Effects of Electrical Stimulation on the Degradation of Azo Dye in Three-Dimensional Biofilm Electrode Reactors
Abstract
:1. Introduction
2. Materials and Methods
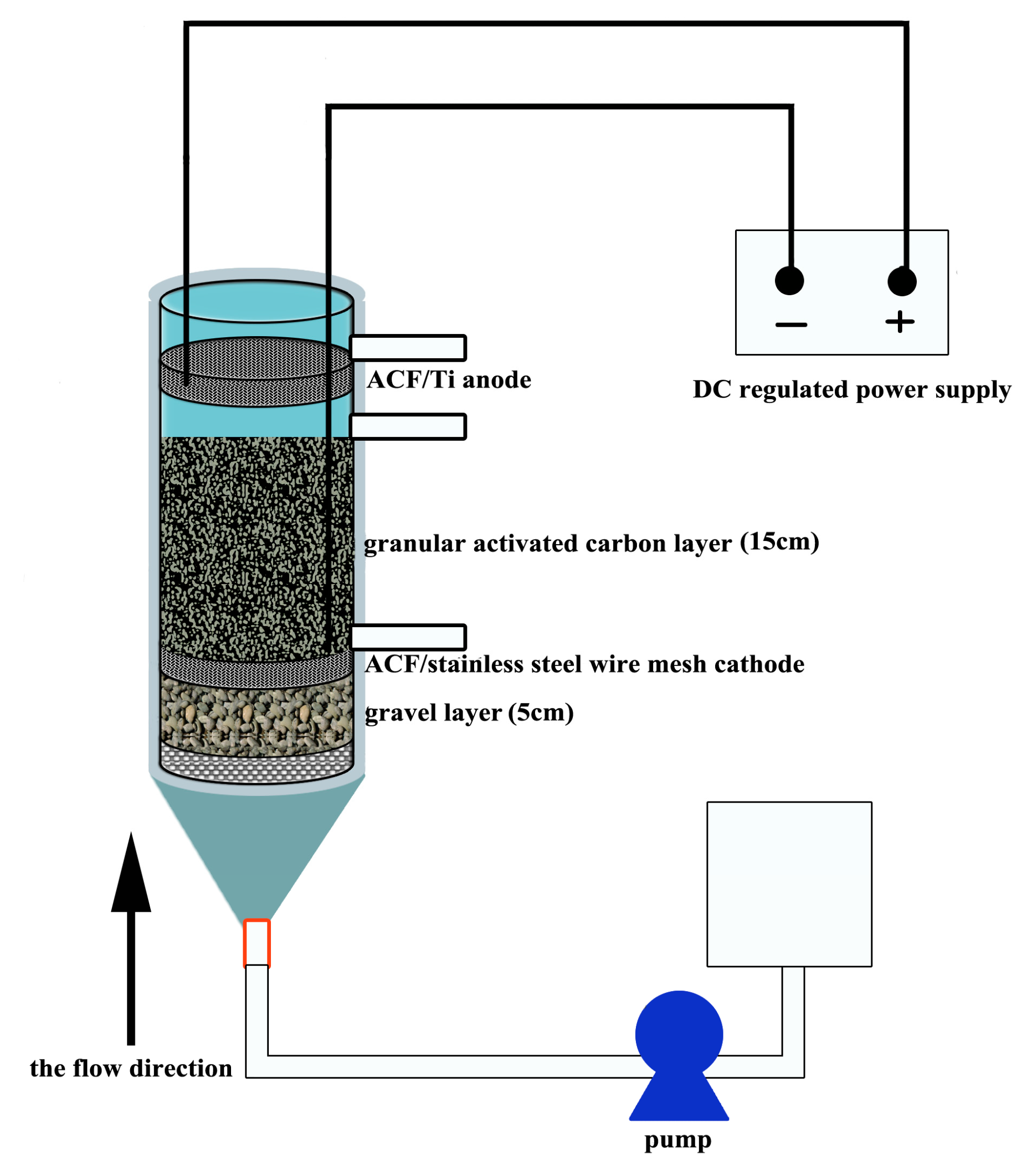
2.1. Reactor Configuration
2.2. System Operation
2.3. Analytics and Calculations
3. Results and Discussion
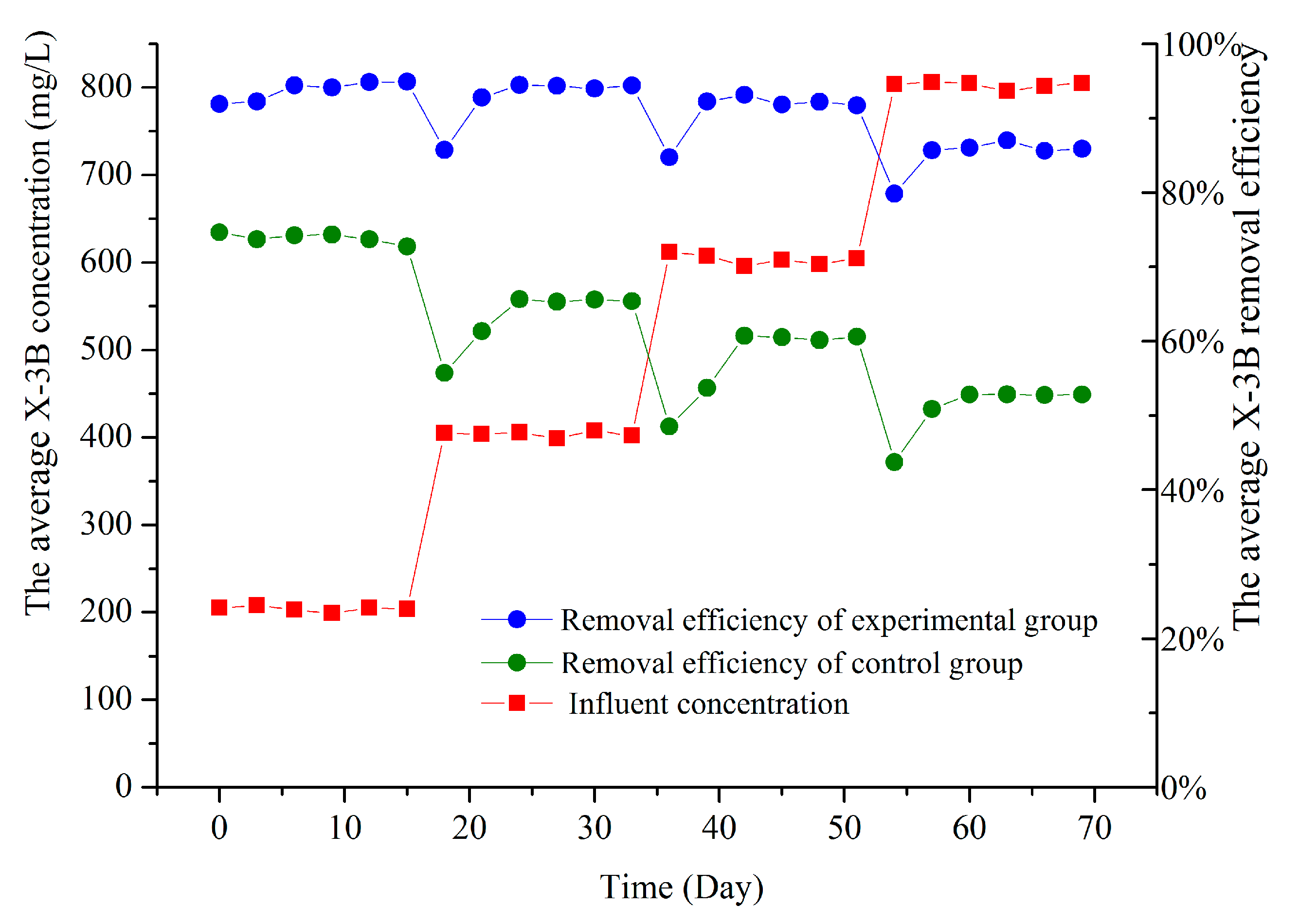
3.1. X-3B Removal Efficiencies at Different Influent Concentrations
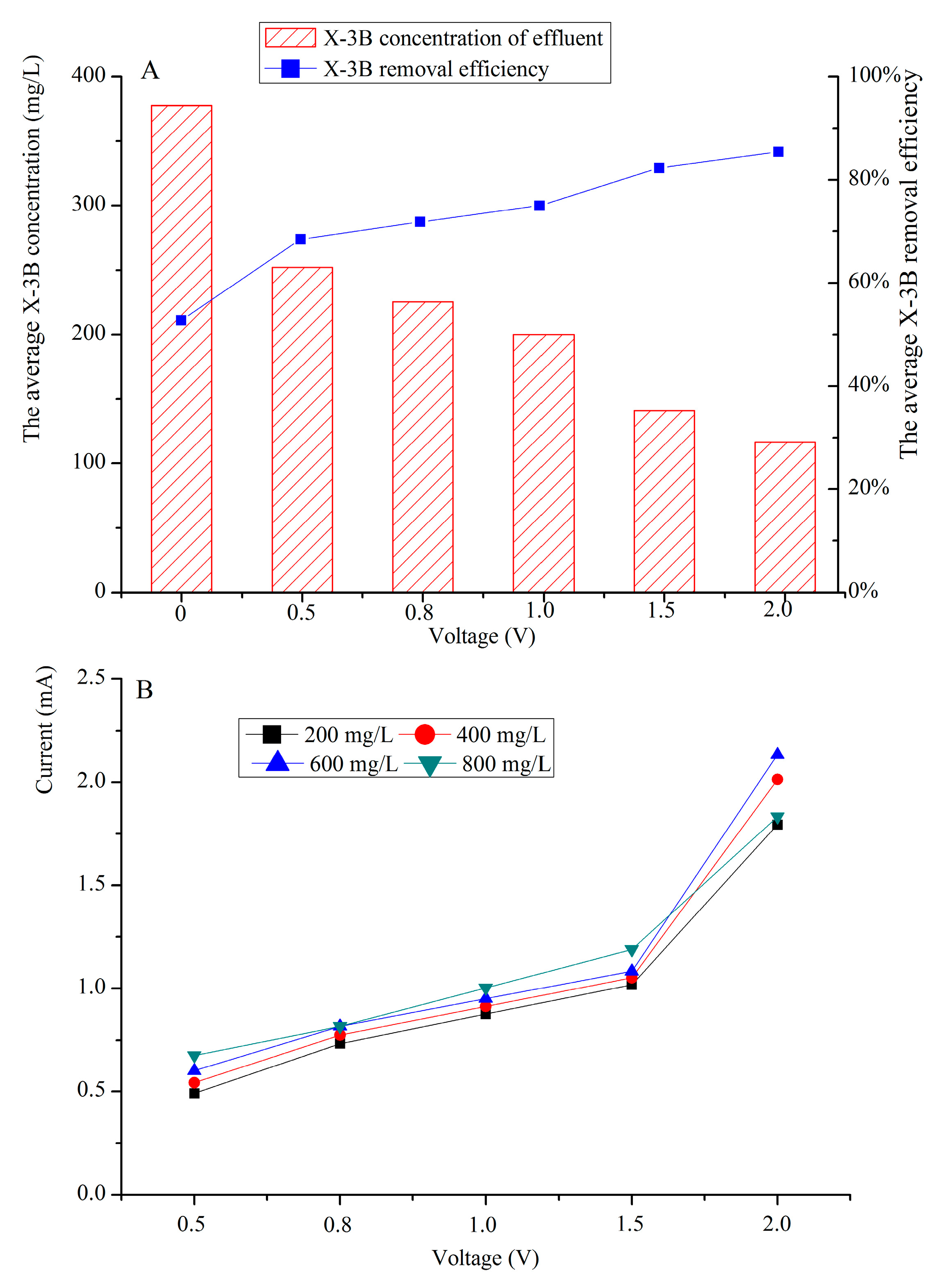
3.2. X-3B Removal Efficiencies under Different External Voltages
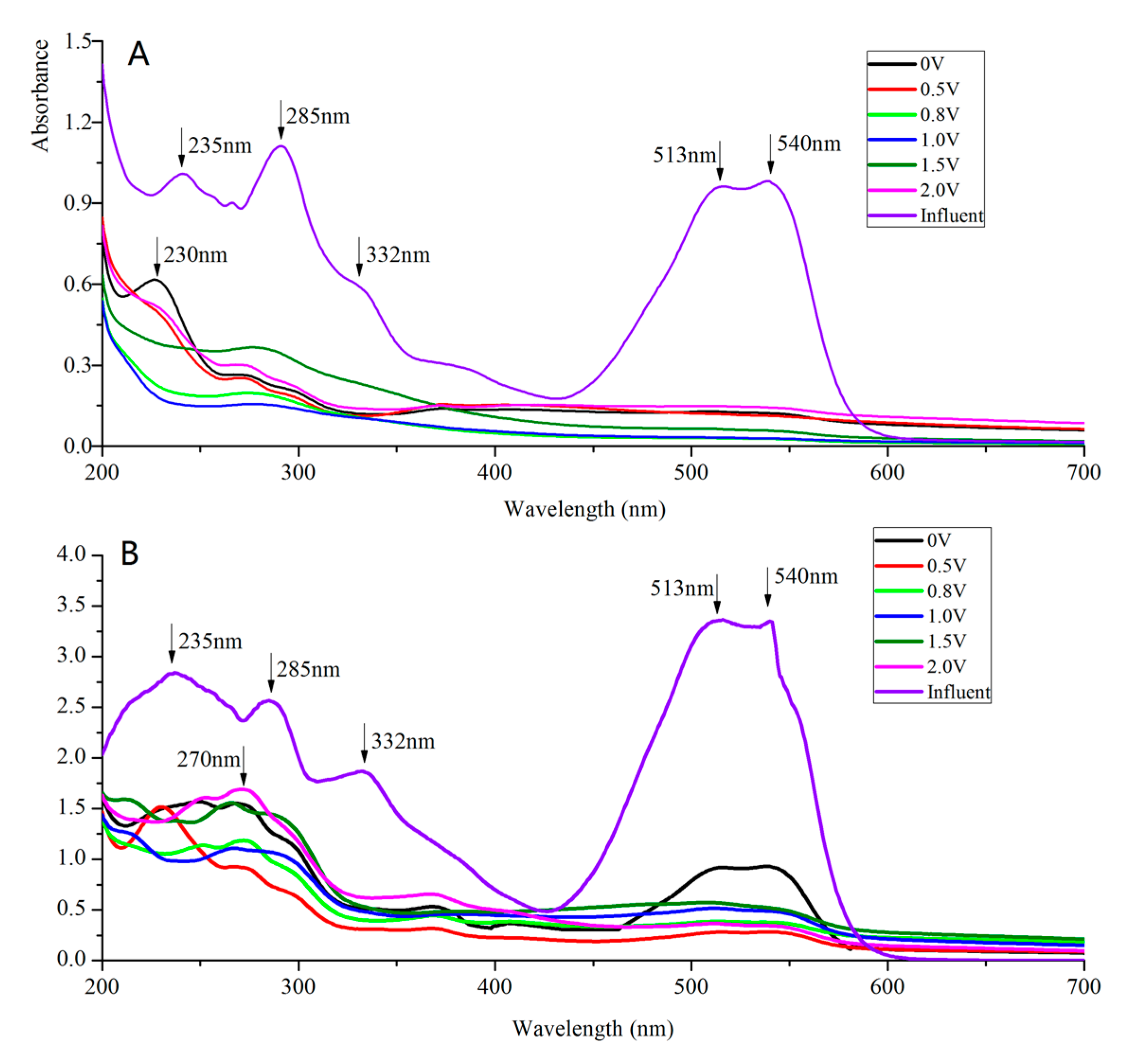
3.3. UV-Vis Spectral Analysis
3.4. Identification of Degradation Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, Y.; Wang, Y.H.; Song, X.S. High-effective denitrification of low C/N wastewater by combined constructed wetland and biofilm-electrode reactor (CW-BER). Bioresour. Technol. 2016, 203, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, X.; Yang, Q.; Wang, D.B.; Yao, F.B.; Li, X.M.; Zhao, J.M.; Xu, Q.X.; Zhang, C.; Zeng, G.M. Complete bromate and nitrate reduction using hydrogen as the sole electron donor in a rotating biofilm-electrode reactor. J. Hazard. Mater. 2016, 307, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fu, W.J.; Gu, H.Y.; Lei, L.C. Nitrate removal from groundwater by a novel three-dimensional electrode biofilm reactor. Electrochim. Acta 2007, 52, 6052–6059. [Google Scholar] [CrossRef]
- Zhang, S.; Song, H.L.; Yang, X.L.; Long, X.Z.; Liu, X.; Chen, T.Q. Behavior of tetracycline and sulfamethoxazole and their corresponding resistance genes in three-dimensional biofilm-electrode reactors with low current. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2017, 52, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Zhang, B.G.; Feng, C.P.; Huang, F.Y.; Zhang, P.; Zhang, Z.Y.; Yang, Y.N.; Sugiura, N. Behavior of autotrophic denitrification and heterotrophic denitrification in an intensified biofilm-electrode reactor for nitrate-contaminated drinking water treatment. Bioresour. Technol. 2012, 107, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Nageswara, R.N.; Rohit, M.; Nitin, G.; Parameswaran, P.N.; Astik, J.K. Kinetics of electrooxidation of landfill leachate in a three-dimensional carbon bed electrochemical reactor. Chemosphere 2009, 76, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.X.; Meng, C.C.; Li, J.B. An integrated process of three-dimensional biofilm-electrode with sulfur autotrophic denitrification (3DBER-SAD) for wastewater reclamation. Appl. Microbiol. Biotechnol. 2016, 100, 7339–7348. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.X.; Li, S.M.; Li, J.B.; Meng, C.C. Denitrification of simulated municipal wastewater treatment plant effluent using a three-dimensional biofilm-electrode reactor: Operating performance and bacterial community. Bioresour. Technol. 2013, 143, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Z.; Yan, S.; Xu, L.N.; Ni, J.R. Removal of Acid Orange 7 in simulated wastewater using a three-dimensional electrode reactor: Removal mechanisms and dye degradation pathway. Chemosphere 2010, 78, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, H.L.; Yang, X.L.; Yang, Y.L.; Yang, K.Y.; Wang, X.Y. Fate of tetracycline and sulfamethoxazole and their corresponding resistance genes in microbial fuel cell coupled constructed wetlands. RSC Adv. 2016, 6, 95999–96005. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.L.; Ning, C.; Li, X.N. Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour. Technol. 2013, 144, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Godini, K.; Nematollahi, D.; Azarian, G.; Maleki, S. Degradation of azo dye C.I. Acid Red 18 using an eco-friendly and continuous electrochemical process. Korean J. Chem. Eng. 2016, 33, 532–538. [Google Scholar] [CrossRef]
- Nigam, P.; Banat, I.M.; Singh, D.; Marchant, R. Microbial process for the decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem. 1996, 31, 435–442. [Google Scholar] [CrossRef]
- Kumar, A.N.; Reddy, C.N.; Mohan, S.V. Biomineralization of azo dye bearing wastewater in periodic discontinuous batch reactor: Effect of microaerophilic conditions on treatment efficiency. Bioresour. Technol. 2015, 188, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Guo, Y.Q.; Lee, H.S.; Cheng, H.Y.; Liang, B.; Kong, F.Y.; Wang, Y.Z.; Huang, L.P.; Xu, M.Y.; Wang, A.J. Efficient azo dye removal in bioelectrochemical system and post-aerobic bioreactor: Optimization and characterization. Chem. Eng. J. 2014, 243, 355–363. [Google Scholar] [CrossRef]
- Reddy, C.N.; Kumar, A.N.; Modestra, J.A.; Mohan, S.V. Induction of anoxic microenvironment in multi-phase metabolic shift strategy during periodic discontinuous batch mode operation enhances treatment of azo dye wastewater. Bioresour. Technol. 2014, 165, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Rao, N.C.; Sarma, P.N. Simulated acid azo dye (Acid black 210) wastewater treatment by periodic discontinuous batch mode operation under anoxic–aerobic–anoxic microenvironment conditions. Ecol. Eng. 2007, 31, 242–250. [Google Scholar] [CrossRef]
- Sreelatha, S.; Velvizhi, G.; Reddy, C.N.; Modestra, J.A.; Mohan, S.V. Solid electron acceptor effect on biocatalyst activity in treating azo dye based wastewater. RSC Adv. 2015, 5, 95926–95938. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, X.M.; Liu, J.; Yang, M.; Zhang, L.P.; Kamagata, Y. Decolorization of reactive brilliant red X-3B by heterogeneous photo-Fenton reaction using an Fe-Ce bimetal catalyst. Catal. Today 2007, 126, 387–393. [Google Scholar] [CrossRef]
- Cao, X.; Gu, F.; Wang, H.; Fang, Z.; Li, X.N. The degradation of azo dye with different cathode and anode structures in biofilm electrode reactors. RSC Adv. 2017, 7, 16854–16860. [Google Scholar] [CrossRef]
- Liu, S.T.; Song, H.L.; Wei, S.Z.; Liu, Q.J.; Li, X.N.; Qian, X.W. Effect of direct electrical stimulation on decolorization and degradation of azo dye reactive brilliant red X-3B in biofilm-electrode reactors. Biochem. Eng. J. 2015, 93, 294–302. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Li, X.Q.; Fang, Z.; Li, X.N. Enhanced degradation of azo dye by a stacked microbial fuel cell-biofilm electrode reactor coupled system. Bioresour. Technol. 2017, 227, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Huang, W.M.; Wang, X.; Li, H.T.; Lu, H.Y.; Lin, H.B. Biofilm-electrode process with high efficiency for degradation of 2,4-dichlorophenol. Environ. Chem. Lett. 2011, 9, 383–388. [Google Scholar] [CrossRef]
- Huang, L.P.; Regan, J.M.; Xie, Q. Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Bioresour. Technol. 2011, 102, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Schaetzle, O.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Strik, D.P.B.T.B.; Barriere, F.; Buisman, C.J.N.; Hamelers, H.V.M. Identifying charge and mass transfer resistances of an oxygen reducing biocathode. Energ. Environ. Sci. 2011, 4, 5035–5043. [Google Scholar] [CrossRef]
- Rau, J.; Knackmuss, H.J.; Stolz, A. Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ. Sci. Technol. 2002, 36, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.S.; Wang, H.; Zhang, X.H.; Qian, Y. Effect of direct electric current on the cell surface properties of phenol-degrading bacteria. Appl. Environ. Microbiol. 2005, 71, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Park, H.I.; Dong, K.K.; Choi, Y.J.; Pak, D. Nitrate reduction using an electrode as direct electron donor in a biofilm-electrode reactor. Process Biochem. 2005, 40, 3383–3388. [Google Scholar] [CrossRef]
- Wang, A.; Qu, J.; Liu, H.; Ru, J. Mineralization of an azo dye Acid Red 14 by photoelectro-Fenton process using an activated carbon fiber cathode. Appl. Catal. B Environ. 2008, 84, 393–399. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo dye treatment with simultaneous electricity production in an anaerobic-aerobic sequential reactor and microbial fuel cell coupled system. Bioresour. Technol. 2010, 101, 4440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Wang, Y. Microwave assisted photocatalytic degradation of high concentration azo dye Reactive Brilliant Red X-3B with microwave electrodeless lamp as light source. Dyes Pigment. 2007, 74, 536–544. [Google Scholar] [CrossRef]
- Feng, W.; Deng, N.; Hua, H. Degradation mechanism of azo dye C. I. reactive red 2 by iron powder reduction and photooxidation in aqueous solutions. Chemosphere 2000, 41, 1233–1238. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, L.; Sun, M.; Xiao, Y.; Su, J. Fast zero-order hydro-cracking reaction of X-3B over crystal Al-Fe alloys: Effect of electrochemical corrosion behaviors. Mater. Des. 2016, 109, 570–579. [Google Scholar] [CrossRef]
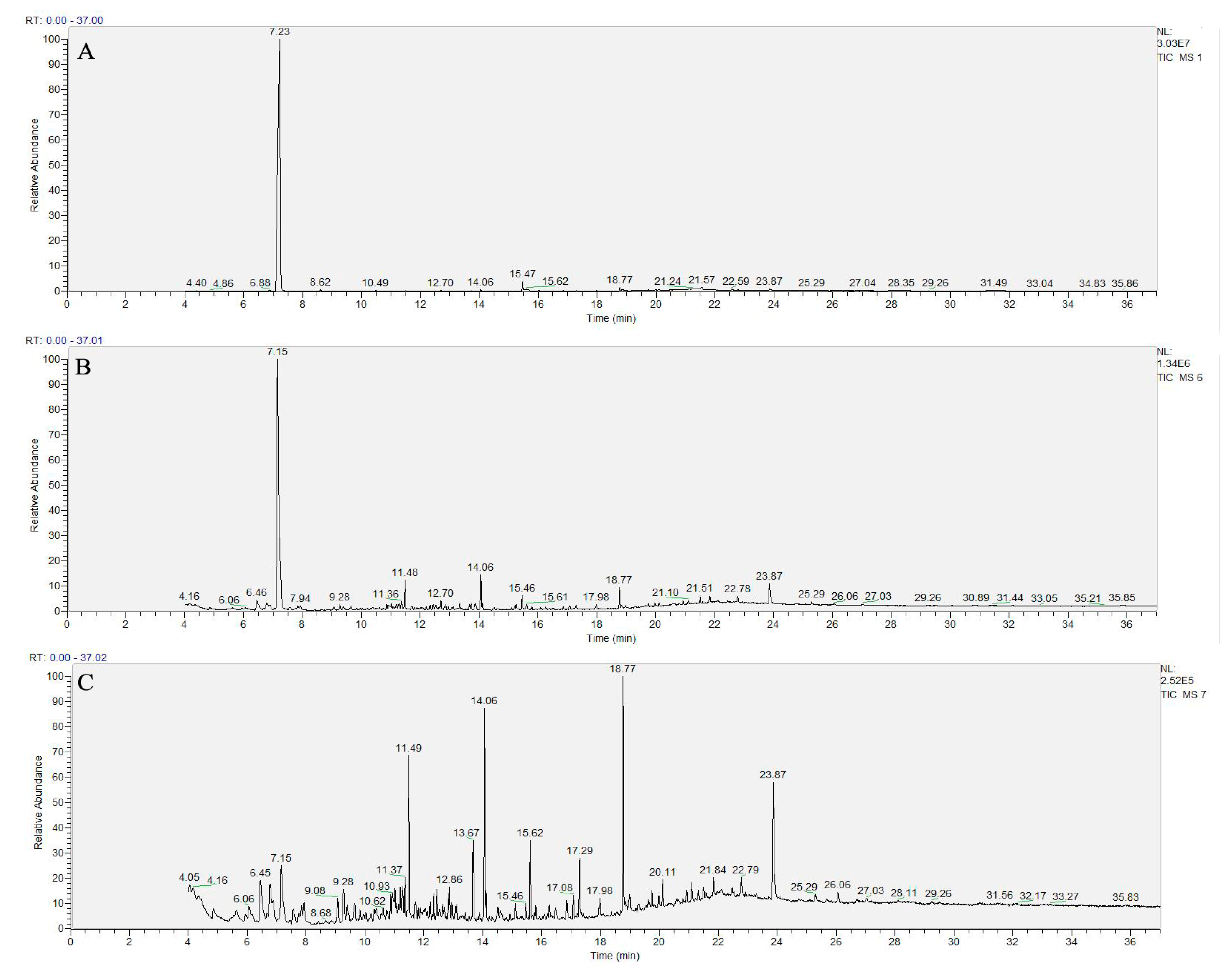
| Effluent | Retention Time (min) | Molecular Formula | Chemical Construction | Chemical Name |
|---|---|---|---|---|
| The cathode area | 7.23 | C6H7N |  | Aniline |
| 14.06 | C15H24O |  | 2,4-bis(1,1-dimethylethyl)-5-methyl-Phenol | |
| 15.47 | C7H7NCl2O |  | 3,5-dichloropyridin-4-ol | |
| 18.77 | C17H24O4 |  | Isobutyl pentan-2-yl phthalate | |
| The top of the activated carbon layer | 6.46 | C13H24 |  | 5-Undecene,7-ethenyl- |
| 7.15 | C6H7N |  | Aniline | |
| 14.06 | C15H24O |  | 2,4-bis(1,1-dimethylethyl)-5-methyl-Phenol | |
| 15.47 | C7H7NCl2O |  | 3,5-dichloropyridin-4-ol | |
| 18.77 | C17H24O4 |  | Isobutyl pentan-2-yl phthalate | |
| 23.87 | C14H26O4 |  | Hexanedioic acid, mono(2-rthylhexyl)ester | |
| The anode area | 6.46 | C13H24 |  | 5-Undecene,7-ethenyl- |
| 7.15 | C6H7N |  | Aniline | |
| 14.06 | C15H24O |  | 2,4-bis(1,1-dimethylethyl)-5-methyl-Phenol | |
| 15.47 | C7H7NCl2O |  | 3,5-dichloropyridin-4-ol | |
| 18.77 | C17H24O4 |  | Isobutyl pentan-2-yl phthalate | |
| 23.87 | C14H26O4 |  | Hexanedioic acid, mono(2-rthylhexyl)ester |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Li, X.; Wang, H.; Zhang, J.; Fang, Z.; Li, X. Effects of Electrical Stimulation on the Degradation of Azo Dye in Three-Dimensional Biofilm Electrode Reactors. Water 2017, 9, 301. https://doi.org/10.3390/w9050301
Cao X, Li X, Wang H, Zhang J, Fang Z, Li X. Effects of Electrical Stimulation on the Degradation of Azo Dye in Three-Dimensional Biofilm Electrode Reactors. Water. 2017; 9(5):301. https://doi.org/10.3390/w9050301
Chicago/Turabian StyleCao, Xian, Xiaoqi Li, Hui Wang, Jingran Zhang, Zhou Fang, and Xianning Li. 2017. "Effects of Electrical Stimulation on the Degradation of Azo Dye in Three-Dimensional Biofilm Electrode Reactors" Water 9, no. 5: 301. https://doi.org/10.3390/w9050301





