Human Bodily Asymmetry Relates to Behavioral Lateralization and May not Reliably Reflect Developmental Instability
Abstract
:1. Introduction
2. Materials and Methods
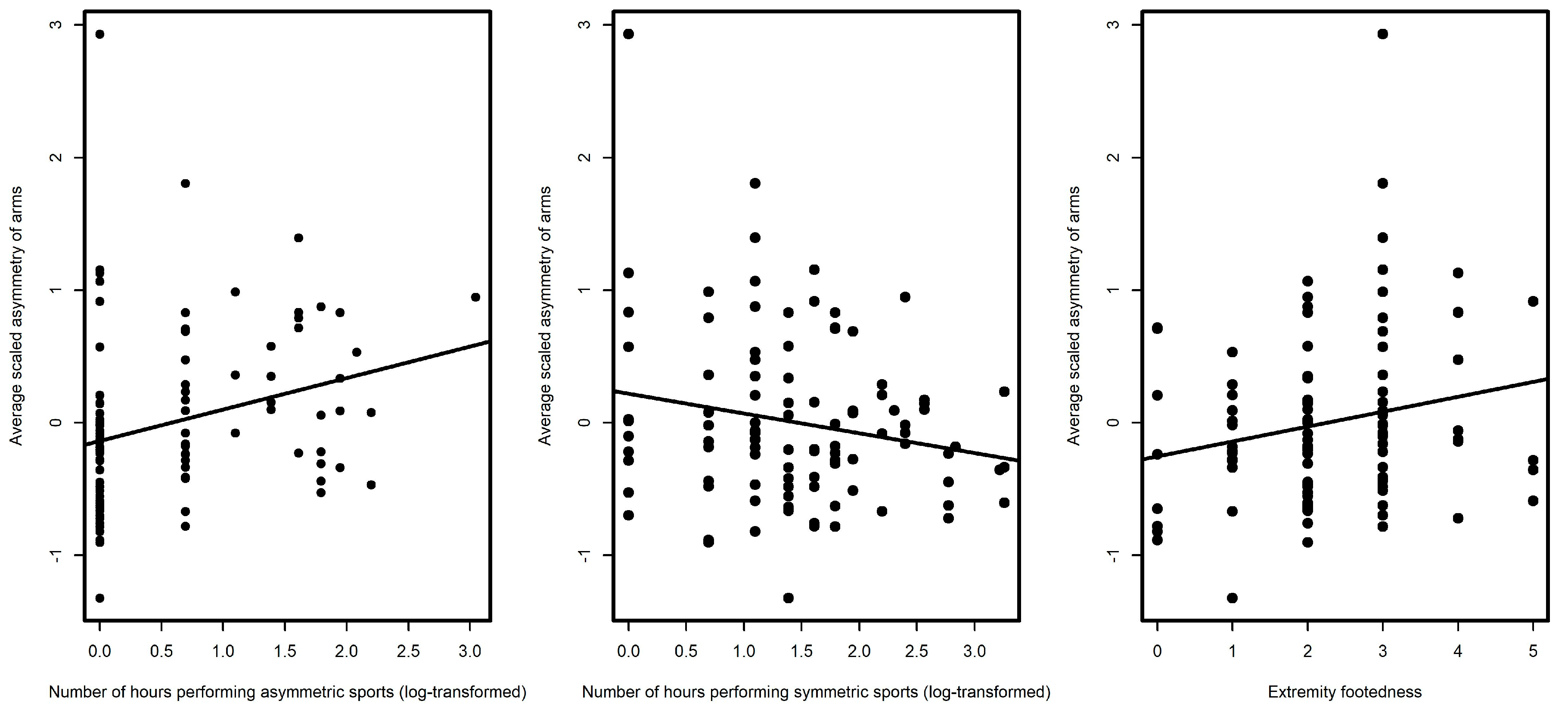
3. Results
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Klingenberg, C.P. A developmental perspective on developmental instability: Theory, models, and mechanisms. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 14–34. [Google Scholar]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Auerbach, B.; Ruff, C.B. Limb bone bilateral asymmetry: Variability and commonality among modern humans. J. Hum. Evol. 2006, 50, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, T.; Kumar, M.; Kumar, P.; Yoganarasimha, K. Skeletal asymmetry. J. Forensic Legal Med. 2008, 15, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Özener, B. Fluctuating Asymmetry of Human Populations: A Review. Symmetry 2016, 8, 154. [Google Scholar] [CrossRef]
- Daly, R.M.; Saxon, L.; Turner, C.H.; Robling, A.G.; Bass, S.L. The relationship between muscle size and bone geometry during growth and in response to exercise. Bone 2004, 34, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Özener, B. Fluctuating and directional asymmetry in young human males: Effect of heavy working condition and socioeconomic status. Am. J. Phys. Anthropol. 2010, 143, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, S.; Cornille, R.; Lens, L. Sex and asymmetry in humans: What is the role of developmental instability. J. Evol. Biol. 2009, 22, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, S.; Gangestad, S.W. Human fluctuating asymmetry in relation to health and quality: A meta-analysis. Evol. Hum. Behav. 2011, 32, 380–398. [Google Scholar] [CrossRef]
- Brown, W.M.; Price, M.E.; Kang, J.S.; Pound, N.; Zhou, Y.; Yu, H. Fluctuating asymmetry and preferences for sex-typical bodily characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 12938–12943. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, S.; Molenberghs, G.; Matthysen, E. The statistical analysis of fluctuating asymmetry: REML estimation of a mixed regression model. J. Evol. Biol. 1999, 12, 94–102. [Google Scholar] [CrossRef]
- LeMay, M. Asymmetries of the skull and handedness. Phrenology revisted. J. Neurol. Sci. 1977, 32, 243–253. [Google Scholar] [CrossRef]
- Plato, C.C.; Wood, J.L.; Norris, A.H. Bilateral asymmetry in bone measurements of the hand and lateral hand dominance. Am. J. Phys. Anthopol. 1980, 52, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Steele, J. Handedness in past human populations: Skeletal markers. Laterality 2000, 5, 193–220. [Google Scholar] [CrossRef] [PubMed]
- Swissa-Sivan, A.; Simkin, A.; Leichter, I.; Nyska, A.; Nyska, M.; Statter, M.; Bivas, A.; Menczel, J.; Samueloff, S. Effect of swimming on bone growth and development in young rats. Bone Miner. 1989, 7, 91–105. [Google Scholar] [CrossRef]

| Trait | Directional Asymmetry Mean (SE) | Signal-to-Noise Ratio | Number of Scan Errors | Number of Outliers | ||
|---|---|---|---|---|---|---|
| FA | ME | %FA | ||||
| Thigh length | −0.06 (0.01) *** | 0.013 | 0.007 | 63% | 0 | 6 |
| Knee height | 0.07 (0.02) ** | 0.042 | 0.008 | 85% | 1 | 5 |
| Knee circumference | 0.02 (0.03) | 0.060 | 0.018 | 77% | 0 | 0 |
| Calf circumference | 0.02 (0.02) | 0.048 | 0.002 | 96% | 1 | 12 |
| Ankle circumference | −0.25 (0.15) | 0.74 | 2.76 | 21% | 5 | 5 |
| Leg volume | −0.04 (0.85) | 0.734 | 0.049 | 95% | 0 | 14 |
| Leg surface area | 0.447 (0.893) | 0.767 | 0.072 | 91% | 0 | 24 |
| Upper arm length | −0.07 (0.05) | 0.137 | 0.193 | 41% | 11 | 12 |
| Forearm length | −0.06 (0.02) ** | 0.032 | 0.011 | 74% | 0 | 3 |
| Armscye circumference | 0.05 (0.07) | 0.250 | 0.588 | 30% | 0 | 14 |
| Biceps circumference | 0.01 (0.04) | 0.100 | 0.061 | 62% | 0 | 5 |
| Elbow circumference | 0.05 (0.02) * | 0.040 | 0.020 | 67% | 0 | 6 |
| Wrist circumference | −0.07 (0.03) * | 0.055 | 0.047 | 54% | 0 | 6 |
| Arm volume | 0.19 (048) | 0.159 | 0.152 | 52% | 0 | 0 |
| Arm surface area | −0.32 (0.60) | 0.243 | 0.258 | 48% | 0 | 4 |
| Trait | Unsigned FA—Trait Size Correlation | Signed FA Correlations with a | Unsigned FA Correlations with a | S1 | S2 | ||
|---|---|---|---|---|---|---|---|
| Hand. | Footed. | Asymm. Sports | Symm. Sports | ||||
| Thigh length | −0.14 | 0.14 | 0.05 | 0.01 | 0.09 | * | |
| Knee height | 0.03 | −0.23 * | −0.05 | 0.20 * | 0.07 | * | |
| Knee circumference | 0.03 | 0.03 | −0.06 | 0.01 | −0.08 | * | * |
| Calf circumference | 0.18 | −0.12 | 0.06 | 0.17 | −0.11 | * | * |
| Ankle circumference | 0.48 *** | - | - | - | - | ||
| Leg volume | 0.30 ** | −0.08 | 0.01 | −0.01 | −0.08 | * | * |
| Leg surface area | 0.07 | 0.12 | 0.22 * | 0.06 | −0.07 | * | * |
| Upper arm length | 0.13 | - | - | - | - | ||
| Forearm length | 0.26 ** | 0.14 | 0.06 | 0.12 | −0.14 | * | |
| Armscye circumference | 0.04 | - | - | - | - | ||
| Biceps circumference | 0.10 | 0.15 | −0.01 | 0.28 ** | −0.12 | * | * |
| Elbow circumference | 0.32 *** | −0.02 | 0.13 | 0.26 * | −0.03 | * | |
| Wrist circumference | 0.05 | 0.15 | −0.05 | 0.15 | −0.20 * | * | |
| Arm volume | 0.19* | 0.20 * | −0.07 | 0.14 | −0.24 * | * | * |
| Arm surface area | 0.04 | - | - | - | |||
| Extremity of Handedness | Extremity of Footedness | Amount of Asymm. Sports | Amount of Symm. Sports | |
|---|---|---|---|---|
| Average asymmetry 1 | 0.01 (0.05) | 0.07 (0.04) | 0.16 (0.06) ** | −0.06 (0.05) |
| Average asymmetry 2 | −0.02 (0.05) | 0.07 (0.04) | 0.13 (0.06) * | −0.10 (0.06) |
| Average arm asymmetry | −0.00 (0.07) | 0.11 (0.05) * | 0.23 (0.08) ** | −0.15 (0.08) * |
| Average leg asymmetry | 0.01 (0.05) | 0.01 (0.04) | 0.10 (0.07) | −0.03 (0.06) |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Dongen, S. Human Bodily Asymmetry Relates to Behavioral Lateralization and May not Reliably Reflect Developmental Instability. Symmetry 2018, 10, 117. https://doi.org/10.3390/sym10040117
Van Dongen S. Human Bodily Asymmetry Relates to Behavioral Lateralization and May not Reliably Reflect Developmental Instability. Symmetry. 2018; 10(4):117. https://doi.org/10.3390/sym10040117
Chicago/Turabian StyleVan Dongen, Stefan. 2018. "Human Bodily Asymmetry Relates to Behavioral Lateralization and May not Reliably Reflect Developmental Instability" Symmetry 10, no. 4: 117. https://doi.org/10.3390/sym10040117





