Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes
Abstract
:1. Introduction
2. Materials and Methods
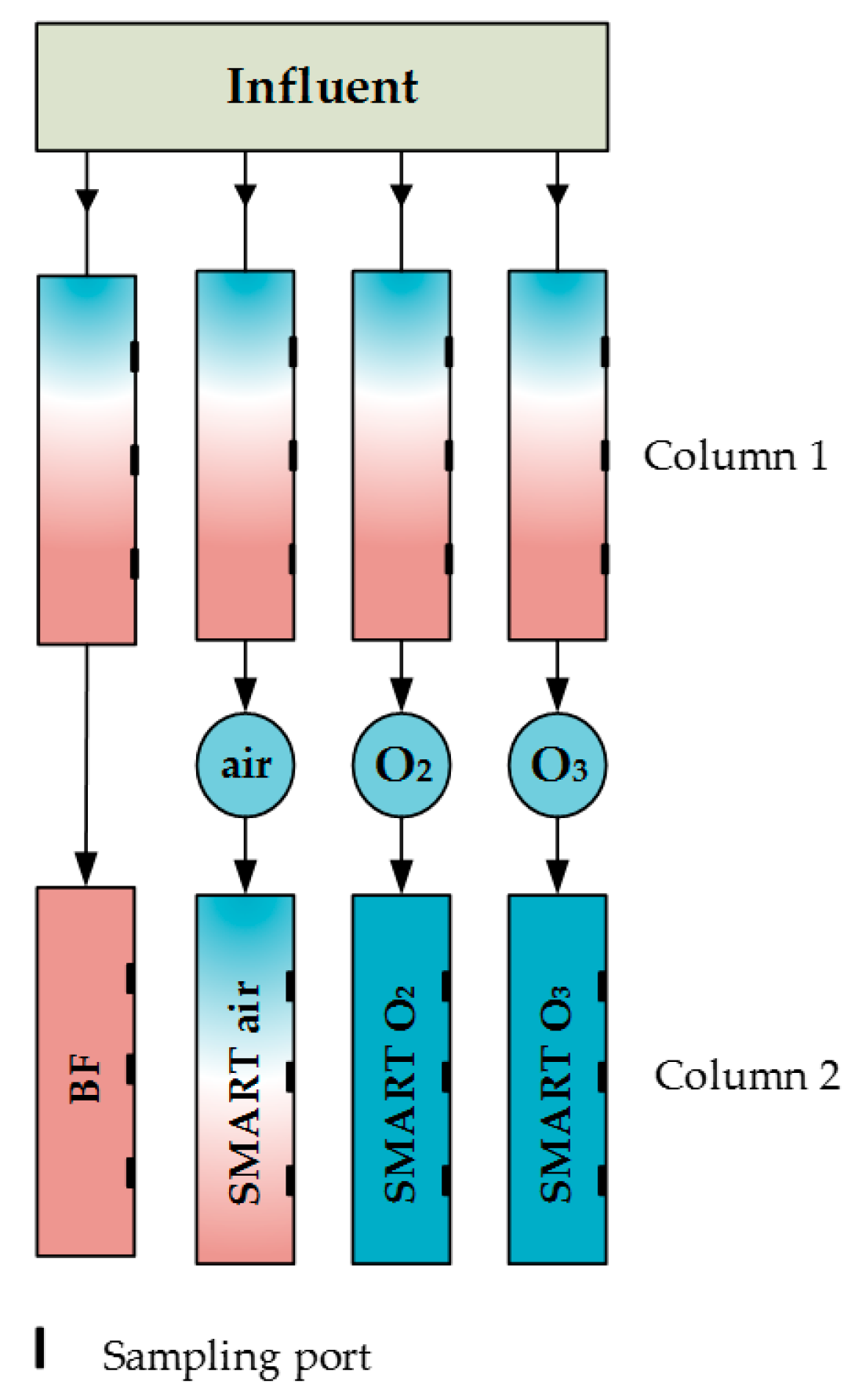
2.1. Soil Column Experiments
2.2. Aeration/Oxidation
2.3. Analytical Methods
3. Results and Discussion
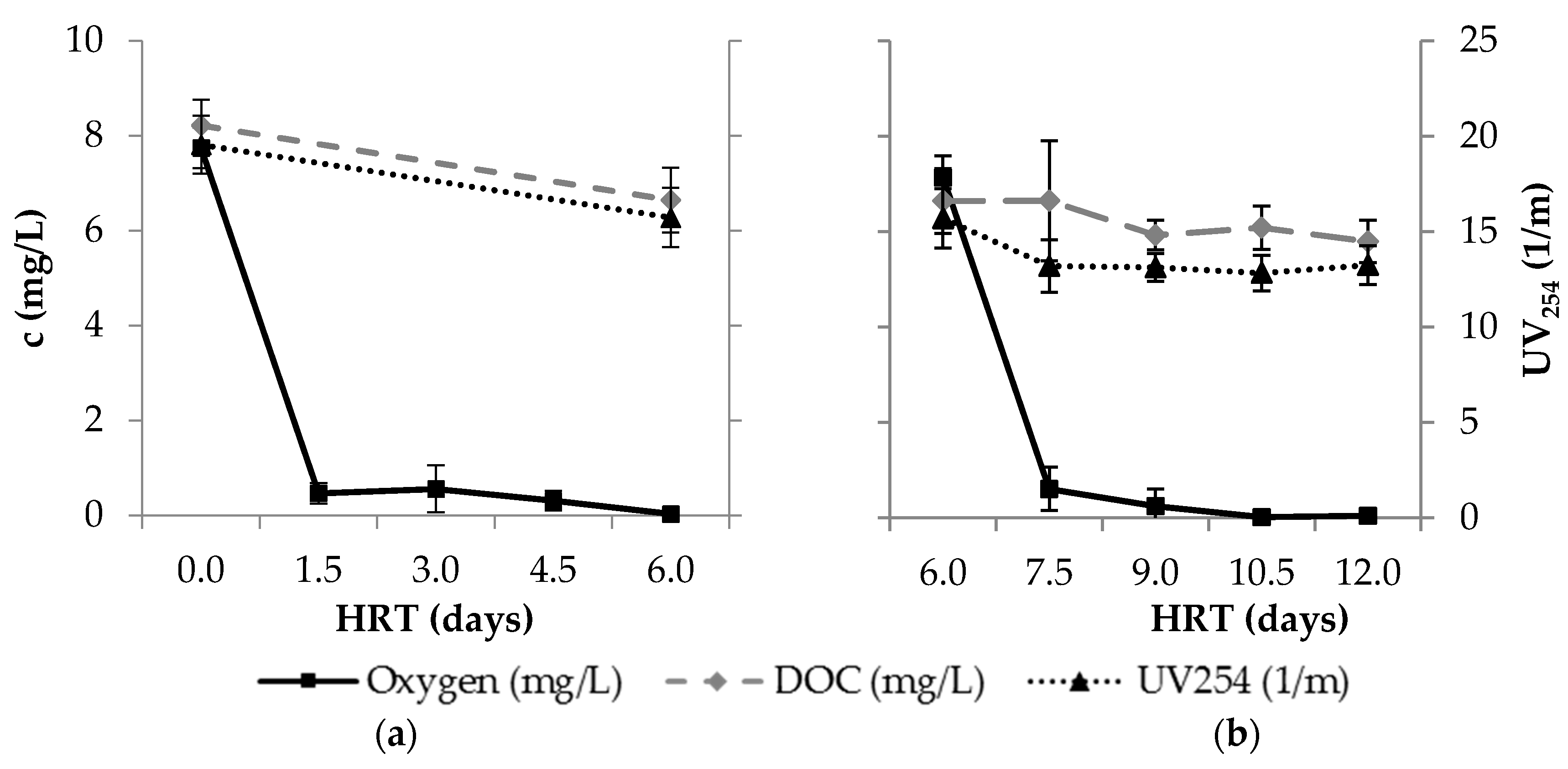
3.1. Redox Conditions
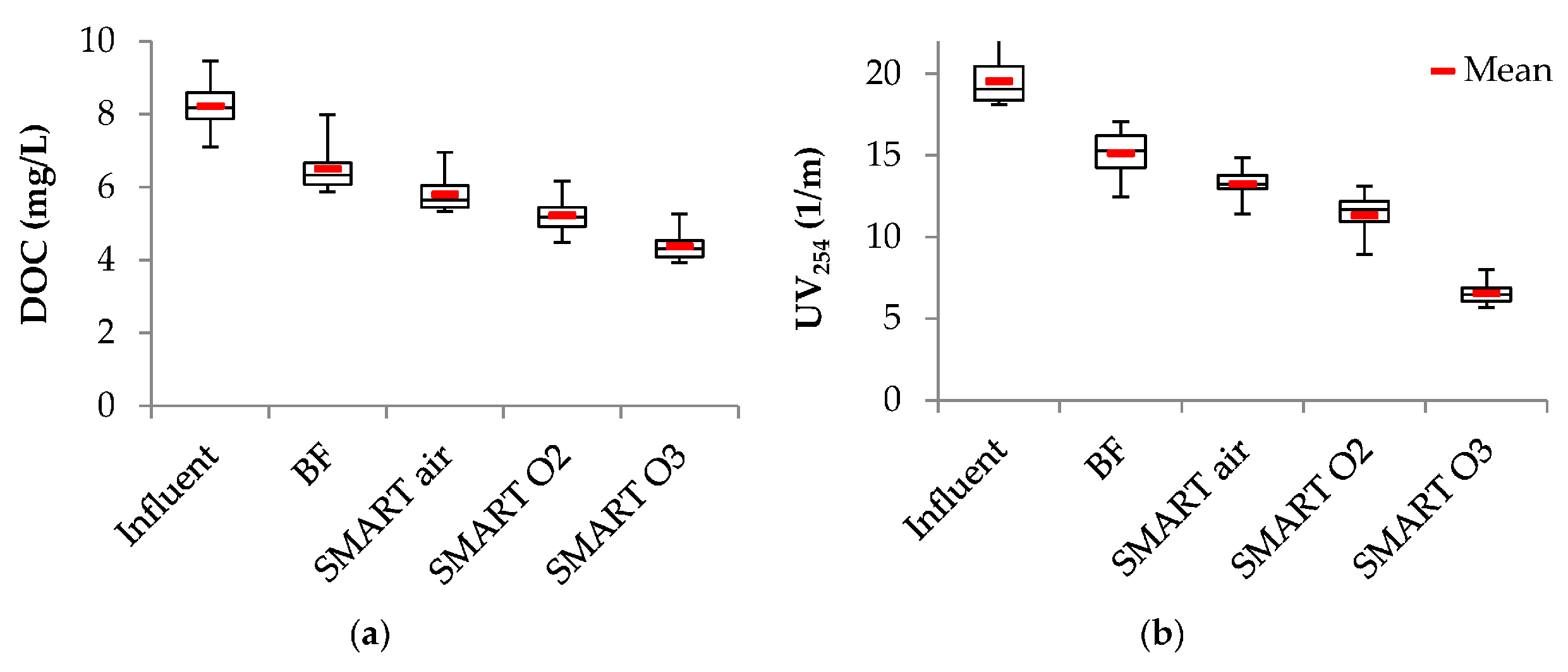
3.2. Removal of Bulk Organic Parameters
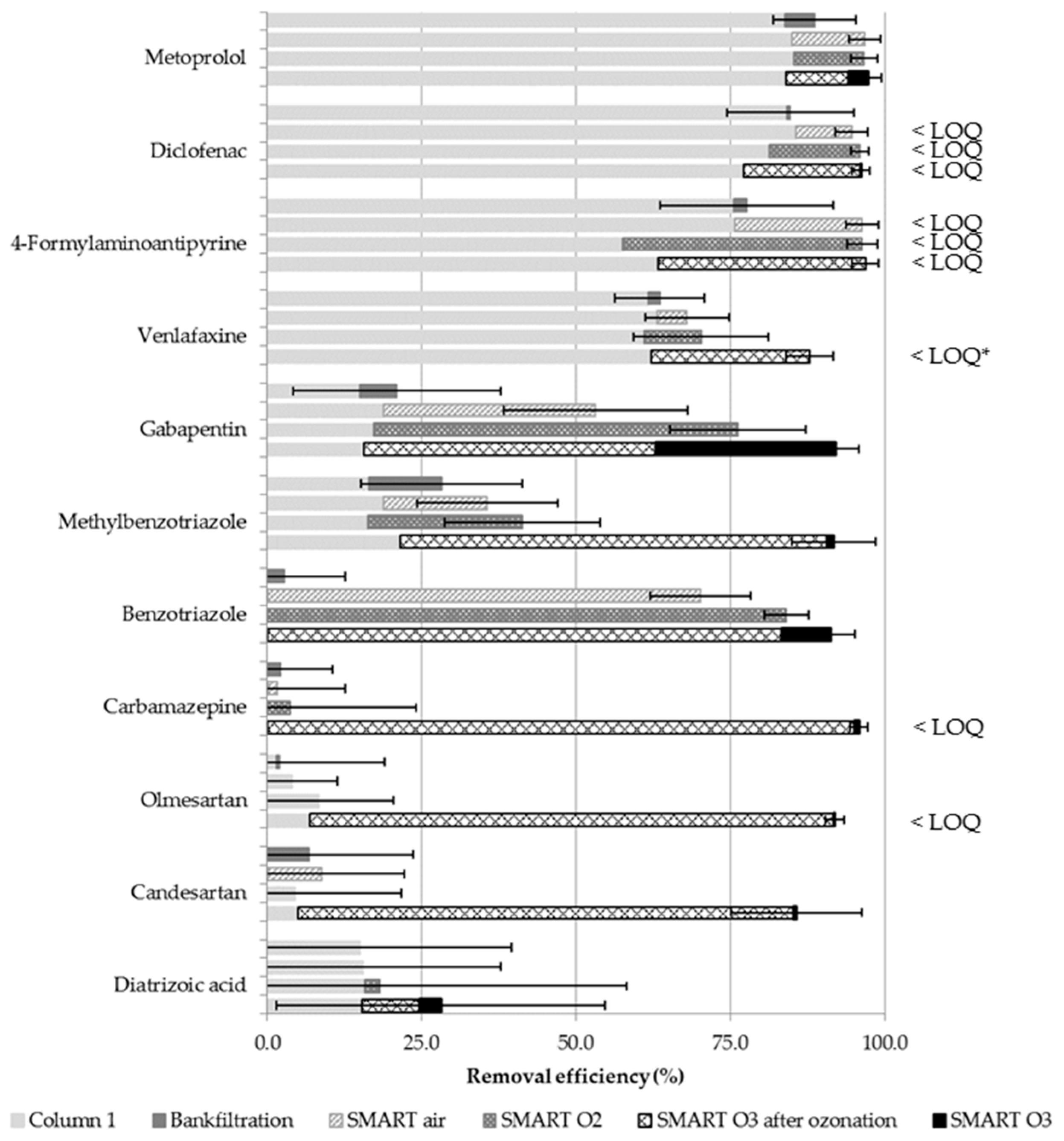
3.3. Transformation of Trace Organic Chemicals as a Function of Different Oxidation Techniques
3.3.1. Consistency and Reproducibility of Observed Degradation
3.3.2. Compound Removal during Anoxic Infiltration
3.3.3. TOrC Removal during SMART
3.3.4. Application of Ozone for Intermediate Aeration and Oxidation
3.3.5. Formation and Fate of the Transformation Product Valsartan Acid
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Cahill, J.D.; Zaugg, S.D.; Werner, S.L.; Meyer, M.T.; Kryak, D.D. Transport of Chemical and Microbial Compounds from Known Wastewater Discharges: Potential for Use as Indicators of Human Fecal Contamination. Environ. Sci. Technol. 2005, 39, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Missimer, T.M.; Drewes, J.E.; Maliva, R.G.; Amy, G. Aquifer Recharge and Recovery: Groundwater Recharge Systems for Treatment, Storage, and Water Reclamation. Ground Water 2011, 49, 771. [Google Scholar] [CrossRef] [PubMed]
- Amy, G.; Drewes, J.E. Soil Aquifer Treatment (SAT) as a Natural and Sustainable Wastewater Reclamation/Reuse Technology: Fate of Wastewater Effluent Organic Matter (EfOM) and Trace Organic Compounds. Environ. Monit. Assess. 2007, 129, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tufenkji, N.; Ryan, J.N.; Elimelech, M. The Promise of Bank Filtration. Environ. Sci. Technol. 2002, 36, 422A–428A. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Jones, C.; Oldham, G.; Drewes, J.E. Attenuation of total organic carbon and unregulated trace organic chemicals in U.S. riverbank filtration systems. Water Res. 2010, 44, 4643–4659. [Google Scholar] [CrossRef] [PubMed]
- Alidina, M.; Li, D.; Drewes, J.E. Investigating the role for adaptation of the microbial community to transform trace organic chemicals during managed aquifer recharge. Water Res. 2014, 56, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Massmann, G.; Dünnbier, U.; Heberer, T.; Taute, T. Behaviour and redox sensitivity of pharmaceutical residues during bank filtration—Investigation of residues of phenazone-type analgesics. Chemosphere 2008, 71, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Grünheid, S.; Amy, G.; Jekel, M. Removal of bulk dissolved organic carbon (DOC) and trace organic compounds by bank filtration and artificial recharge. Water Res. 2005, 39, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Alidina, M.; Ouf, M.; Sharp, J.O.; Saikaly, P.; Drewes, J.E. Microbial community evolution during simulated managed aquifer recharge in response to different biodegradable dissolved organic carbon (BDOC) concentrations. Water Res. 2013, 47, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Jones, C.; Dickenson, E.R.V.; Drewes, J.E. The role of microbial adaptation and biodegradable dissolved organic carbon on the attenuation of trace organic chemicals during groundwater recharge. Sci. Total Environ. 2012, 437, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Rauch-Williams, T.; Hoppe-Jones, C.; Drewes, J.E. The role of organic matter in the removal of emerging trace organic chemicals during managed aquifer recharge. Water Res. 2010, 44, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Regnery, J.; Barringer, J.; Wing, A.D.; Hoppe-Jones, C.; Teerlink, J.; Drewes, J.E. Start-up performance of a full-scale riverbank filtration site regarding removal of DOC, nutrients, and trace organic chemicals. Chemosphere 2015, 127, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Regnery, J.; Wing, A.D.; Kautz, J.; Drewes, J.E. Introducing sequential managed aquifer recharge technology (SMART)—From laboratory to full-scale application. Chemosphere 2016, 154, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.M.; Göbel, A.; Joss, A.; Hermann, N.; Löffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef] [PubMed]
- Kozyatnyk, I.; Swietlik, J.; Raczyk-Stanislawiak, U.; Dabrowska, A.; Klymenko, N.; Nawrocki, J. Influence of oxidation on fulvic acids composition and biodegradability. Chemosphere 2013, 92, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Salhi, E.; Köster, O.; Kaiser, H.-P.; Egli, T.; von Gunten, U. Mechanistic and kinetic evaluation of organic disinfection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res. 2006, 40, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Hübner, U.; Kuhnt, S.; Jekel, M.; Drewes, J.E. Fate of bulk organic carbon and bromate during indirect water reuse involving ozone and subsequent aquifer recharge. J. Water Reuse Desalin. 2016, 6, 413–420. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Worch, E.; Altmann, J.; Ruhl, A.S.; Sperlich, A.; Meinel, F.; Jekel, M. Impact of EfOM size on competition in activated carbon adsorption of organic micro-pollutants from treated wastewater. Water Res. 2014, 65, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Massmann, G.; Nogeitzig, A.; Taute, T.; Pekdeger, A. Seasonal and spatial distribution of redox zones during lake bank filtration in Berlin, Germany. Environ. Geol. 2008, 54, 53–65. [Google Scholar] [CrossRef]
- Regnery, J.; Wing, A.; Alidina, M.; Drewes, J.E. Biotransformation of trace organic chemicals during groundwater recharge: How useful are first-order rate constants? J. Contam. Hydrol. 2015, 179, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, B.; Jährig, J.; Reemtsma, T.; Jekel, M. Long term laboratory column experiments to simulate bank filtration: Factors controlling removal of sulfamethoxazole. Water Res. 2011, 45, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Polesel, F.; Andersen, H.R.; Trapp, S.; Plosz, B.G. Removal of Antibiotics in Biological Wastewater Treatment Systems—A Critical Assessment Using the Activated Sludge Modeling Framework for Xenobiotics (ASM-X). Environ. Sci. Technol. 2016, 50, 10316–10334. [Google Scholar] [CrossRef] [PubMed]
- Wiese, B.; Massmann, G.; Jekel, M.; Heberer, T.; Dünnbier, U.; Orlikowski, D.; Grützmacher, G. Removal kinetics of organic compounds and sum parameters under field conditions for managed aquifer recharge. Water Res. 2011, 45, 4939–4950. [Google Scholar] [CrossRef] [PubMed]
- Kormos, J.L.; Schulz, M.; Ternes, T.A. Occurrence of iodinated X-ray contrast media and their biotransformation products in the urban water cycle. Environ. Sci. Technol. 2011, 45, 8723–8732. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Buser, H.-R.; Kahle, M.; Müller, M.D.; Poiger, T. Ubiquitous Occurrence of the Artificial Sweetener Acesulfame in the Aquatic Environment: An Ideal Chemical Marker of Domestic Wastewater in Groundwater. Environ. Sci. Technol. 2009, 43, 4381–4385. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Storck, F.R.; Graf, C.; Brauch, H.-J.; Ruck, W.; Lev, O.; Lange, F.T. Correlation of six anthropogenic markers in wastewater, surface water, bank filtrate, and soil aquifer treatment. J. Environ. Monit. 2011, 13, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.; Zwiener, C.; Zemann, M. Tracking artificial sweeteners and pharmaceuticals introduced into urban groundwater by leaking sewer networks. Sci. Total Environ. 2012, 430, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Mamane, H.; Cikurel, H.; Jekel, M.; Hübner, U.; Avisar, D. A hybrid process of biofiltration of secondary effluent followed by ozonation and short soil aquifer treatment for water reuse. Water Res. 2015, 84, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Nödler, K.; Tsakiri, M.; Licha, T. The impact of different proportions of a treated effluent on the biotransformation of selected micro-contaminants in river water microcosms. Int. J. Environ. Res. Public Health 2014, 11, 10390–10405. [Google Scholar] [CrossRef] [PubMed]
- Nödler, K.; Tsakiri, M.; Aloupi, M.; Gatidou, G.; Stasinakis, A.S.; Licha, T. Evaluation of polar organic micropollutants as indicators for wastewater-related coastal water quality impairment. Environ. Pollut. 2016, 211, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, G.D.; Roseth, R.; Sparrevik, M.; Hartnig, T.; Hem, L.J. Persistence of the De-Icing Additive Benzotriazole at an Abandoned Airport. Water Air Soil Pollut. Focus 2003, 3, 91–101. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Biodegradation of three selected benzotriazoles under aerobic and anaerobic conditions. Water Res. 2011, 45, 5005–5014. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.D.; Patterson, B.M.; McKinley, A.J.; Reeder, A.Y.; Furness, A.J.; Donn, M.J. Fate of benzotriazole and 5-methylbenzotriazole in recycled water recharged into an anaerobic aquifer: Column studies. Water Res. 2015, 70, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Escher, B.I.; Richle, P.; Schaffner, C.; Alder, A.C. Elimination of beta-blockers in sewage treatment plants. Water Res. 2007, 41, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.K.; Sharma, S.K.; Lekkerkerker-Teunissen, K.; Amy, G.L. Occurrence and fate of bulk organic matter and pharmaceutically active compounds in managed aquifer recharge: A review. Water Res. 2011, 45, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Nödler, K.; Hillebrand, O.; Idzik, K.; Strathmann, M.; Schiperski, F.; Zirlewagen, J.; Licha, T. Occurrence and fate of the angiotensin II receptor antagonist transformation product valsartan acid in the water cycle—A comparative study with selected beta-blockers and the persistent anthropogenic wastewater indicators carbamazepine and acesulfame. Water Res. 2013, 47, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Hübner, U.; Miehe, U.; Jekel, M. Optimized removal of dissolved organic carbon and trace organic contaminants during combined ozonation and artificial groundwater recharge. Water Res. 2012, 46, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Onesios, K.M.; Bouwer, E.J. Biological removal of pharmaceuticals and personal care products during laboratory soil aquifer treatment simulation with different primary substrate concentrations. Water Res. 2012, 46, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Lester, Y.; Mamane, H.; Zucker, I.; Avisar, D. Treating wastewater from a pharmaceutical formulation facility by biological process and ozone. Water Res. 2013, 47, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Iobbi-Nivol, C.; Pommier, J.; Simala-Grant, J.; Méjean, V.; Giordano, G. High substrate specificity and induction characteristics of trimethylamine-N-oxide reductase of Escherichia coli. Biochim. et Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1996, 1294, 77–82. [Google Scholar] [CrossRef]
- Maeda, S.; Uchida, S.; Kisaki, T. Microbial Degradation of Nicotine-N′-oxide I Degradation Products. Agric. Biol. Chem. 2014, 42, 1455–1460. [Google Scholar]
- Buffle, M.-O.; von Gunten, U. Phenols and Amine Induced HO· Generation during the Initial Phase of Natural Water Ozonation. Environ. Sci. Technol. 2006, 40, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Real, F.J.; Benitez, F.J.; Acero, J.L.; Sagasti, J.J.P.; Casas, F. Kinetics of the Chemical Oxidation of the Pharmaceuticals Primidone, Ketoprofen, and Diatrizoate in Ultrapure and Natural Waters. Ind. Eng. Chem. Res. 2009, 48, 3380–3388. [Google Scholar] [CrossRef]
- Hübner, U.; von Gunten, U.; Jekel, M. Evaluation of the persistence of transformation products from ozonation of trace organic compounds—A critical review. Water Res. 2015, 68, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Letzel, T.; Bayer, A.; Schulz, W.; Heermann, A.; Lucke, T.; Greco, G.; Grosse, S.; Schüssler, W.; Sengl, M.; Letzel, M. LC-MS screening techniques for wastewater analysis and analytical data handling strategies: Sartans and their transformation products as an example. Chemosphere 2015, 137, 198–206. [Google Scholar] [CrossRef] [PubMed]
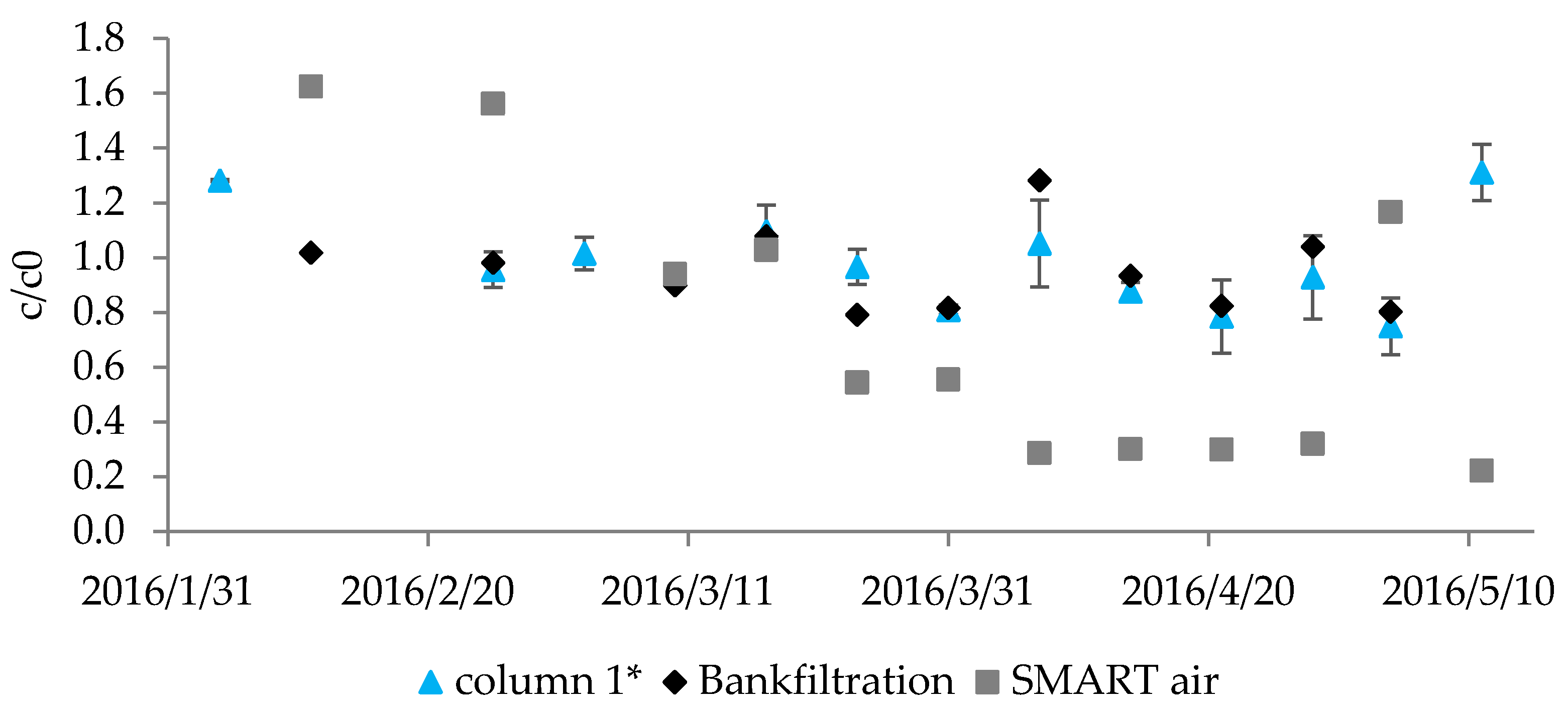
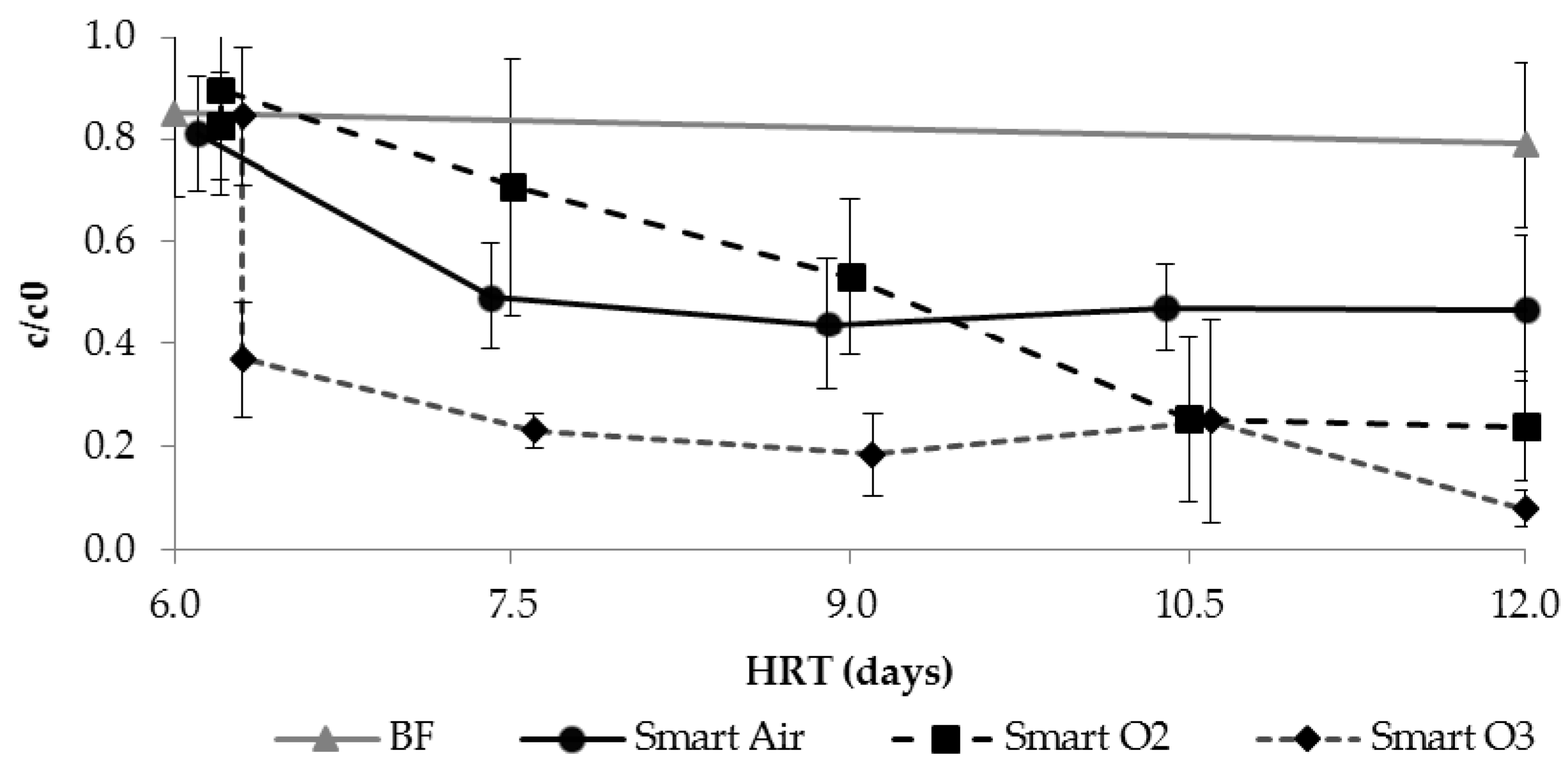
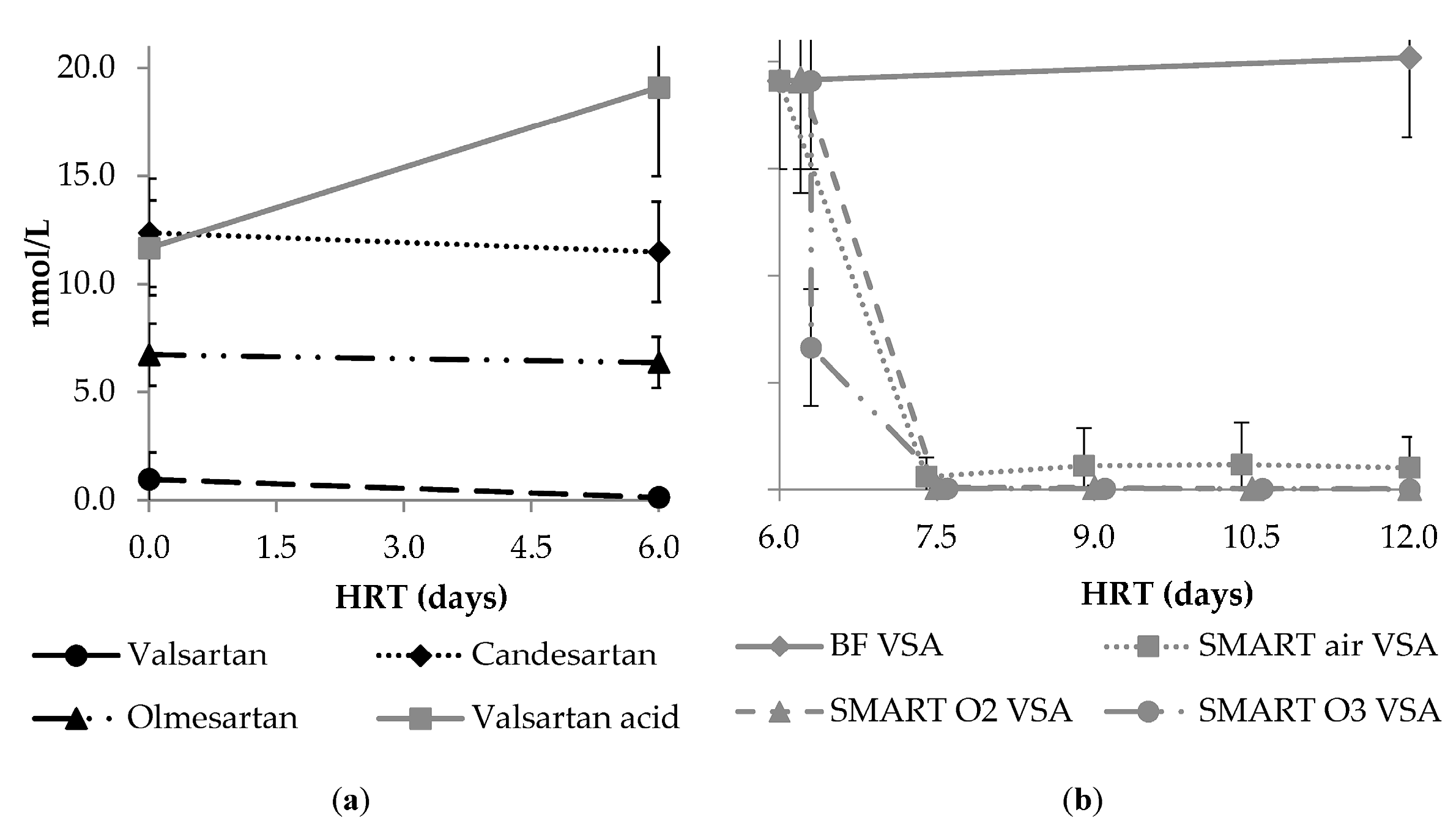
| Para- Meter | Influent n ≥ 17 | Column 1 n ≥ 64 | After Aeration n ≥ 16 | BF n ≥ 15 | Smart Air n ≥ 15 | Smart O2 n ≥ 14 | Smart O3 n ≥ 15 | |
|---|---|---|---|---|---|---|---|---|
| Air | O2 and O3 | |||||||
| DOC (mg/L) | 8.2 ± 0.6 | 6.6 ± 0.7 | - | 6.3 ± 0.5 * | 6.5 ± 0.6 | 5.8 ± 0.4 | 5.2 ± 0.5 | 4.4 ± 0.4 |
| O2 (mg/L) | 7.74 ± 0.43 | 0.02 ± 0.09 | 7.14 ± 0.45 | 22.01 ± 5.76 | 0.03 ± 0.08 | 0.04 ± 0.10 | 14.32 ± 4.50 | 16.12 ± 2.08 |
| NO3-N (mg/L) | 4.8 ± 0.8 | 3.8 ± 0.7 | - | 3.8 ± 0.7 | 3.5 ± 0.8 | 3.7 ± 0.7 | 3.9 ± 0.6 | 4.0 ± 0.7 |
| NO2-N (mg/L) | 0.0 ± 0.0 | 0.3 ± 0.1 | - | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Fe (mg/L) | 0.2 ± 0.1 | 0.1 ± 0.1 | - | - | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Mn (mg/L) | 0.1 ± 0.1 | 1.1 ± 0.4 | - | - | 0.6 ± 0.2 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Prevailing Redox Conditions | anoxic | anoxic | anoxic–suboxic | oxic | oxic | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellauer, K.; Mergel, D.; Ruhl, A.S.; Filter, J.; Hübner, U.; Jekel, M.; Drewes, J.E. Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes. Water 2017, 9, 221. https://doi.org/10.3390/w9030221
Hellauer K, Mergel D, Ruhl AS, Filter J, Hübner U, Jekel M, Drewes JE. Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes. Water. 2017; 9(3):221. https://doi.org/10.3390/w9030221
Chicago/Turabian StyleHellauer, Karin, Dorothea Mergel, Aki S. Ruhl, Josefine Filter, Uwe Hübner, Martin Jekel, and Jörg E. Drewes. 2017. "Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes" Water 9, no. 3: 221. https://doi.org/10.3390/w9030221
APA StyleHellauer, K., Mergel, D., Ruhl, A. S., Filter, J., Hübner, U., Jekel, M., & Drewes, J. E. (2017). Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes. Water, 9(3), 221. https://doi.org/10.3390/w9030221






