Sediment-Water Methane Flux in a Eutrophic Pond and Primary Influential Factors at Different Time Scales
Abstract
:1. Introduction
2. Materials and Methods
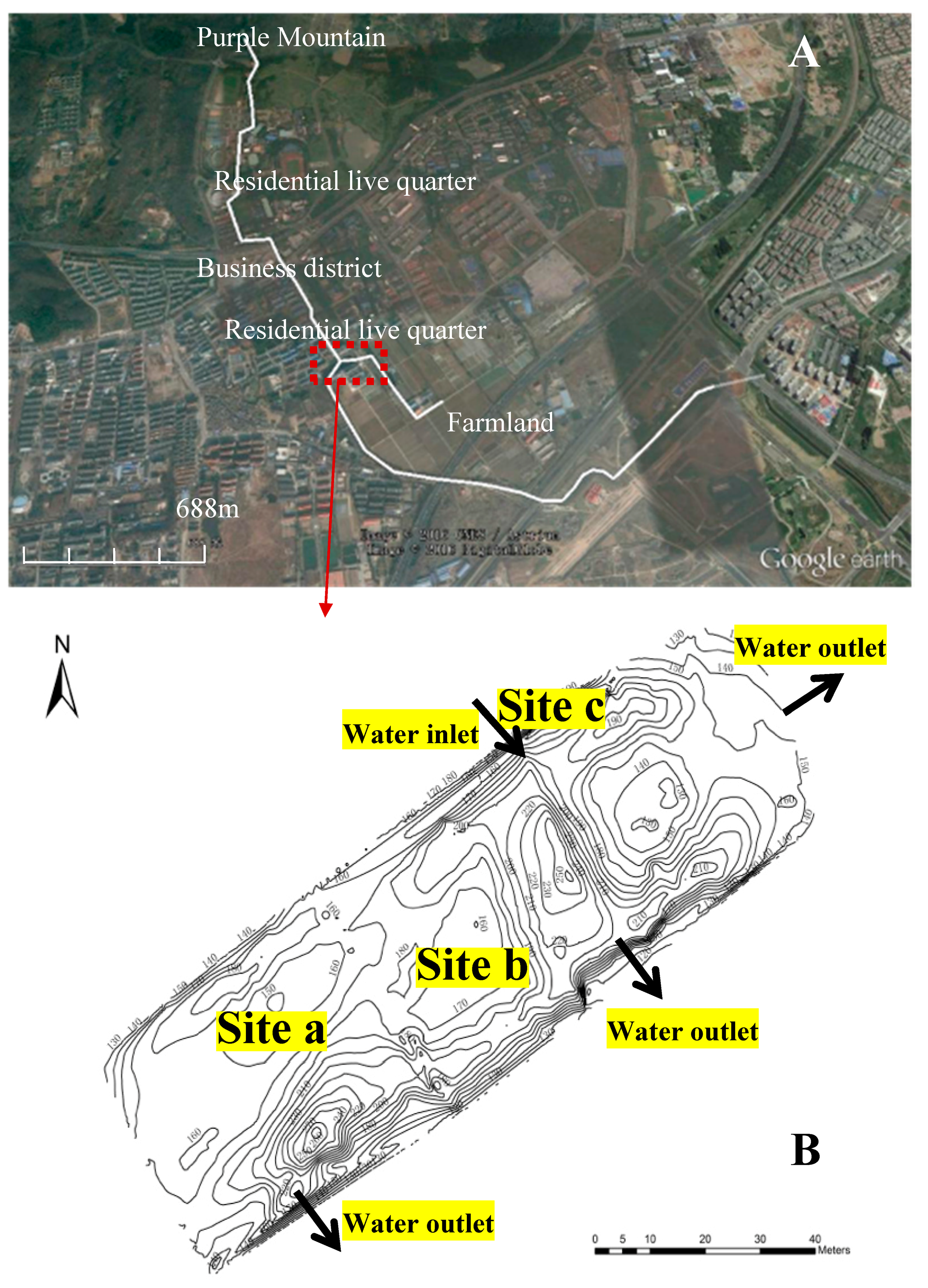
2.1. Characteristics of the Eutrophic Water Body and Sampling Point Settings
2.2. Sampling Campaign
2.3. Statistical Analysis
3. Results and Analysis
3.1. Annual Variation Characteristics and Diel Variation of Methane Flux
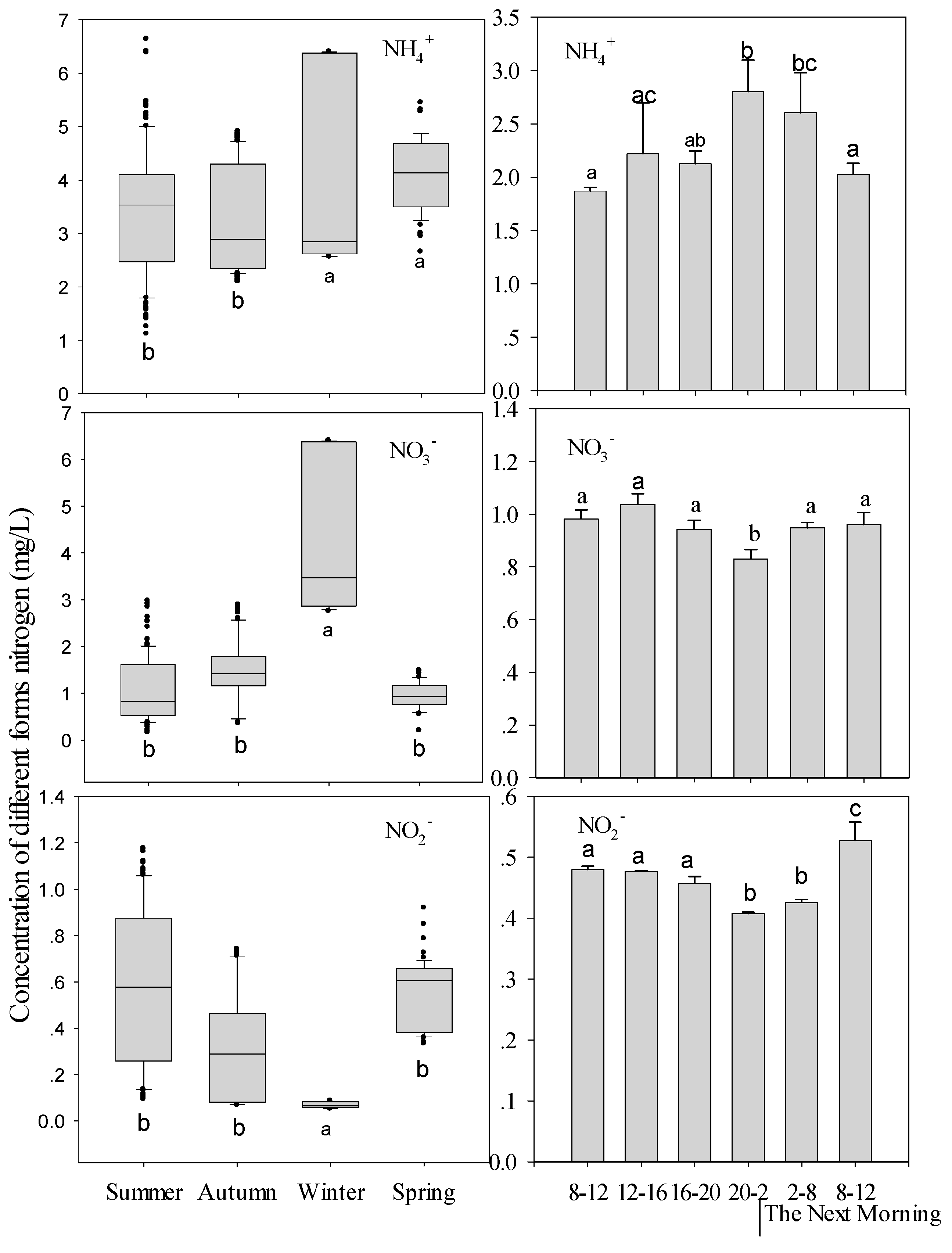
3.2. Environmental Parameter Variation
3.3. Influential Factor Analysis
4. Discussion
4.1. A Eutrophic Pond Is a Significant Waterbody of Methane Release
4.2. Effects of Temperature and DO on Other Parameters
4.3. Factors Correlated with Methane Release
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alford, D.P.; Delaune, R.D.; Lindau, C.W. Methane flux from Mississippi river deltaic plaine wetland. Biogeochemistry 1997, 37, 227–236. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Houghton, T.; Ding, Y.; Griggs, D.J.; Noguer, M.P.; van der Linden, J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change: The Scientific Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001. [Google Scholar]
- Whalen, S.C. Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ. Eng. Sci. 2005, 22, 73–94. [Google Scholar] [CrossRef]
- IPCC. IPCC Fourth Assessment Report: Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Qu, J.; Fan, M. The current state of water quality and technology development for water pollution control in China. Crit. Rev. Environ. Sci. Technol. 2010, 40, 519–560. [Google Scholar] [CrossRef]
- Chen, W.Y. Environmental externalities of urban river pollution and restoration: A hedonic analysis in Guangzhou (China). Landsc. Urban Plan. 2017, 157, 170–179. [Google Scholar] [CrossRef]
- Uggetti, E.; García, J.; Lind, S.E.; Martikainen, P.J.; Ferrer, I. Quantification of greenhouse gas emissions from sludge treatment wetlands. Water Res. 2012, 46, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; He, Y.X.; Yuan, X.Z.; Chen, H.; Peng, C.H.; Yue, J.S.; Zhang, Q.Y.; Diao, Y.B.; Liu, S.S. Greenhouse gases concentrations and fluxes from subtropical small reservoirs in relation with water shed urbanization. Atmos. Environ. 2017, 154, 225–235. [Google Scholar] [CrossRef]
- Liu, K.H.; Hu, Z.B.; Wei, J.Q.; Jiang, Z.; Lu, H.; Wang, C. Analysis of methane flux produced by city black odor river in summer-an example of Chaoyang creek in Nanjing City, China. Earth Environ. 2015, 43, 415–419. (In Chinese) [Google Scholar]
- Ding, W.X.; Cai, Z.C. Effects of soil organic matter and exogenous organic materials on methane production in and emission from wetlands. Acta Ecol. Sin. 2002, 22, 1672–1679. (In Chinese) [Google Scholar]
- Wassmann, R.; Neue, H.U.; Bueno, C.; Lantin, R.S.; Alberto, M.C.R.; Buendia, L.V.; Bronson, K.; Papen, H. Methane production capacities of different rice soils derived from inherent and exogenous substrates. Plant Soil 1998, 203, 227–237. [Google Scholar] [CrossRef]
- Bender, M. Kinetics of methane oxidation in oxic soils. Chemosphere 1993, 26, 687–696. [Google Scholar] [CrossRef]
- Conrad, R.; Rothfuss, F. Methane oxidation in the soil surface layer of a flooded rice field and the effect of ammonium. Biol. Fertil. Soils 1991, 12, 28–32. [Google Scholar] [CrossRef]
- Mason, I. Methane as a carbon source in biological denitrification. Water Pollut. Control Fed. 1977, 49, 855–857. [Google Scholar]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettwig, K.F.; Butler, M.K.; Paslier, D.L.; Pelletier, E.; Mangenot, S.; Kuypers, M.M.M.; Schreiber, F.; Dutilh, B.E.; Zedelius, J.; Beer, D.D.; et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010, 464, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, E.; Lucotte, M.; Canuel, R.; Chamberland, A. Production of the greenhouse gases CH4 and CO2 by hydroelectric reservoirs of the boreal region. Glob. Biogeochem. Cycles 1995, 9, 529–540. [Google Scholar] [CrossRef]
- Smith, L.K.; Lewis, W.M. Seasonality of methane emissions from five lakes and associated wetlands of the coloeado rockies. Glob. Biogeochem. Cycles 1992, 6, 323–338. [Google Scholar] [CrossRef]
- Mazumdar, A.; Peketi, A.; Joao, H.M.; Dewangan, P. Pore-water chemistry of sediment cores off Mahanadi Basin, Bay of Bengal: Possible link to deep seated methane hydrate deposit. Mar. Pet. Geol. 2014, 49, 162–175. [Google Scholar] [CrossRef]
- Bastviken, D.; Jonathan, C.; Michael, P.; Tranvik, L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Keiski, R.L.; Desponds, O.; Chang, Y.F.; Somorjai, G.A. Kinetics of the water-gas shift reaction over several alkane activation and water-gas shift catalysts. Appl. Catal. A-Gen. 1993, 101, 317–338. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Shi, K.; Liu, J.J.; Deng, J.M.; Qin, B.Q.; Zhu, G.W.; Zhou, Y.Q. Meteorological and hydrological conditions driving the formation and disappearance of black blooms, an ecological disaster phenomena of eutrophication and algal blooms. Sci. Total Environ. 2016, 569–570, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, X.H.; Yi, N.; Wang, Y.; Guo, J.Y.; Zhang, Z.H.; Yan, S.H. Estimation of N2 and N2O ebullition from eutrophic water using an improved bubble trap device. Ecol. Eng. 2013, 57, 403–412. [Google Scholar] [CrossRef]
- Liu, X.H.; Gao, Y.; Zhao, Y.; Wang, Y.; Yi, N.; Zhang, Z.; Yan, S. Supplemental tests of gas trapping device for N2 flux measurement. Ecol. Eng. 2016, 93, 9–12. [Google Scholar] [CrossRef]
- Liu, X.H.; Gao, Y.; Wang, H.L.; Guo, J.Y.; Yan, S.H. Applying a new method for direct collection, volume quantification and determination of N2 emission from water. J. Environ. Sci. 2015, 27, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.; Ledo, L.; Soares, S.; Barbosa, I.R.; Alvarenga, P. Spatial and temporal variability of the water and sediments quality in the Alqueva reservoir (Guadiana Basin; southern Portugal). Sci. Total Environ. 2014, 470, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Z.H.; Liu, X.H.; Yi, N.; Zhang, L.; Song, W.; Wang, Y.; Mazumder, A.; Yan, S.H. Seasonal and diurnal dynamics of physicochemical parameters and gas production in vertical water column of a eutrophic pond. Ecol. Eng. 2016, 87, 313–323. [Google Scholar] [CrossRef]
- Martinez, D.; Anderson, M.A. Methane production and ebullition in a shallow, artificially aerated, eutrophic temperate lake (Lake Elsinore, CA). Sci. Total Environ. 2013, 454, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Lewis, W.M.; Jeffrey, P.; Cronin, G.; Hamilton, S.K. Methane emissions from the Orinoco River floodplain, Venezuela. Biogeochemistry 2000, 51, 113–140. [Google Scholar] [CrossRef]
- Delsontro, T.; McGinnis, D.F.; Sobek, S.; Ostrovsky, I.; Wehrli, B. Extreme methane emissions from a Swiss hydropower reservoir: Contribution from bubbling sediments. Environ. Sci. Technol. 2010, 44, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.B.; Wang, Y.C.; Liu, D.F.; Yang, Z.J.; Lei, D.; Zhang, C. Diel and seasonal variation of methane and carbon dioxide fluxes at Site Guojiaba, the Three Gorges Reservoir. J. Environ. Sci. 2013, 25, 2065–2071. [Google Scholar] [CrossRef]
- Li, Z.; Yao, X.; He, P.; Wang, Q.; Guo, J.S.; Chen, Y.B. Diel variations of air-water CO2 and CH4 diffusive fluxes in the Pengxi River, Three Gorges Reservoir. J. Lake Sci. 2014, 26, 576–584. (In Chinese) [Google Scholar]
- Yang, W.Y.; Song, C.C.; Zhang, J.B. Seasonal dynamics of dissolved org anic carbon and nitrogen and correlativity between their concentrations and methane flux in the fresh water marsh. Acta Sci. Circumstantiae 2006, 26, 1745–1750. (In Chinese) [Google Scholar]
- Su, M.M.; Kuang, F.H.; Lv, Y.; Shi, X.J.; Liu, X.J.; Shen, J.B.; Zhang, F.F. Nitrous oxide and methane emissions from paddy soils in southwest China. Geoderma Reg. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Higgins, T.M.; McCutchan, J.H.; Lewis, W.M. Nitrogen ebullition in a Colorado plains river. Biogeochemistry 2008, 89, 367–377. [Google Scholar] [CrossRef]
- Olguin, E.J.; Galvan, G.S.; Melo, F.J.; Hernandez, V.J.; Portela, R.E.G. Long-term assessment at field scale of floating treatment wetlands for improvement of water quality and provision of ecosystem services in a eutrophic urban pond. Sci. Total Environ. 2017, 584–585, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, M.; Gennaro, P.; Mercatali, I.; Persia, E.; Solari, D.; Porrello, S. Physico-chemical and nutrient variable stratifications in the water column and in macroalgal thalli as a result of his biomass mats in a non-tidal shallow-water lagoon. Mar. Pollut. Bull. 2013, 75, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Glover, H.E.; Keller, M.D.; Spinrad, R.W. The effects of light quality and intensity on photosynthesis and growth of marine eukaryotic and prokaryotic phytoplankton clones. J. Exp. Mar. Biol. Ecol. 1987, 105, 137–159. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Javanaud, C.; Aigle, A.; Michotey, V.D.; Guasco, S.; Deborde, J.; Deflandre, B.; Anschutz, P.; Bonin, P.C. Anaerobic nitrification–denitrification mediated by Mn-oxides in meso-tidal sediments:Implications for N2 and N2O production. J. Mar. Syst. 2015, 144, 1–8. [Google Scholar] [CrossRef]
- Hutsch, B.W.; Webster, C.P.; Powlson, D.S. Methane oxidation in soil as affected by land use, soil pH and N fertilization. Soil Biol. Biochem. 1994, 26, 1613–1622. [Google Scholar] [CrossRef]
- Deshmukh, C.; Serça, D.; Delon, C.; Tardif, R.; Demarty, M.; Jarnot, C.; Meyerfeld, Y.; Chanudet, V.; Guédant, P.; Rode, W.; et al. Physical controls on CH4 emissions from a newly flooded subtropical freshwater hydroelectric reservoir: Nam Theun 2. Biogeosciences 2014, 11, 4151–4269. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Koba, K.; Yoh, M. Strong inhibitory effect of nitrate on atmospheric methane oxidation in forest soils. Soil Biol. Biochem. 2012, 50, 164–166. [Google Scholar] [CrossRef]
- Modin, O.; Fukushi, K.; Yamamoto, K. Denitrification with methane as external carbon source. Water Res. 2007, 41, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Roland, F.A.E.; Darchambeau, F.; Morana, C.; Bouillon, S. Emission and oxidation of methane in a meromictic, eutrophic and temperate lake (Dendre, Belgium). Chemosphere 2017, 168, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Tanaka, Y.; Osada, T.; Susuki, K. Effects of nitrite and ammonium on methane-dependent denitrification. Appl. Microbiol. Biothechnol. 2002, 59, 338–343. [Google Scholar]
- Liikanen, A.; Martikainen, P.J. Effect of ammonium and oxygen on methane and nitrous oxide fluxes across sediment-water interface in a eutrophic lake. Chemosphere 2003, 52, 1287–1293. [Google Scholar] [CrossRef]
| Parameters | NO3− (mg kg−1) | NO2− (mg kg−1) | NH4+ (mg kg−1) | Total Nitrogen (TN) % | Total Phosphorus (TP) (%) | Organic Matter % | pH |
|---|---|---|---|---|---|---|---|
| Content | 3.64 ± 1.41 | 0.95 ± 0.23 | 169.06 ± 30.42 | 0.71 ± 0.08 | 0.46 ± 0.05 | 3.56 ± 0.71 | 7.53 ± 0.22 |
| Parameters | Time | CH4 Flux Rate (mmol m−2 h−1) |
|---|---|---|
| Seasonal variation | Summer | 2.81 ± 0.19 |
| Autumn | 0.63 ± 0.10 | |
| Winter | zero | |
| Spring | 0.62 ± 0.14 | |
| Diel variation in Summer | 8:00–12:00 | 2.75 ± 0.14 a |
| 12:00–16:00 | 3.41 ± 0.89 a | |
| 16:00–20:00 | 6.19 ± 0.44 b | |
| 20:00–2:00 | 11.48 ± 0.84 c | |
| 2:00–8:00 | 6.46 ± 1.35 b | |
| 8:00–12:00 | 2.93 ± 1.55 a |
| Parameters | Time | Temperature °C | Saturation Percentage of Dissolved Oxygen % | pH | Chlorophyll-a μg L−1 | TN mg L−1 | TP mg L−1 |
|---|---|---|---|---|---|---|---|
| Seasonal variation | Summer | 26.46 ± 0.22 | 12.55 ± 1.14 | 7.41 ± 0.05 | 150.56 ± 9.47 | 5.21 ± 0.15 | 0.56 ± 0.03 |
| Autumn | 19.86 ± 0.53 | 46.21 ± 1.97 | 7.95 ± 0.03 | 75.44 ± 5.38 | 4.77 ± 0.11 | 0.45 ± 0.04 | |
| Winter | 7.25 ± 0.21 | 92.41 ± 13.03 | 8.13 ± 0.16 | 76.22 ± 18.16 | 8.15 ± 0.65 | 1.00 ± 0.10 | |
| Spring | 17.28 ± 0.56 | 27.27 ± 3.13 | 8.04 ± 0.03 | 182.97 ± 18.46 | 5.43 ± 0.18 | 0.57 ± 0.02 | |
| Diurnal variation in Summer | 8:00–12:00 | 31.3 ± 0.00 a | 17.43 ± 3.50 a | 7.56 ± 0.1 a | 52.33 ± 4.39 a | 2.85 ± 0.04 a | 0.66 ± 0.00 a |
| 12:00–16:00 | 31.2 ± 0.00 b | 16.8 ± 2.38 a | 7.76 ± 0.08 a | 54.6 ± 3.03 a | 3.03 ± 0.06 a | 0.63 ± 0.01 a | |
| 16:00–20:00 | 31.25 ± 0.07 b | 4.7 ± 0.44 b | 7.44 ± 0.03 a | 94.19 ± 4.95 b | 3.25 ± 0.05 a | 0.62 ± 0.01 a | |
| 20:00–2:00 | 31.25 ± 0.07 b | 1.67 ± 0.20 c | 7.33 ± 0.04 b | 75.96 ± 3.82 b | 3.44 ± 0.02 a | 0.60 ± 0.00 a | |
| 2:00–8:00 | 31.35 ± 0 a | 13.68 ± 2.01 a | 7.59 ± 0.07 a | 88.27 ± 5.59 b | 3.18 ± 0.02 a | 0.60 ± 0.02 a | |
| 8:00–12:00 | 31.15 ± 0.07 a | 3.97 ± 1.40 b | 7.52 ± 0.05 a | 73.48 ± 0.81 b | 3.32 ± 0.03 a | 0.54 ± 0.03 a |
| Parameters | Time | Equation | R2 | p-Value |
|---|---|---|---|---|
| NO3−-N concentration | Different seasons | y = −0.33x + 2.23 | 0.05 | 0.01 |
| The whole Summer | y = −1.21x + 4.33 | 0.32 | <0.0001 | |
| Diel variation | y = −34.99x + 39.08 | 0.68 | <0.001 | |
| NO2−-N concentration | Different seasons | y = 0.68x + 1.37 | 0.01 | 0.12 |
| The whole Summer | y = −0.92x + 3.37 | 0.01 | 0.45 | |
| Diel variation | y = −41.896x + 24.912 | 0.36 | <0.01 | |
| NH4+-N concentration | Different seasons | y = 0.259x + 1.428 | 0.03 | <0.02 |
| The whole Summer | y = 0.796x + 0.532 | 0.19 | <0.01 | |
| Diel variation | y = 5.2067x − 7.4312 | 0.87 | <0.01 | |
| Temperature | Different seasons | y = 0.227x − 3.345 | 0.44 | <0.001 |
| The whole Summer | y = 0.0376x + 1.753 | 0 | 0.73 | |
| Diel variation | y = 18.680x − 577.962 | 0.41 | <0.02 | |
| Dissolved oxygen | Different seasons | y = −0.303x + 2.536 | 0.13 | <0.001 |
| The whole Summer | y = −0.3172x + 3.2479 | 0.07 | 0.08 | |
| Diel variation | y = −2.5061x + 7.4835 | 0.25 | 0.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Gao, Y.; Zhang, Z.; Luo, J.; Yan, S. Sediment-Water Methane Flux in a Eutrophic Pond and Primary Influential Factors at Different Time Scales. Water 2017, 9, 601. https://doi.org/10.3390/w9080601
Liu X, Gao Y, Zhang Z, Luo J, Yan S. Sediment-Water Methane Flux in a Eutrophic Pond and Primary Influential Factors at Different Time Scales. Water. 2017; 9(8):601. https://doi.org/10.3390/w9080601
Chicago/Turabian StyleLiu, Xinhong, Yan Gao, Zhenhua Zhang, Jia Luo, and Shaohua Yan. 2017. "Sediment-Water Methane Flux in a Eutrophic Pond and Primary Influential Factors at Different Time Scales" Water 9, no. 8: 601. https://doi.org/10.3390/w9080601






