Determination of Growth Stage-Specific Crop Coefficients (Kc) of Sunflowers (Helianthus annuus L.) under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Characteristics and Field Experiment Design
2.2. Model Descriptions
2.2.1. Basic Principles
2.2.2. Infiltration
2.2.3. Groundwater Recharge
2.2.4. Root Distribution
2.2.5. Root Water Uptake
2.2.6. Evapotranspiration and Crop Coefficients (Kc)
2.2.7. Modeling Process
2.3. Statistical Analysis
3. Results
3.1. Meteorological Conditions
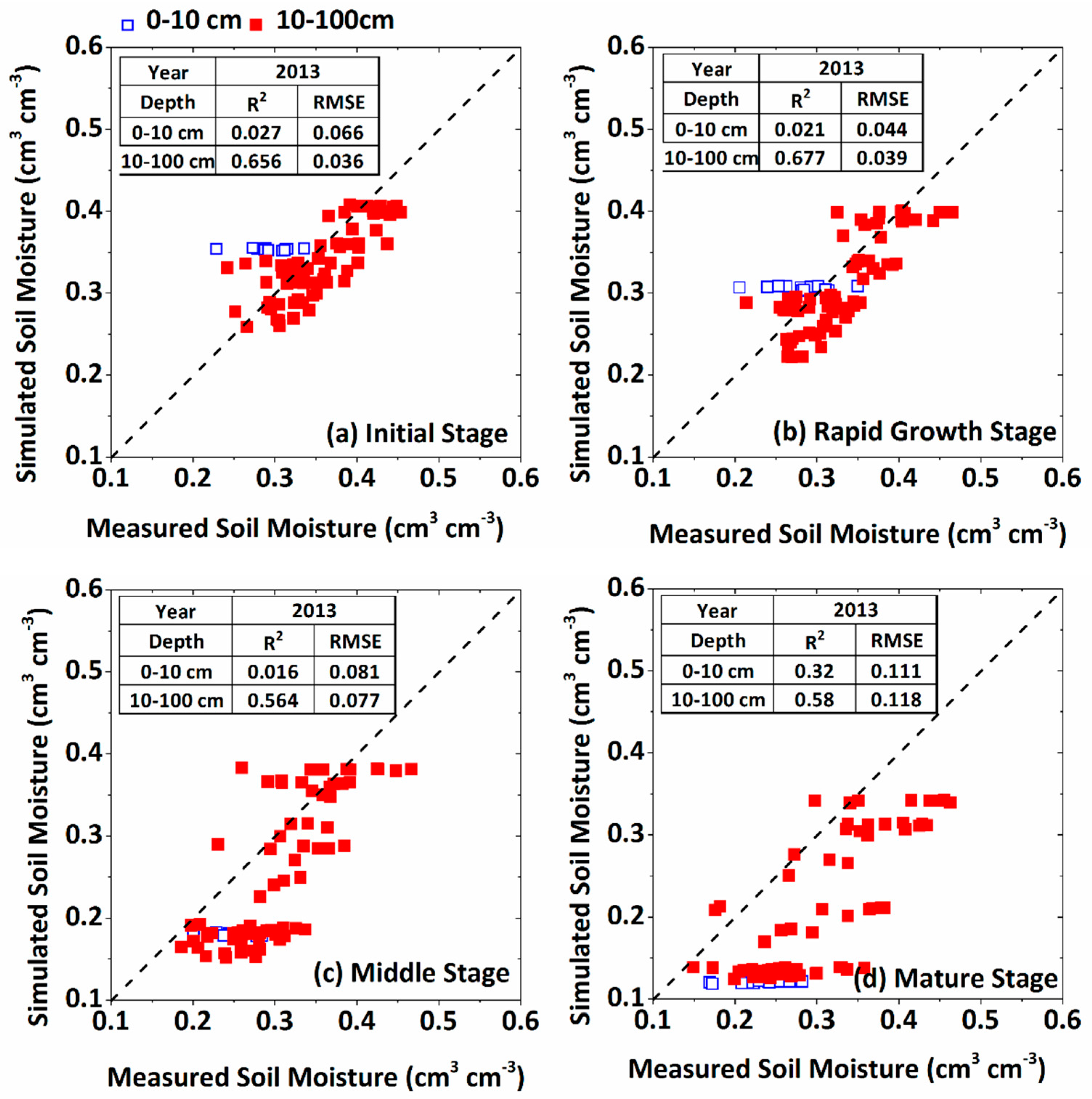
3.2. Calibration of the WFSP Model
3.3. Establishment and Evaluation of the Locally-Based Sunflower Kc Values
4. Discussion
4.1. Performance of the WFSP Model
4.2. Sunflower’s Kc Values in Saline Soils
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demir, A.O.; Goksoy, A.T.; Buyukcangaz, H.; Turan, Z.M.; Koksal, E.S. Deficit irrigation of sunflower (Helianthus annuus L.) in a sub-humid climate. Irrig. Sci. 2006, 24, 279–289. [Google Scholar] [CrossRef]
- Shi, H.; Akae, A.; Kong, D.; Zhang, L.; Chen, Y.; Wei, Z.; Li, Y.; Zhang, Y. In study on sunflower response to soil water and salt stress in the Hetao area, China. In Water-Saving Agriculture and Sustainable use of Water and Land Resources; Shaanxi Science and Technology Press: Xi’an, China, 2003; pp. 111–116. [Google Scholar]
- Feng, Z.Z.; Wang, X.K.; Feng, Z.W. Soil n and salinity leaching after the autumn irrigation and its impact on groundwater in Hetao irrigation district, China. Agric. Water Manag. 2005, 71, 131–143. [Google Scholar] [CrossRef]
- Li, L.; Shi, H.; Wang, C.; Liu, H. Simulation of water and salt transport of uncultivated land in Hetao irrigation district in Inner Mongolia. Trans. Chin. Soc. Agric. Eng. 2010, 26, 31–35. [Google Scholar]
- Ertek, A.; Kara, B. Yield and quality of sweet corn under deficit irrigation. Agric. Water Manag. 2013, 129, 138–144. [Google Scholar] [CrossRef]
- Cakir, R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crop Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Dagdelen, N.; Yilmaz, E.; Sezgin, F.; Gurbuz, T. Water-yield relation and water use efficiency of cotton (Gossypium hirsutum L.) and second crop corn (Zea mays L.) in western Turkey. Agric. Water Manag. 2006, 82, 63–85. [Google Scholar] [CrossRef]
- Irmak, S.; Djaman, K.; Rudnick, D.R. Effect of full and limited irrigation amount and frequency on subsurface drip-irrigated maize evapotranspiration, yield, water use efficiency and yield response factors. Irrig. Sci. 2016, 34, 271–286. [Google Scholar] [CrossRef]
- Kiziloglu, F.M.; Sahin, U.; Kuslu, Y.; Tunc, T. Determining water-yield relationship, water use efficiency, crop and pan coefficients for silage maize in a semiarid region. Irrig. Sci. 2009, 27, 129–137. [Google Scholar] [CrossRef]
- Payero, J.O.; Melvin, S.R.; Irmak, S.; Tarkalson, D. Yield response of corn to deficit irrigation in a semiarid climate. Agric. Water Manag. 2006, 84, 101–112. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Xu, C.; Wu, J.W.; Huang, J.S. Soil salt leaching under different irrigation regimes: HYDRUS-1D modelling and analysis. J. Arid Land 2014, 6, 44–58. [Google Scholar] [CrossRef]
- Alcaras, L.M.A.; Rousseaux, M.C.; Searles, P.S. Responses of several soil and plant indicators to post-harvest regulated deficit irrigation in olive trees and their potential for irrigation scheduling. Agric. Water Manag. 2016, 171, 10–20. [Google Scholar] [CrossRef]
- Pereira, L.S.; Allen, R.G.; Smith, M.; Raes, D. Crop evapotranspiration estimation with FAO56: Past and future. Agric. Water Manag. 2015, 147, 4–20. [Google Scholar] [CrossRef]
- Vellidis, G.; Tucker, M.; Perry, C.; Wen, C.; Bednarz, C. A real-time wireless smart sensor array for scheduling irrigation. Comput. Electron. Agric. 2008, 61, 44–50. [Google Scholar] [CrossRef]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; p. D05109. [Google Scholar]
- Jensen, M.E. Water consumption by agricultural plants (Chapter 1). In Plant Water Consumption and Response. Water Deficits and Plant Growth; Academic Press: New York, NY, USA, 1968; Volume II, pp. 1–22. [Google Scholar]
- Howell, T.A.; Evett, S.R.; Tolk, J.A.; Copeland, K.S.; Marek, T.H. Evapotranspiration, water productivity and crop coefficients for irrigated sunflower in the US southern high plains. Agric. Water Manag. 2015, 162, 33–46. [Google Scholar] [CrossRef]
- Reddy, K.C.; Arunajyothy, S.; Mallikarjuna, P. Crop coefficients of some selected crops of Andhra Pradesh. J. Inst. Eng. (India): Ser. A 2015, 96, 123–130. [Google Scholar] [CrossRef]
- Tyagi, N.K.; Sharma, D.K.; Luthra, S.K. Determination of evapotranspiration and crop coefficients of rice and sunflower with lysimeter. Agric. Water Manag. 2000, 45, 41–54. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Xu, C.; Huang, J.S.; Wu, J.W.; Ma, T. Emergence rate, yield, and nitrogen-use efficiency of sunflowers (Helianthus annuus) vary with soil salinity and amount of nitrogen applied. Commun. Soil Sci. Plant Anal. 2015, 46, 1006–1023. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Xu, C.; Wu, J.W.; Huang, J.S.; Zhao, Q.; Wu, M.S. Impacts of salinity and nitrogen on the photosynthetic rate and growth of sunflowers (Helianthus annuus L.). Pedosphere 2014, 24, 635–644. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Wu, J.W.; Hoffmann, M.P.; Xu, C.; Ma, T.; Huang, J.S. Testing the apsim sunflower model on saline soils of Inner Mongolia, China. Field Crop Res. 2016, 192, 42–54. [Google Scholar] [CrossRef]
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722. [Google Scholar] [CrossRef]
- Du, B.; Qu, Z.; Yu, J.; Sun, G.; Shi, J. Experimental study on crop coefficient under mulched drip irrigation in Hetao irrigation district of Inner Mongolia. J. Irrig. Drain. 2014, 33, 16–20. [Google Scholar]
- Miller, W.P.; Miller, D.M. A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plant Anal. 1987, 18, 1–15. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2011; p. 496. [Google Scholar]
- Institute of Soil Science. Analysis of Soil Physico-Chemical Properties; Shanghai Scientific & Technical Publishers: Shanghai, China, 1978. [Google Scholar]
- Forrester, J.W. Industrial dynamics: A major breakthrough for decision makers. Harv. Bus. Rev. 1958, 37–66. [Google Scholar]
- Forrester, J.W. Industrial Dynamics; MIT Press: Cambridge, UK, 1961. [Google Scholar]
- Khan, S.; Luo, Y.F.; Ahmad, A. Analysing complex behaviour of hydrological systems through a system dynamics approach. Environ. Modell. Softw. 2009, 24, 1363–1372. [Google Scholar] [CrossRef]
- Miller, G.R.; Cable, J.M.; McDonald, A.K.; Bond, B.; Franz, T.E.; Wang, L.X.; Gou, S.; Tyler, A.P.; Zou, C.B.; Scott, R.L. Understanding ecohydrological connectivity in savannas: A system dynamics modelling approach. Ecohydrology 2012, 5, 200–220. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Chang, L.-C.; Ho, C.-C. Application of system dynamics with impact analysis to solve the problem of water shortages in Taiwan. Water Resour. Manag. 2008, 22, 1561–1577. [Google Scholar] [CrossRef]
- Eberlein, R.L.; Peterson, D.W. Understanding models with Vensim™. Eur. J. Oper. Res. 1992, 59, 216–219. [Google Scholar] [CrossRef]
- Systems, V. Vensim Reference Mannual; Ventana Systems: Harvard, MA, USA, 2007. [Google Scholar]
- Systems, V. Vensim Version 5 User’s Guide; Ventana Systems: Harvard, MA, USA, 2007. [Google Scholar]
- Igboekwe, M.U.; Adindu, R.U. Use of Kostiakov’s Infiltration Model on Michael Okpara University of Agriculture, Umudike Soils, Southeastern, Nigeria. J. Water Resour. Prot. 2014, 6, 888–894. [Google Scholar] [CrossRef]
- Vangenuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Vangenuchten, M.T.; Nielsen, D.R. On describing and predicting the hydraulic-properties of unsaturated soils. Ann. Geophys. 1985, 3, 615–627. [Google Scholar]
- Schaap, M.G.; Leij, F.J.; van Genuchten, M.T. ROSETTA: A computer program for estimating soil hydraulic parameters with hierarchical pedotransfer functions. J. Hydrol. 2001, 251, 163–176. [Google Scholar] [CrossRef]
- Liang, J.; Li, R.; Shi, H.; Li, Z.; Lu, X.; Bu, H. Effect of mulching on transfer and distribution of salinizated soil nutrient in Hetao irrigation district. Trans. Chin. Soc. Agric. Mach. 2016, 2, 113–121. [Google Scholar]
- Li, F. Experimental study of groudwater availability in relation to crops. Groundwater 1992, 197–202. [Google Scholar]
- Feng, G.; Liu, C. Analysis of root system growth in relation to soil water extraction pattern by winter wheat under water-limiting conditions. J. Nat. Resour. 1998, 3, 234–241. [Google Scholar]
- Chen, H.; Gao, Z.; Wang, S.; Hu, Y. Modeling on impacts of climate change and human activities variability on the shallow groundwater level using modflow. J. Hydraul. Eng. 2012, 43, 344–353. [Google Scholar]
- Gale, M.R.; Grigal, D.F. Vertical root distributions of northern tree species in relation to successional status. Can. J. For. Res. 1987, 17, 829–834. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of field water use and crop yield. Soil Sci. 1980, 129, 193. [Google Scholar]
- Feddes, R.A.; Kowalik, P.; Kolinska-Malinka, K.; Zaradny, H. Simulation of field water uptake by plants using a soil water dependent root extraction function. J. Hydrol. 1976, 31, 13–26. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X. Soil and Crop Science; China Water & Power Press: Beijing, China, 2012. [Google Scholar]
- Putnam, H. Trial and error predicates and the solution to a problem of Mostowski. J. Symb. Logic 1965, 30, 49–57. [Google Scholar] [CrossRef]
- Yuting, Y.; Songhao, S. Comparison of dual-source evapotranspiration models in estimating potential evaporation and transpiration. Trans. Chin. Soc. Agric. Eng. 2012, 28, 85–91. [Google Scholar]
- Šimůnek, J.; Van Genuchten, M.T.; Sejna, M. The Hydrus-1d Software Package for Simulating the Movement of Water, Heat, and Multiple Solutes in Variably Saturated Media, Version 3.0, Hydrus Software Series 1; Department of Environmental Sciences, University of California Riverside: Riverside, CA, USA, 2005. [Google Scholar]
- Zhang, Z.Y.; Wang, W.K.; Yeh, T.C.J.; Chen, L.; Wang, Z.F.; Duan, L.; An, K.D.; Gong, C.C. Finite analytic method based on mixed-form richards’ equation for simulating water flow in vadose zone. J. Hydrol. 2016, 537, 146–156. [Google Scholar] [CrossRef]
- Zha, Y.Y.; Shi, L.S.; Ye, M.; Yang, J.Z. A generalized ross method for two- and three-dimensional variably saturated flow. Adv. Water Resour. 2013, 54, 67–77. [Google Scholar] [CrossRef]
- Zha, Y.Y.; Yang, J.Z.; Shi, L.S.; Song, X.H. Simulating one-dimensional unsaturated flow in heterogeneous soils with water content-based richards equation. Vadose Zone J. 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Ren, D.; Xu, X.; Hao, Y.; Huang, G. Modeling and assessing field irrigation water use in a canal system of Hetao, upper Yellow River basin: Application to maize, sunflower and watermelon. J. Hydrol. 2016, 532, 122–139. [Google Scholar] [CrossRef]
- He, X.; Yang, P.L.; Ren, S.M.; Li, Y.K.; Jiang, G.Y.; Li, L.H. Quantitative response of oil sunflower yield to evapotranspiration and soil salinity with saline water irrigation. Int. J. Agric. Biol. Eng. 2016, 9, 63–73. [Google Scholar]
- Sun, M.; Zhang, X.; Huo, Z.; Feng, S.; Huang, G.; Mao, X. Uncertainty and sensitivity assessments of an agricultural–hydrological model (RZWQM2) using the GLUE method. J. Hydrol. 2016, 534, 19–30. [Google Scholar] [CrossRef]
- Wiekenkamp, I.; Huisman, J.A.; Bogena, H.R.; Lin, H.S.; Vereecken, H. Spatial and temporal occurrence of preferential flow in a forested headwater catchment. J. Hydrol. 2016, 534, 139–149. [Google Scholar] [CrossRef]
- Dai, J.; Shi, H.; Tian, D.; Xia, Y.; Li, M. Determined of crop coefficients of main grain and oil crops in Inner Mongolia Hetao irrigated area. J. Irrig. Drain. 2011, 23–27. [Google Scholar]
- Homaee, M.; Dirksen, C.; Feddes, R.A. Simulation of root water uptake: I. Non-uniform transient salinity using different macroscopic reduction functions. Agric. Water Manag. 2002, 57, 89–109. [Google Scholar] [CrossRef]
- Skaggs, T.H.; van Genuchten, M.T.; Shouse, P.J.; Poss, J.A. Macroscopic approaches to root water uptake as a function of water and salinity stress. Agric. Water Manag. 2006, 86, 140–149. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity mineral nutrient relations in horticultural crops. Sci. Hortic-Amst. 1999, 78, 127–157. [Google Scholar] [CrossRef]
- Bernstein, L.; Hayward, H. Physiology of salt tolerance. Annu. Rev. Plant Physiol. 1958, 9, 25–46. [Google Scholar] [CrossRef]
- Katerji, N.; van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Salt tolerance classification of crops according to soil salinity and to water stress day index. Agric. Water Manag. 2000, 43, 99–109. [Google Scholar] [CrossRef]
- Zeng, W.Z. Research and Simulation for the Coupling Effects of Water, Nitrogen, and Salt on Sunflower; Wuhan University: Wuhan, China, 2015. [Google Scholar]
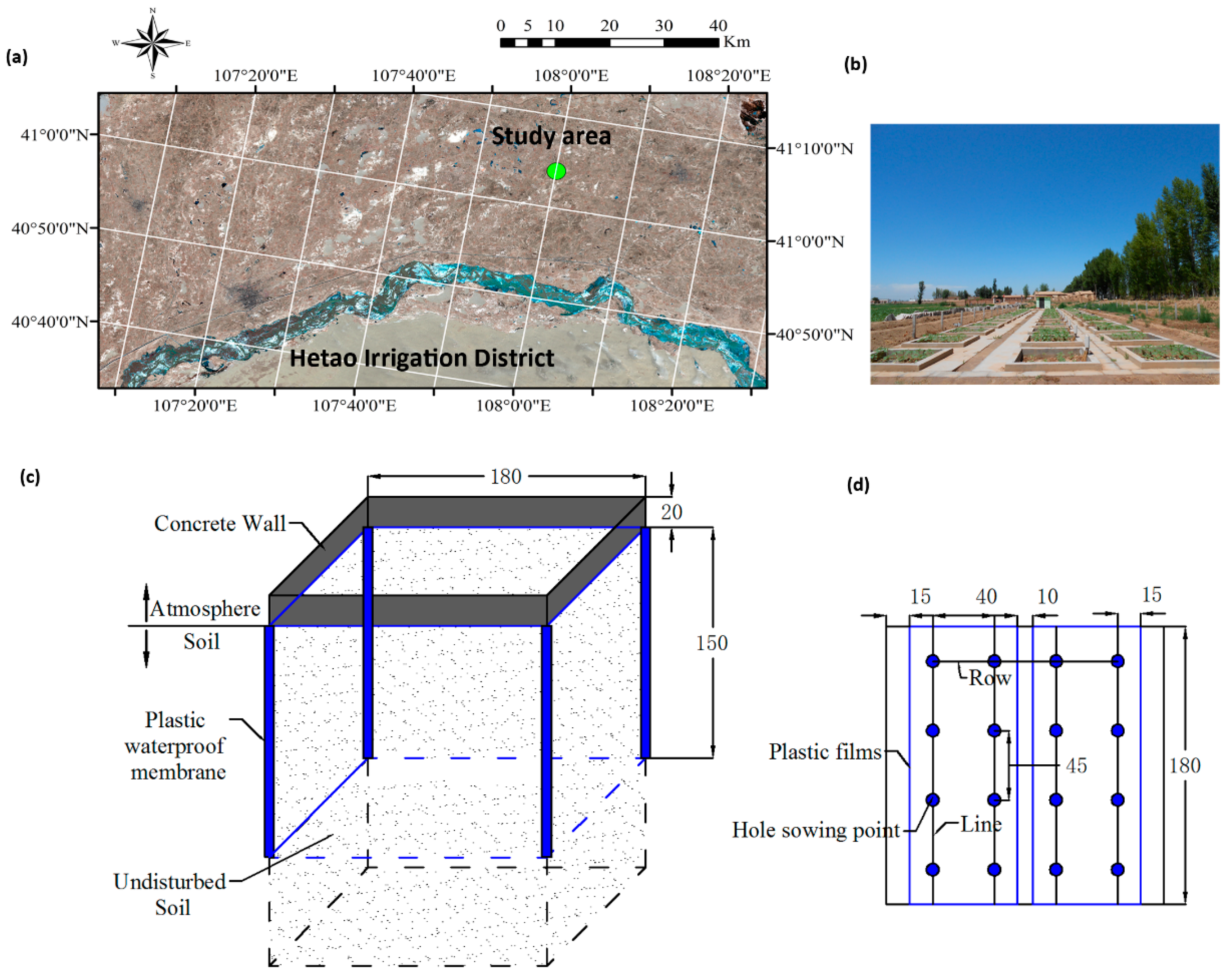
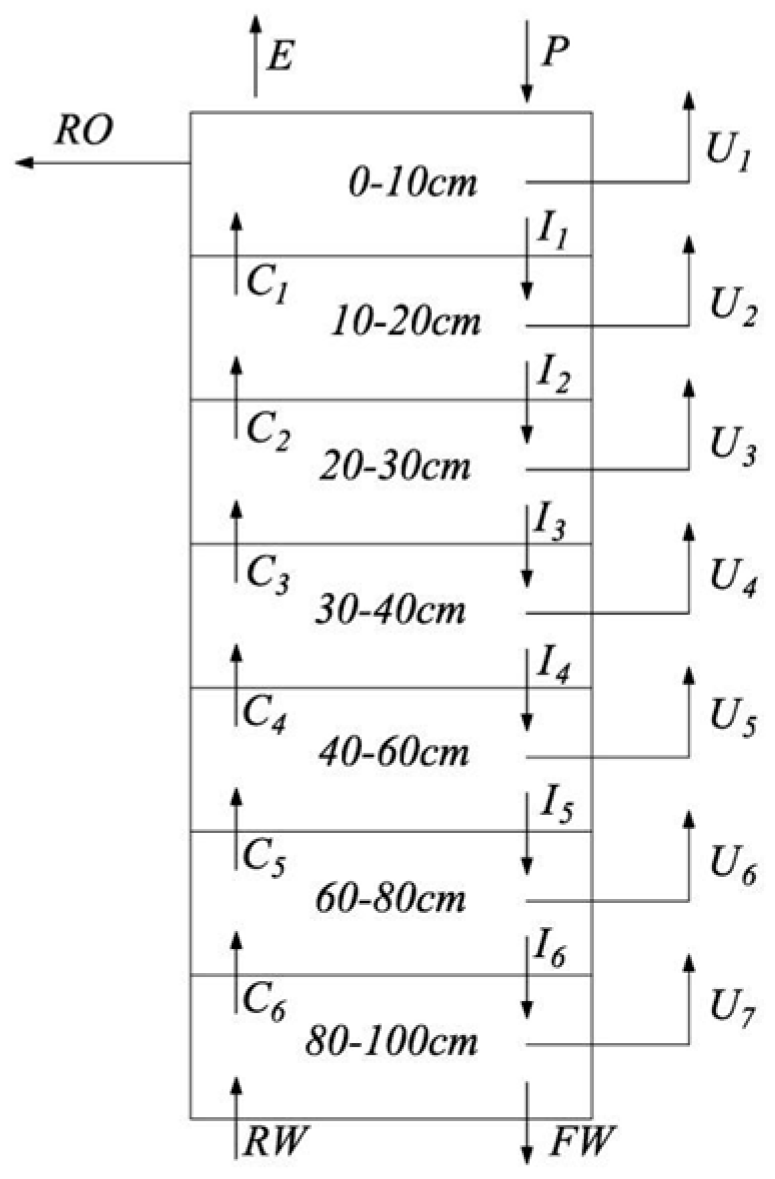
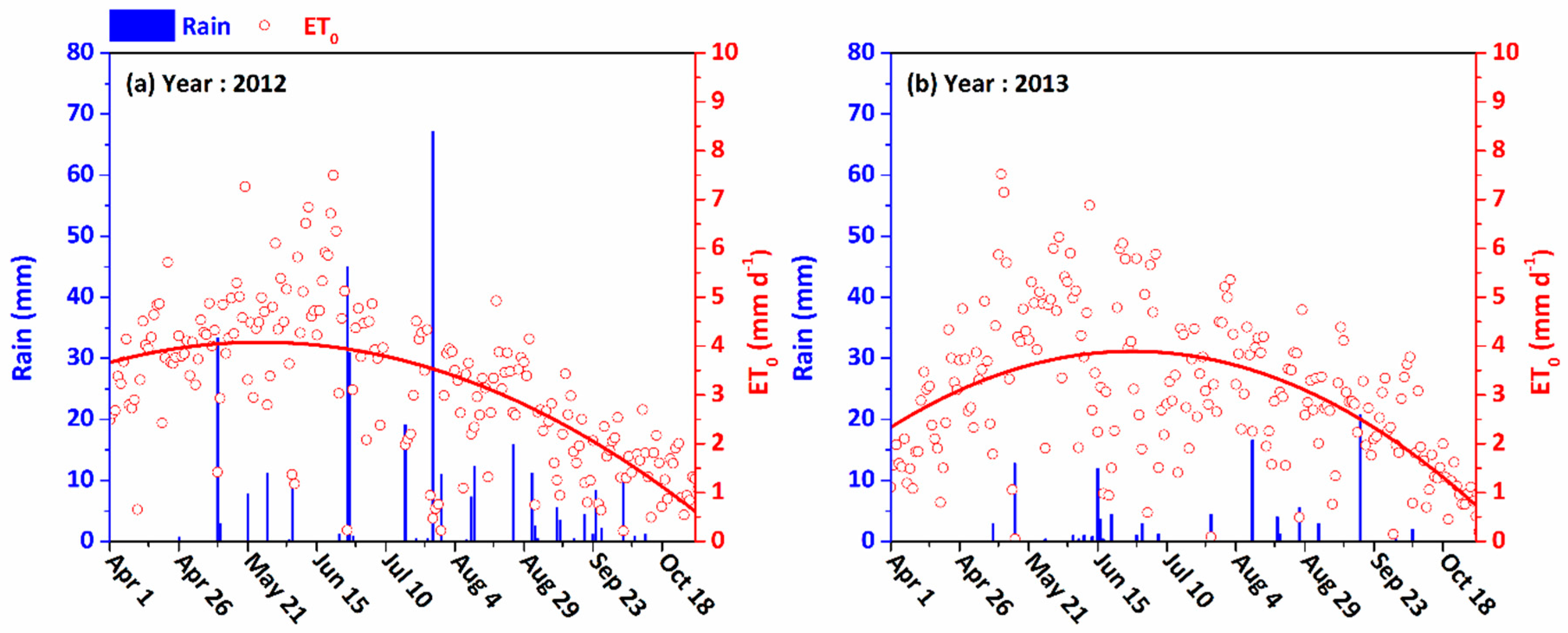

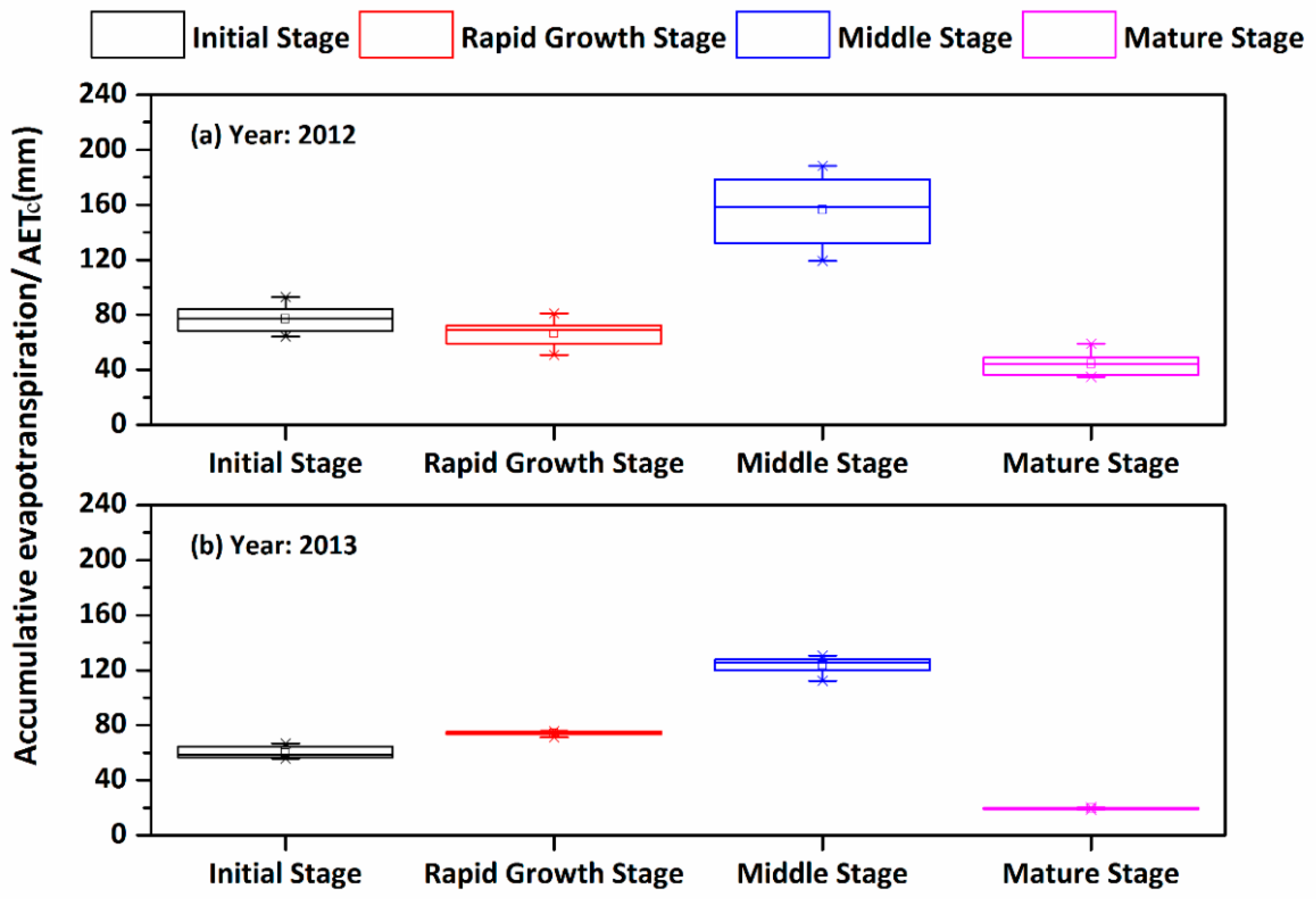

| Depth (cm) | Soil Particle Size Distribution/Mean ± S.D.* (%) | Bulk Density (g/cm3) | ||
|---|---|---|---|---|
| Sand (0.05–2.0 mm) | Silt (0.002–0.05 mm) | Clay (< 0.002 mm) | ||
| 0–10 | 12.58 ± 4.30 | 54.94 ± 12.13 | 32.48 ± 13.86 | 1.35 |
| 10–20 | 11.79 ± 3.97 | 52.81 ± 13.53 | 35.40 ± 14.67 | 1.35 |
| 20–30 | 12.02 ± 4.24 | 52.32 ± 12.43 | 35.66 ± 14.05 | 1.35 |
| 30–40 | 12.27 ± 6.86 | 53.77 ± 12.10 | 33.96 ± 15.49 | 1.44 |
| 40–60 | 8.91 ± 12.96 | 69.77 ± 11.41 | 21.32 ± 12.29 | 1.48 |
| 60–80 | 12.48 ± 13.91 | 70.57 ± 11.80 | 16.95 ± 10.61 | 1.51 |
| 80–100 | 16.69 ± 16.69 | 69.59 ± 13.28 | 13.72 ± 6.17 | 1.51 |
| Depth | θr | θs | α | n | Ks |
|---|---|---|---|---|---|
| cm3·cm−3 | cm3·cm−3 | 1/cm | - | mm/d | |
| 0–10 cm | 0.0883 | 0.4691 | 0.0082 | 1.5057 | 121.9 |
| 10–20 cm | 0.0919 | 0.4774 | 0.0093 | 1.4737 | 125.4 |
| 20–30 cm | 0.0921 | 0.4776 | 0.0094 | 1.4702 | 125.9 |
| 30–40 cm | 0.0901 | 0.4733 | 0.0087 | 1.4893 | 123.5 |
| 40–60 cm | 0.0763 | 0.4540 | 0.0058 | 1.6156 | 131.2 |
| 60–80 cm | 0.0687 | 0.4477 | 0.0051 | 1.6582 | 176.0 |
| 80–100 cm | 0.0616 | 0.4431 | 0.0046 | 1.6903 | 235.6 |
| Parameter | Units | Range | Value | Description | Reference |
|---|---|---|---|---|---|
| θwilt | cm3·cm−3 | 0.06–0.10 | 0.10 | Wilting point | [49] |
| θfc | cm3·cm−3 | 0.32–0.42 | 0.39 | Field capacity | [49] |
| θstress | cm3·cm−3 | (0.45–0.9)·θfc | 0.6·θfc | Stress point | [32] |
| Item | Initial Stage | Rapid Growth Stage | Middle Stage | Mature Stage | R2 | Location | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 0.52 | 1.1 | 1.32 | 0.41 | 0.55 | Karnal, India (29°43′ N, 76°58′ E) | [20] |
| 2 | 0.697 | 0.751 | 0.804 | 0.35 | 0.55 | Dengkou County, Hetao (40°24′ N, 107°02′ E) | [60] |
| 3 | 0.194 | 0.779 | 1.26 | 0.315 | 0.67 | Linhe County, Hetao (40°43′ N, 76°58′ E) | [25] |
| 4 | 0.14 | — | 1.08 | 0.24 | 0.66 | Yangchang, Hetao (40°48′ N, 107°05′ E) | [56] |
| 5 | 0.35 | — | 1–1.15 | 0.35 | 0.64 | California, US | [16] |
| 6 | Equation (20) | 0.659 | 1.156 | 0.324 | 0.7 | Wuyuan County, Hetao (41°04′ N, 108°00′ E) | WFSP model |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Zeng, W.; Ma, T.; Lei, G.; Zha, Y.; Fang, Y.; Wu, J.; Huang, J. Determination of Growth Stage-Specific Crop Coefficients (Kc) of Sunflowers (Helianthus annuus L.) under Salt Stress. Water 2017, 9, 215. https://doi.org/10.3390/w9030215
Hong M, Zeng W, Ma T, Lei G, Zha Y, Fang Y, Wu J, Huang J. Determination of Growth Stage-Specific Crop Coefficients (Kc) of Sunflowers (Helianthus annuus L.) under Salt Stress. Water. 2017; 9(3):215. https://doi.org/10.3390/w9030215
Chicago/Turabian StyleHong, Minghai, Wenzhi Zeng, Tao Ma, Guoqing Lei, Yuanyuan Zha, Yuanhao Fang, Jingwei Wu, and Jiesheng Huang. 2017. "Determination of Growth Stage-Specific Crop Coefficients (Kc) of Sunflowers (Helianthus annuus L.) under Salt Stress" Water 9, no. 3: 215. https://doi.org/10.3390/w9030215







