Pty Ltd. (Eltham, Victoria, Australia). The chemical consumption and plant operation time were calculated based on the data recorded by the supervisory control and data acquisition (SCADA) system. The required critical values for each barrier are listed in
Table 1.
Following operation for 6 months, SCADA faults were corrected and adjustments made to the plant based on operational performance during the first operational period. Trend analysis of operational performance was formally captured on the SCADA for each barrier in the AWTP and fault analysis was recorded in the operator log. The plant did not operate during days 160–170 (Easter holiday period) or around day 180 when serious maintenance issues at SPWWTP resulted in feed water quality exceeding the set limits. Periods of high flow because of rainfall also resulted in high feed turbidity and the plant was shut down.
2.2.3. Plant Challenge for Disinfection By-Products
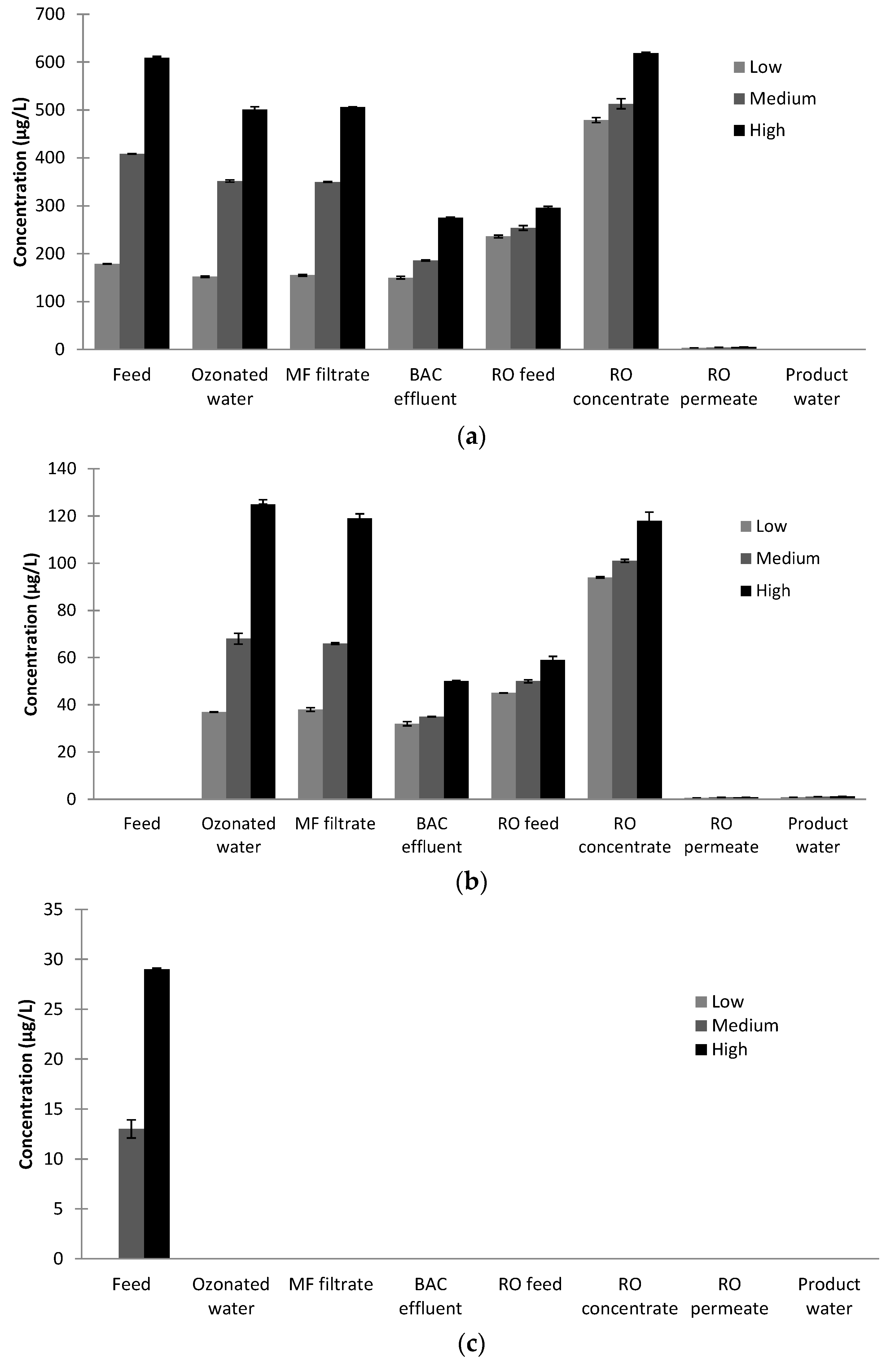
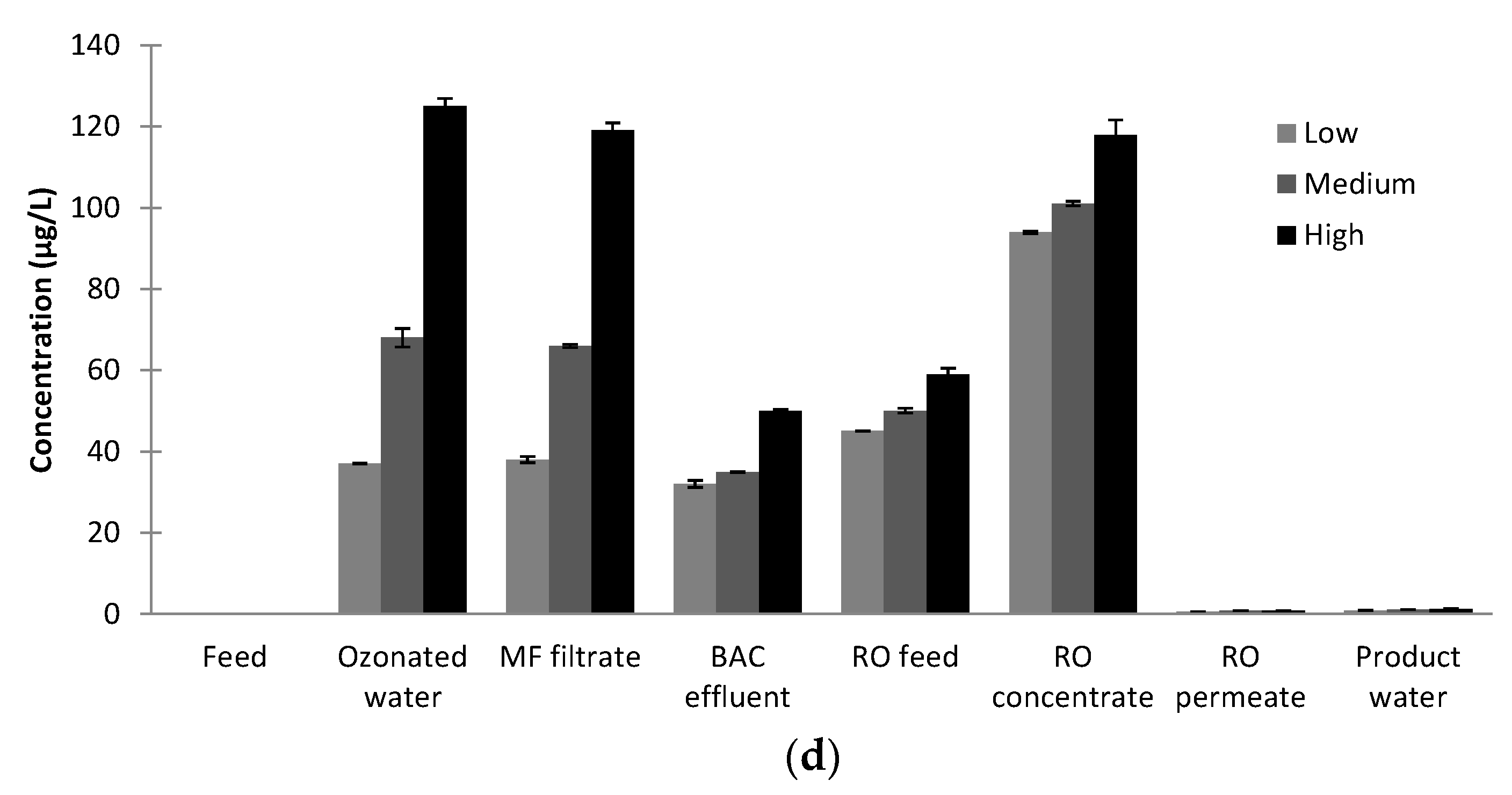
A risk identified for AWTP was the formation of brominated and iodated disinfection by-products as ozone was used on the feedwater. While the current plant was to be operated at Davis Station, it was envisaged the same AWTP design could also be used at other locations for potable water recycling. Therefore, the AWTP was challenged with increased concentrations of bromide (Br−) and iodide (I−) to ensure efficient removal of disinfection by-products (DBPs) could be achieved. The AWTP feed water was spiked with bromide and iodide to produce three different feedwater concentrations: low (200 µg/L Br−, 9 µg/L I−), medium (490 µg/L Br−, 37 µg/L I−) and high (693 µg/L Br−, 63 µg/L I−). The high feedwater concentrations were chosen to be similar to the concentrations found in high bromide and iodide natural waters. Spiking with bromide and iodide occurred prior to any oxidative process and chlorination was the final disinfection process. Samples were taken throughout the plant (Plant Feed, Post Ozone, Post MF, Post BAC, RO Feed, RO Concentrate, RO Permeate and Product Water) and analysed for a variety of DPBs (bromate (BrO3−), iodate (IO3−), Adsorbable Organic Halides (AOCl, AOBr and AOI), trihalomethanes (THMs) and haloacetic acids (HAAs)). Duplicate measurements were carried out for all samples.
Samples (24) were collected in amber bottles. Residual ozone in the Post ozone and Post MF samples was quenched with sodium sulphite during collection, while Product Water samples were quenched for chlorine.
Ion chromatography (IC) (Dionex (Sunnyvale, CA, USA) ICS3000 ion chromatograph) was used to analyse for halides (Br− and I−) and oxyhalides (BrO3− and IO3−). The IC was fitted with an anion exchange column (Dionex IonPac® (Sunnyvale, CA, USA) AS9-HC 4 × 250 mm), used sodium carbonate as the eluent and utilised conductivity and UV for detection. Filtered samples (500 µL) were injected into the IC and the anions were measured simultaneously. Br− and I− were detected using conductivity. BrO3− and IO3− were detected using an online post-column reaction (using acidified potassium iodide, catalysed by heptamolybdate) with UV/Vis detection.
The method of Kristiana, et al. [
7] was used to analyse for specific adsorbable organic halides (AOCl, AOBr and AOI). Acidified (pH 2) 50 mL of samples were passed through two activated carbon columns in series, the activated carbon columns combusted (Mitsubishi AQF-100), the hydrogen halide gases collected in MilliQ water and subsequently analysed in an IC system using an anion exchange column (Dionex IonPac
® (Sunnyvale, CA, USA) AS19-HC 4 × 250 mm) and conductivity detector.
Head-space solid phase micro-extraction (SPME), followed by gas chromatography separation and mass spectrometry detection (GC–MS) was used to analyse for 10 trihalomethanes (THMs) according to a published method [
8]. The THMs analysed were Iodoform (CHI
3), Bromodiiodomethane (CHBrI
2), Dichloroiodomethane (CHCl
2I), Bromochloroiodomethane (CHBrClI), Dibromoiodomethane (CHBr
2I), Chlorodiiodomethane (CHClI
2), Bromoform (CHBr
3), Bromodichloromethane (CHBrCl
2), Chlorodibromomethane (CHBr
2Cl), and Chloroform (CHCl
3).
Liquid–liquid extraction (LLE) with methyl-tert-butyl-ether (MtBE), subsequent derivatisation with acidic methanol, followed by quantification using GC-was used to analyse the haloacetic acid concentrations. The nine haloacetic acids (HAAs) measured were Bromodichloroacetic acid (BDCAA), Tribromoacetic acid (TBAA), Dibromoacetic acid (DBAA), Chlorodibromoacetic acid (CDBAA), Bromochloroacetic acid (BCAA), Bromoacetic acid (MBAA), Trichloroacetic acid (TCAA), Dichloroacetic acid (DCAA) and Chloroacetic acid (MCAA).
Specific ultraviolet absorbance at 254 nm (SUVA254), defined as the ultra-violet absorbance at 254 nm (UV254) divided by dissolved organic carbon (DOC) concentration, was measured for all DBP Plant Feed and Post Ozone samples. SUVA254 was used as a measure of the aromatic content of the organic matter (i.e., strong reactive sites). A Shimadzu TOC-Vws Total Organic Carbon (TOC) analyser was used to measure DOC concentrations and UV254 was measured using a Cary 60 UV-Vis Spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).
2.2.4. Screening for Trace Organic Chemicals (TrOCs)
An Automated Identification and Quantification System database method (AIQS-DB) was linked to gas chromatographic–mass spectrometric (GC–MS) and liquid chromatographic-time of flight mass spectrometric (LC-TOF-MS) methods to allow the determination of more than 1250 trace organic chemicals (TrOCs) in extracted water samples. These methods differ from many current operations, where a few chemicals (surrogates) are often chosen to be representative of many, because of the difficulty and cost of assessment of the very large range of chemicals that could be present in secondary effluent.
Samples of the feed, reject and product water were collected in 1 L amber glass bottles on a monthly basis and kept on ice until prepared for analysis (within 48 h). After addition of appropriate buffers and/or internal standards or other reagents, the samples were extracted by solid phase extraction (SPE): Empore SDB-XC disks for GC–MS; Waters Sep Pak PS-2 and AC-2 SPE cartridges (for LC-TOF-MS). SPE extracts were refrigerated until they were further analysed. Full methodological details can be found in [
9,
10].
The AIQS-DB method uses internal standard calibration curves, obtained under set operating conditions, to identify and quantify chemical substances using retention times and mass spectra. The GC–MS or LC-TOF-MS instrument conditions are required to be adjusted to the designated conditions used to compile the database in order to obtain accurate results. The results obtained from performance check standards were evaluated against three criteria (spectrum validity, inertness of column and inlet liner, and stability of response) and the difference between the predicted and actual retention times will be less than 3 s. The method detection limits (MDL) for target substances are estimated from the concentration ratio and the instrument detection limit (IDL) of model compounds and are in the range 0.01 to 0.1 µg/L for GC–MS, and 2.5–5 ng/L for LC-TOF-MS. The AIQS-DB GC–MS method can detect 940 semi-volatile substances including a variety of polychlorinated biphenyl compounds (PCBs); halogenated and non-halogenated hydrocarbons; pharmaceutical and personal care products (PPCPs); polycyclic aromatic hydrocarbons (PAHs); and agricultural compounds. The AIQS-DB LC-TOF-MS method can analyse 265 polar and non-volatile compounds, including 180 agricultural compounds and 70 pharmaceuticals.