Metabolomic Signature of Diabetic Kidney Disease in Cerebrospinal Fluid and Plasma of Patients with Type 2 Diabetes Using Liquid Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
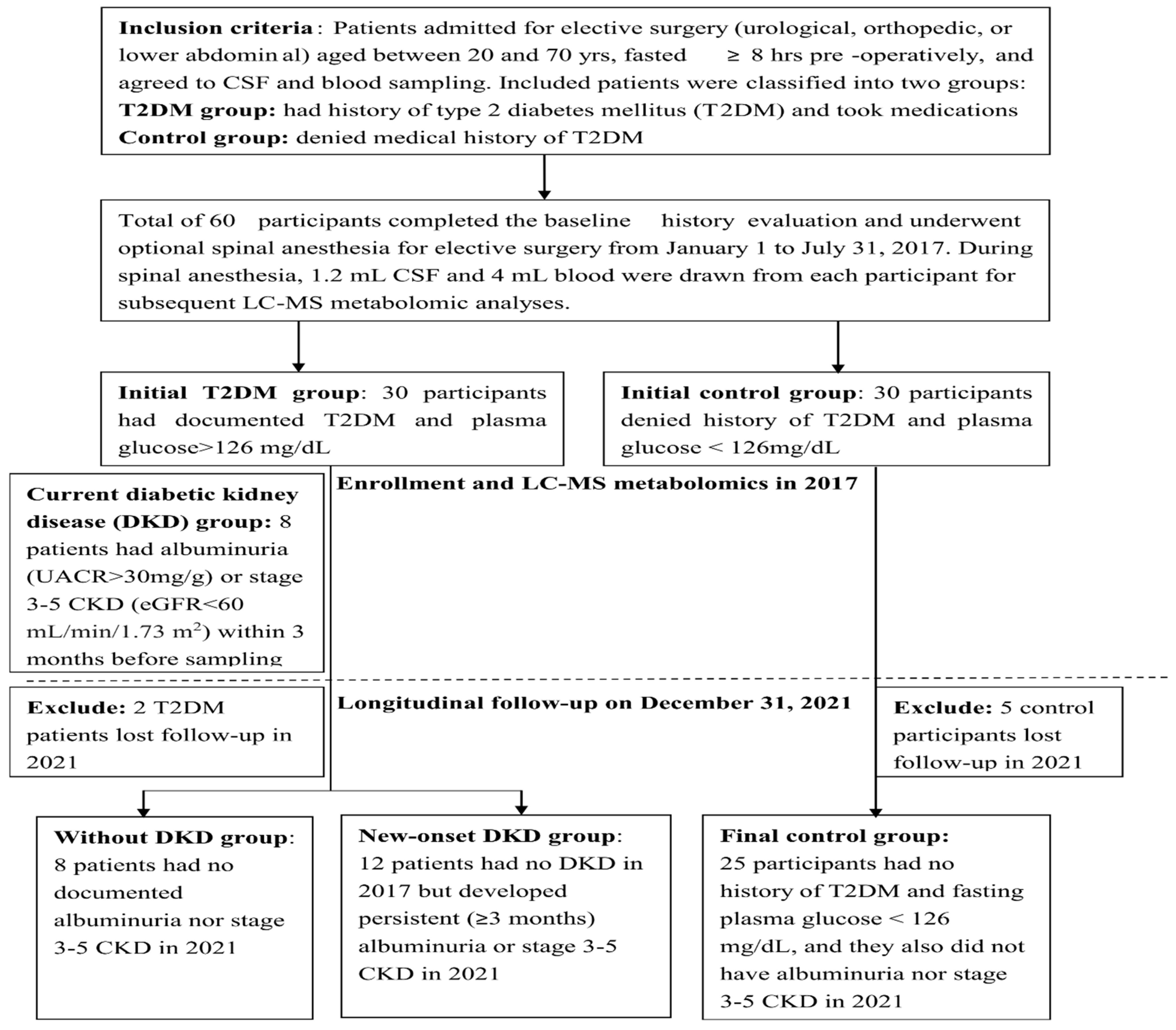
2.1. Study Population
2.2. CSF and Blood Sampling Procedures
2.3. Biochemical Analyses of the CSF and Plasma Samples
2.4. Sample Preparation for the LC-MS
2.5. Metabolites Identification and the Statistical Analysis
3. Results
3.1. Group Separation and Their Demographic Comparison
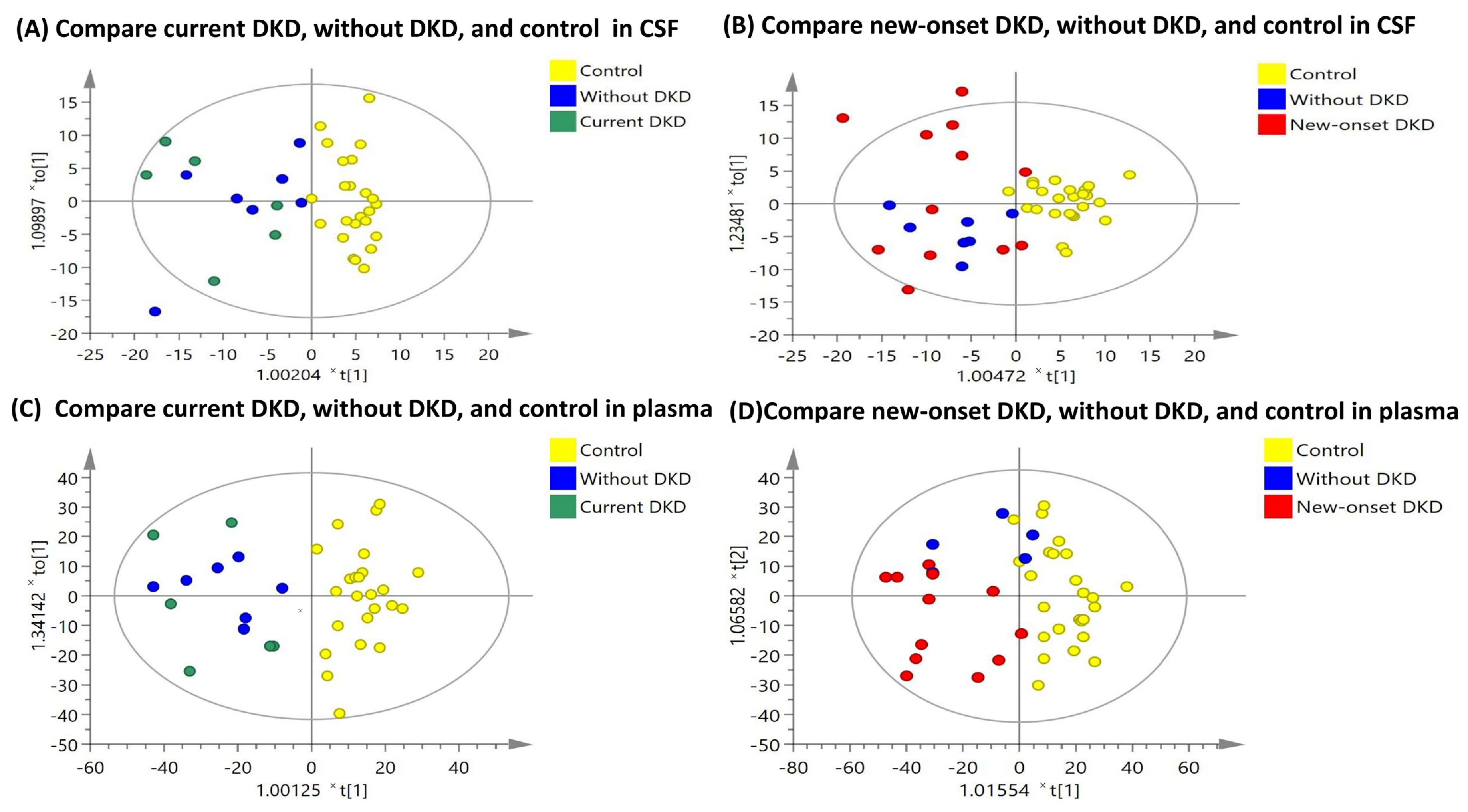
3.2. OPLS-DA Score Plots
3.3. Metabolomic Comparison between the Patients with DKD versus the Control Participants
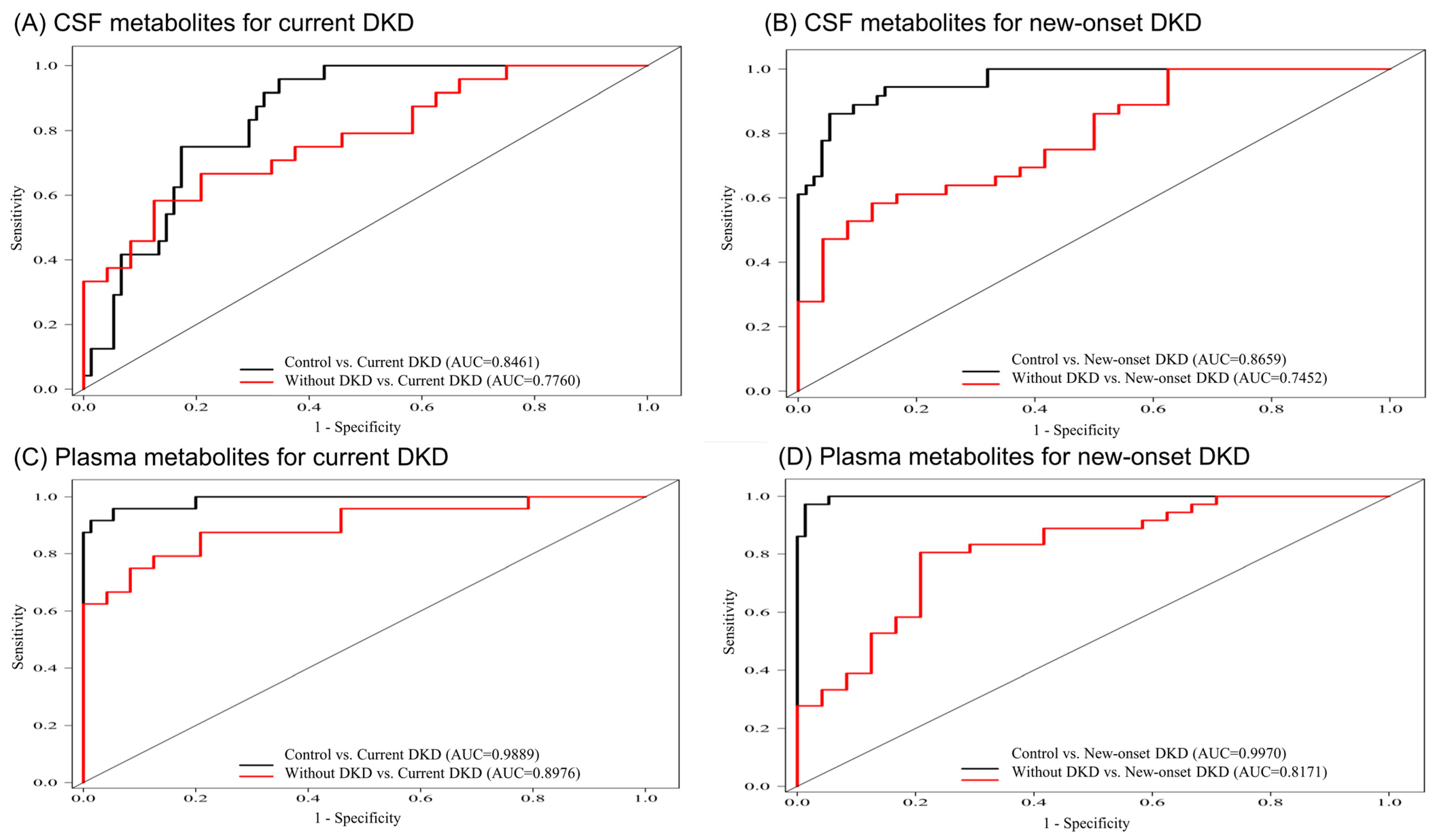
3.4. Metabolite Combinations for Correlating with DKD
3.5. Correlation Analysis of the Altered Metabolites with UACR and eGFR
3.6. Enrichment Analysis and Metabolic Pathways of the Altered Metabolites in DKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qiao, S.; Shi, C.; Wang, S.; Ji, G. Metabolomics window into diabetic complications. J. Diabetes Investig. 2018, 9, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khater, S.I.; Mohamed, A.A.; Arisha, A.H.; Ebraheim, L.L.M.; El-Mandrawy, S.A.M.; Nassan, M.A.; Mohammed, A.T.; Abdo, S.A. Stabilized-chitosan selenium nanoparticles efficiently reduce renal tissue injury and regulate the expression pattern of aldose reductase in the diabetic-nephropathy rat model. Life Sci. 2021, 279, 119674. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.; Tiribelli, C.; Bellarosa, C. Translational Approach to the Protective Effect of Bilirubin in Diabetic Kidney Disease. Biomedicines 2022, 10, 696. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Yang, H.; Liu, S.; Lu, Y.; Fu, P.; Liu, J. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of DKD in T2DM patients. Mol. Biosyst. 2017, 13, 2392–2400. [Google Scholar] [CrossRef]
- Solini, A.; Manca, M.L.; Penno, G.; Pugliese, G.; Cobb, J.E.; Ferrannini, E. Prediction of Declining Renal Function and Albuminuria in Patients With Type 2 Diabetes by Metabolomics. J. Clin. Endocrinol. Metab. 2016, 101, 696–704. [Google Scholar] [CrossRef]
- Xu, T.; Xu, X.; Zhang, L.; Zhang, K.; Wei, Q.; Zhu, L.; Yu, Y.; Xiao, L.; Lin, L.; Qian, W.; et al. Lipidomics Reveals Serum Specific Lipid Alterations in Diabetic Nephropathy. Front. Endocrinol. 2021, 12, 781417. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Cheng, M.L.; Lo, C.J.; Lin, G.; Lin, S.F.; Yeh, J.T.; Ho, H.Y.; Lin, J.R.; Liu, F.C. (1)H Nuclear Magnetic Resonance (NMR)-Based Cerebrospinal Fluid and Plasma Metabolomic Analysis in Type 2 Diabetic Patients and Risk Prediction for Diabetic Microangiopathy. J. Clin. Med. 2019, 8, 874. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Ghosh, S.; Kovalik, J.P.; Ching, J.; Choi, H.W.; Tavintharan, S.; Ong, C.N.; Sum, C.F.; Summers, S.A.; Tai, E.S.; et al. Profiling of Plasma Metabolites Suggests Altered Mitochondrial Fuel Usage and Remodeling of Sphingolipid Metabolism in Individuals With Type 2 Diabetes and Kidney Disease. Kidney Int. Rep. 2017, 2, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breneman, C.B.; Tucker, L. Dietary fibre consumption and insulin resistance—The role of body fat and physical activity. Br. J. Nutr. 2013, 110, 375–383. [Google Scholar] [CrossRef] [Green Version]
- The Human Metabolome Database (HMDB). Available online: http://www.hmdb.ca/ (accessed on 18 May 2022).
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Jove, M.; Mate, I.; Naudi, A.; Mota-Martorell, N.; Portero-Otin, M.; De la Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Li, H.; Zhang, L.; Wang, Y.; Long, Y.; Li, R.; Qiu, W.; Lu, Z.; Hu, X.; Peng, F. Elevated cerebrospinal fluid uric acid during relapse of neuromyelitis optica spectrum disorders. Brain Behav. 2017, 7, e00584. [Google Scholar] [CrossRef]
- Chang, S.H.; Tian, X.B.; Wang, J.; Liu, M.Q.; Huang, C.N.; Qi, Y.; Zhang, L.J.; Gao, C.L.; Zhang, D.Q.; Sun, L.S.; et al. Increased Cerebrospinal Fluid Uric Acid Levels in Guillain-Barre Syndrome. Front. Neurol. 2020, 11, 589928. [Google Scholar] [CrossRef]
- Gul, A.; Zager, P. Does Altered Uric Acid Metabolism Contribute to Diabetic Kidney Disease Pathophysiology? Curr. Diab. Rep. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Tofte, N.; Persson, F.; Rossing, P. Omics research in diabetic kidney disease: New biomarker dimensions and new understandings? J. Nephrol. 2020, 33, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Tu, Y.; Jiang, F.; Wang, J.; Zhang, R.; Sun, X.; Wang, T.; Wang, S.; Bao, Y.; Hu, C.; et al. Uric Acid is independently associated with diabetic kidney disease: A cross-sectional study in a Chinese population. PLoS ONE 2015, 10, e0129797. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bellini, S.; Canepa, S.; Durazzo, M.; Gruden, G. Novel biomarkers of diabetic kidney disease: Current status and potential clinical application. Acta Diabetol. 2021, 58, 819–830. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, R.; Cui, W.; Wang, L.; Xiao, J.; Shang, J.; Zhao, Z. Untargeted serum metabolomics and tryptophan metabolism profiling in type 2 diabetic patients with diabetic glomerulopathy. Ren. Fail. 2021, 43, 980–992. [Google Scholar] [CrossRef]
- Chou, C.A.; Lin, C.N.; Chiu, D.T.; Chen, I.W.; Chen, S.T. Tryptophan as a surrogate prognostic marker for diabetic nephropathy. J. Diabetes Investig. 2018, 9, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Crotty, G.F.; Maciuca, R.; Macklin, E.A.; Wang, J.; Montalban, M.; Davis, S.S.; Alkabsh, J.I.; Bakshi, R.; Chen, X.; Ascherio, A.; et al. Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: A metabolomic study. Neurology 2020, 95, e3428–e3437. [Google Scholar] [CrossRef]
- Sachse, K.T.; Jackson, E.K.; Wisniewski, S.R.; Gillespie, D.G.; Puccio, A.M.; Clark, R.S.; Dixon, C.E.; Kochanek, P.M. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J. Cereb Blood Flow Metab. 2008, 28, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Mu, X.; Yang, M.; Ling, P.; Wu, A.; Zhou, H.; Jiang, J. Acylcarnitines: Can They Be Biomarkers of Diabetic Nephropathy? Diabetes Metab. Syndr. Obes. 2022, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Esmati, P.; Najjar, N.; Emamgholipour, S.; Hosseinkhani, S.; Arjmand, B.; Soleimani, A.; Kakaii, A.; Razi, F. Mass spectrometry with derivatization method for concurrent measurement of amino acids and acylcarnitines in plasma of diabetic type 2 patients with diabetic nephropathy. J. Diabetes Metab. Disord. 2021, 20, 591–599. [Google Scholar] [CrossRef]
- Uludag, K.; Oguzhan, N.; Arikan, T.; Boz, G. Serum bilirubin level and its impact on the progression of chronic kidney disease. Int. Urol. Nephrol. 2018, 50, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Weber, W.M.; Mak, T.D.; Chung, J.; Doyle-Eisele, M.; Melo, D.R.; Brenner, D.J.; Guilmette, R.A.; Fornace, A.J., Jr. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, cesium-137, using a shotgun LC-MS(E) approach. J. Proteome Res. 2015, 14, 374–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauer, M.; Doria, A. Uric acid and risk of diabetic kidney disease. J. Nephrol. 2020, 33, 995–999. [Google Scholar] [CrossRef] [PubMed]


| Variables | Control (n = 25) | Without DKD (n = 8) | Current DKD (n = 8) c | New-Onset DKD (n = 12) c | p |
|---|---|---|---|---|---|
| Male sex, N (%) | 11 (45.83%) | 7 (87.50%) | 5 (62.50%) | 10 (83.33%) | 0.010 * |
| Age (mean ± SD, years) | 50.72 ± 14.67 | 59.37 ± 8.75 | 51.25 ± 10.49 | 60.33 ± 8.95 | 0.086 |
| BMI (kg/m2) | 21.99 ± 3.49 | 26.89 ± 4.19 | 27.86 ± 5.66 | 24.55 ± 3.79 | 0.002 * |
| DM duration (years) | NA | 4.87 ± 2.10 | 10.62 ± 4.59 | 11.08 ± 8.08 | 0.074 |
| Current medications, N (%) | |||||
| Insulin injection | NA | 1 (12.50%) | 6 (75.00%) | 7 (58.33%) | 0.030 * |
| Anti-hypertensive agents | 1 (4.16%) | 4 (50.00%) | 2 (25.00%) | 5 (41.66%) | 0.012 * |
| Lipid-modifying agents | 1 (4.16%) | 2 (25.00%) | 3 (37.50%) | 5 (41.66%) | 0.028 * |
| Biochemical parameters | |||||
| CSF glucose (mg/dL) | 55.23 ± 21.12 | 85.90 ± 32.57 | 79.79 ± 31.95 | 78.79 ± 22.59 | 0.006 * |
| CSF insulin (mU/L) | 0.21 ± 0.10 | 0.36 ± 0.21 | 0.21 ± 0.18 | 0.26 ± 0.14 | 0.195 |
| Plasma glucose (mg/dL) | 93.07 ± 18.37 | 181.68 ± 69.21 | 158.89 ± 84.33 | 160.43 ± 73.11 | <0.001 * |
| Plasma HbA1c (%) a | 5.73 ± 0.32 | 10.08 ± 2.62 | 11.46 ± 2.76 | 9.95 ± 2.31 | 0.017 * |
| Plasma insulin (mU/L) | 7.25 ± 4.78 | 12.65 ± 5.96 | 7.20 ± 5.07 | 7.88 ± 2.72 | 0.044 * |
| Plasma HOMA-IR b | 2.03 ± 1.65 | 5.66 ± 4.09 | 3.46 ± 4.65 | 3.18 ± 1.76 | 0.024 * |
| Renal function in 2017 | |||||
| Serum creatinine (mg/dL) | 0.78 ± 0.26 | 1.02 ± 0.28 | 1.62 ± 1.04 | 1.08 ± 0.46 | 0.002 * |
| eGFR (ml/min per1.73 m2) | 109.04 ± 25.56 | 93.75 ± 24.78 | 78.12 ± 35.39 | 98.00 ± 17.85 | 0.033 * |
| UACR | NA | 14.24 ± 10.06 | 33.02 ± 18.86 | 2191.75 ± 1419.63 | 0.002 * |
| Dialysis in 2017 | NA | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Renal function in 2021 | |||||
| Serum creatinine (mg/dL) | 0.81 ± 0.18 | 0.82 ± 0.31 | 6.20 ± 4.26 | 1.69 ± 1.03 | <0.001 * |
| eGFR (ml/min per1.73 m2) | 95.64 ± 24.15 | 125.75 ± 85.18 | 26.63 ± 34.84 | 57.92 ± 27.70 | <0.001 * |
| UACR | NA | 15.30 ± 12.87 | 5480.87± 2706.05 | 1420.00 ± 1234.02 | <0.001 * |
| Dialysis in 2021 | NA | 0 (0%) | 3 (37.50%) | 0 (0%) | 0.034 * |
| Diabetic retinopathy | NA | 2 (25.00%) | 7 (87.50%) | 9 (75.00%) | 0.039 * |
| Diabetic neuropathy | NA | 0 (0%) | 3 (37.50%) | 5 (41.66%) | 0.111 |
| Metabolites in CSF | LC-MS Signal Integration (Mean ± SD) (×10−3 a.u.) | Adjusted Fold Change a,# | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Without DKD | Current DKD | New-Onset DKD | Compared with Control | Compared with without DKD | |||
| Current DKD | New-Onset DKD | Current DKD | New-Onset DKD | |||||
| Proline betaine | 10.12 ± 4.48 | 61.74 ± 8.85 | 62.16 ± 8.13 | 52.17 ± 6.57 | 5.139 * | 5.155 * | 1.035 | 0.845 |
| Tryptophan | 36.12 ± 5.16 | 54.63 ± 10.18 | 52.16 ± 7.07 | 112.74 ± 7.56 | 1.423 * | 3.121 * | 0.968 | 2.063 * |
| D-glucose | 83.76 ± 1.94 | 98.05 ± 3.82 | 103.93 ± 3.94 | 95.99 ± 2.83 | 1.245 * | 1.145 * | 1.066 | 0.979 |
| Phenylalanine | 135.66 ± 3.65 | 143.32 ± 7.21 | 132.82 ± 6.35 | 147.24 ± 5.35 | 0.954 | 1.085 | 0.997 | 1.027 |
| Uric acid | 52.31 ± 2.19 | 46.67 ± 4.32 | 72.03 ± 4.10 | 55.63 ± 3.21 | 1.363 * | 1.063 | 1.523 * | 1.192 |
| L-acetylcarnitine | 29.96 ± 1.37 | 31.98 ± 2.69 | 37.65 ± 2.33 | 28.57 ± 2.00 | 1.208 * | 0.953 | 1.192 | 0.893 |
| Paraxanthine | 19.89 ± 2.87 | 14.89 ± 5.67 | 2.43 ± 5.19 | 17.32 ± 4.21 | 0.056 * | 0.871 | 0.074 * | 1.163 |
| Hypoxanthine | 30.79 ± 0.87 | 31.28 ± 1.72 | 29.64 ± 1.46 | 25.79 ± 1.27 | 0.985 | 0.837 * | 0.916 | 0.825 * |
| Creatinine | 14.34 ± 0.32 | 13.81 ± 0.64 | 13.67 ± 0.61 | 11.77 ± 0.47 | 0.957 | 0.821 * | 1.015 | 0.852 * |
| Metabolites in Plasma | LC-MS Signal Integration (Mean ± SD) (×10−3 a.u.) | Adjusted Fold Change a,# | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Without DKD | Current DKD | New-Onset DKD | Compared with Control | Compared with without DKD | |||
| Current DKD | New-Onset DKD | Current DKD | New-Onset DKD | |||||
| Proline betaine | 9.02 ± 4.46 | 53.74 ± 8.79 | 62.97 ± 7.39 | 50.93 ± 6.53 | 6.187 * | 5.646 * | 1.285 | 0.947 |
| Uric acid | 10.92 ± 0.68 | 14.85 ± 1.35 | 24.67 ± 1.08 | 20.95 ± 1.00 | 2.253 * | 1.918 * | 1.668 * | 1.411 * |
| D-glucose | 37.27 ± 1.38 | 59.35 ± 2.74 | 61.27 ± 2.78 | 65.02 ± 2.03 | 1.664 * | 1.744 * | 1.049 | 1.096 |
| L-acetylcarnitine | 28.16 ± 1.29 | 37.84 ± 2.55 | 47.81 ± 2.38 | 39.36 ± 1.89 | 1.649 * | 1.398 * | 1.163 * | 1.042 |
| Phenylalanine | 28.40 ± 1.04 | 35.78 ± 2.06 | 32.44 ± 1.25 | 39.50 ± 1.53 | 1.134 * | 1.391 * | 0.944 | 1.103 |
| Bilirubin | 3.63 ± 0.23 | 3.28 ± 0.45 | 1.85 ± 0.43 | 2.38 ± 0.33 | 0.476 * | 0.656 * | 0.561 * | 0.726 |
| Edetic acid | 538.88 ± 19.47 | 331.87 ± 38.43 | 406.57 ± 36.52 | 337.50 ± 28.51 | 0.773 * | 0.626 * | 1.109 | 1.059 |
| PE 38:4 | 1.79 ± 0.53 | 7.85 ± 1.06 | 4.35 ± 0.91 | 7.99 ± 0.78 | 2.075 * | 4.459 * | 0.388 * | 1.018 |
| PC 34:1 | 256.56 ± 8.13 | 349.48 ± 16.05 | 325.19 ± 18.49 | 356.50 ± 11.91 | 1.134 | 1.389 * | 0.914 | 1.020 |
| PC 32:0 | 9.42 ± 0.72 | 20.09 ± 1.41 | 13.04 ± 1.29 | 18.37 ± 1.05 | 1.191 | 1.949 * | 0.578 * | 0.914 |
| PC 36:1 | 13.41 ± 1.74 | 28.32 ± 3.42 | 16.74 ± 3.51 | 25.66 ± 2.54 | 0.966 | 1.915 * | 0.583 * | 0.906 |
| PC 38:6 | 40.26 ± 2.38 | 25.86± 4.71 | 22.82 ± 4.44 | 14.37 ± 3.49 | 0.543 * | 0.357 * | 0.806 | 0.558 * |
| PC 36:4 | 84.35 ± 6.46 | 77.60 ± 12.76 | 27.48 ± 2.44 | 20.19 ± 9.46 | 0.358 * | 0.239 * | 0.390 * | 0.226 * |
| PC 38:3 | 19.03 ± 2.93 | 30.59 ± 5.58 | 12.72 ± 4.42 | 34.77 ± 4.14 | 0.546 | 1.827 * | 0.405 * | 1.136 |
| LysoPC 16:0 | 37.22 ± 0.85 | 31.11 ± 1.69 | 32.24 ± 1.36 | 29.18 ± 1.25 | 0.884 * | 0.784 * | 1.072 | 0.938 |
| LysoPC 20:4 | 62.53 ± 2.73 | 43.35 ± 5.39 | 38.15 ± 4.38 | 46.91 ± 4.00 | 0.638 * | 0.750 * | 0.889 | 1.082 |
| LysoPC 18:2 | 346.08 ± 9.54 | 227.07 ± 18.84 | 212.09 ± 16.21 | 172.17 ± 13.97 | 0.636 * | 0.497 * | 0.965 | 0.758 * |
| Significantly Changed Metabolites in CSF | AIC # | AUC | Adjusted OR a,# | AIC # | AUC | Adjusted OR a,# |
|---|---|---|---|---|---|---|
| Correlating with current DKD | Control vs. Current DKD | Without DKD vs. Current DKD | ||||
| Paraxanthine | 98.80 | 0.73 | 0.919 * | 68.52 | 0.63 | 0.990 |
| Uric acid | 89.26 | 0.84 | 1.048 * | 60.33 | 0.74 | 1.026 |
| Paraxanthine, uric acid | 85.27 | 0.85 | 2.718 * | 62.31 | 0.75 | 2.718 * |
| Correlating with new-onset DKD | Control vs. New-onset DKD | Without DKD vs. New-onset DKD | ||||
| Tryptophan | 80.19 | 0.86 | 2.718 * | 73.46 | 0.745 | 1.018 * |
| Creatinine | 101.32 | 0.85 | 0.559 * | 82.06 | 0.57 | 0.772 * |
| Hypoxanthine | 127.36 | 0.71 | 0.867 * | 78.34 | 0.63 | 0.949 |
| Creatinine, Tryptophan | 58.68 | 0.95 | 2.718 * | 74.47 | 0.74 | 2.718 * |
| Creatinine, hypoxanthine, tryptophan | 55.52 | 0.96 | 2.718 * | 70.47 | 0.79 | 2.718 * |
| Significantly Changed Metabolites in Plasma | AIC # | AUC | Adjusted OR a,# | AIC# | AUC | Adjusted OR a,# |
|---|---|---|---|---|---|---|
| Correlating with current DKD | Control vs. Current DKD | Without DKD vs. Current DKD | ||||
| Bilirubin | 101.95 | 0.72 | 0.605 * | 64.54 | 0.65 | 0.663 * |
| PC 36:4 | 90.03 | 0.77 | 0.823 * | 63.87 | 0.66 | 0.885 * |
| PE 38:4 | 83.71 | 0.82 | 1.502 * | 62.67 | 0.69 | 0.879 * |
| Uric acid | 56.72 | 0.93 | 1.366 * | 50.09 | 0.88 | 1.241 * |
| L-acetylcarnitine | 56.72 | 0.93 | 1.237 * | 64.59 | 0.77 | 1.057 * |
| L-Acetylcarnitine, Uric acid | 34.73 | 0.98 | 2.718 * | 51.68 | 0.88 | 2.718 * |
| L-Acetylcarnitine, Uric acid, PC 36:4 | 36.32 | 0.98 | 2.718 * | 52.30 | 0.87 | 2.717 * |
| L-Acetylcarnitine, uric acid, PC 36:4, PE 38:4 | 11.62 | 0.99 | 2.718 * | 48.26 | 0.90 | 2.718 * |
| Correlating with new-onset DKD | Control vs. New-onset DKD | Without DKD vs. New-onset DKD | ||||
| LysoPC 18:2 | 56.39 | 0.95 | 0.9681 * | 83.18 | 0.59 | 0.993 * |
| Uric acid | 88.51 | 0.92 | 1.3038 * | 78.27 | 0.63 | 1.138 * |
| PC 36:4 | 92.03 | 0.88 | 0.9641 * | 72.05 | 0.72 | 0.969 * |
| PC 38:6 | 101.38 | 0.85 | 0.9234 * | 80.29 | 0.65 | 0.967 * |
| Uric acid, PC 38:6 | 22.26 | 0.99 | 2.718 * | 75.30 | 0.72 | 2.718 * |
| Uric acid, PC 36:4 | 75.68 | 0.94 | 2.718 * | 64.61 | 0.82 | 2.718 * |
| Uric acid, PC 38:6, PC 36:4 | 23.96 | 0.99 | 2.718 | 68.47 | 0.82 | 2.718 * |
| Renal Function Measurement | Urinary Albumin/Creatinine Ratio | eGFR | ||
|---|---|---|---|---|
| Correlation with Log2 Transformed Metabolites | Adjusted r a,# | Adjusted r a,# | ||
| Significantly changed metabolites in CSF | 2017 | 2021 | 2017 | 2021 |
| Tryptophan | −0.132 | −0.083 | −0.075 | −0.086 |
| D-glucose | −0.136 | −0.407 * | 0.084 | 0.343 * |
| Uric acid | 0.316 * | 0.353 * | −0.244 * | −0.076 |
| Paraxanthine | −0.228 * | −0.309 * | 0.363 * | 0.051 |
| Hypoxanthine | 0.252 * | 0.381 * | −0.403 * | −0.135 |
| Creatinine | 0.021 | −0.073 | 0.343 * | 0.313 * |
| Significantly changed metabolites in plasma | 2017 | 2021 | 2017 | 2021 |
| Uric acid | 0.232 * | 0.306 * | −0.375 * | −0.302 * |
| L-Acetylcarnitine | 0.186 | 0.514 * | −0.513 * | −0.221 * |
| Bilirubin | −0.073 | 0.029 | 0.170 | 0.375 *- |
| LysoPC 18:2 | 0.159 | −0.144 | 0.239 * | 0.299 |
| PC 38:6 | −0.136 | −0.109 | 0.122 | 0.229 * |
| PC 36:4 | −0.321 * | −0.250 * | 0.253 * | 0.042 |
| PE 38:4 | −0.429 * | −0.344 * | 0.300 * | 0.279 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-T.; Cheng, M.-L.; Lo, C.-J.; Lin, G.; Liu, F.-C. Metabolomic Signature of Diabetic Kidney Disease in Cerebrospinal Fluid and Plasma of Patients with Type 2 Diabetes Using Liquid Chromatography-Mass Spectrometry. Diagnostics 2022, 12, 2626. https://doi.org/10.3390/diagnostics12112626
Lin H-T, Cheng M-L, Lo C-J, Lin G, Liu F-C. Metabolomic Signature of Diabetic Kidney Disease in Cerebrospinal Fluid and Plasma of Patients with Type 2 Diabetes Using Liquid Chromatography-Mass Spectrometry. Diagnostics. 2022; 12(11):2626. https://doi.org/10.3390/diagnostics12112626
Chicago/Turabian StyleLin, Huan-Tang, Mei-Ling Cheng, Chi-Jen Lo, Gigin Lin, and Fu-Chao Liu. 2022. "Metabolomic Signature of Diabetic Kidney Disease in Cerebrospinal Fluid and Plasma of Patients with Type 2 Diabetes Using Liquid Chromatography-Mass Spectrometry" Diagnostics 12, no. 11: 2626. https://doi.org/10.3390/diagnostics12112626







