Synergies of Radiomics and Transcriptomics in Lung Cancer Diagnosis: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.1.1. Radiomics Dataset
2.1.2. Transcriptomics Datasets
2.2. Radiotranscriptomics Feature Extraction
2.2.1. Meta-Radiomics Signature Extraction
2.2.2. Transcriptomics Signature Extraction
2.2.3. p-Metaomics Signature Modeling
2.3. p-Metaomics Model Evaluation
2.4. Enrichment Analysis
3. Results
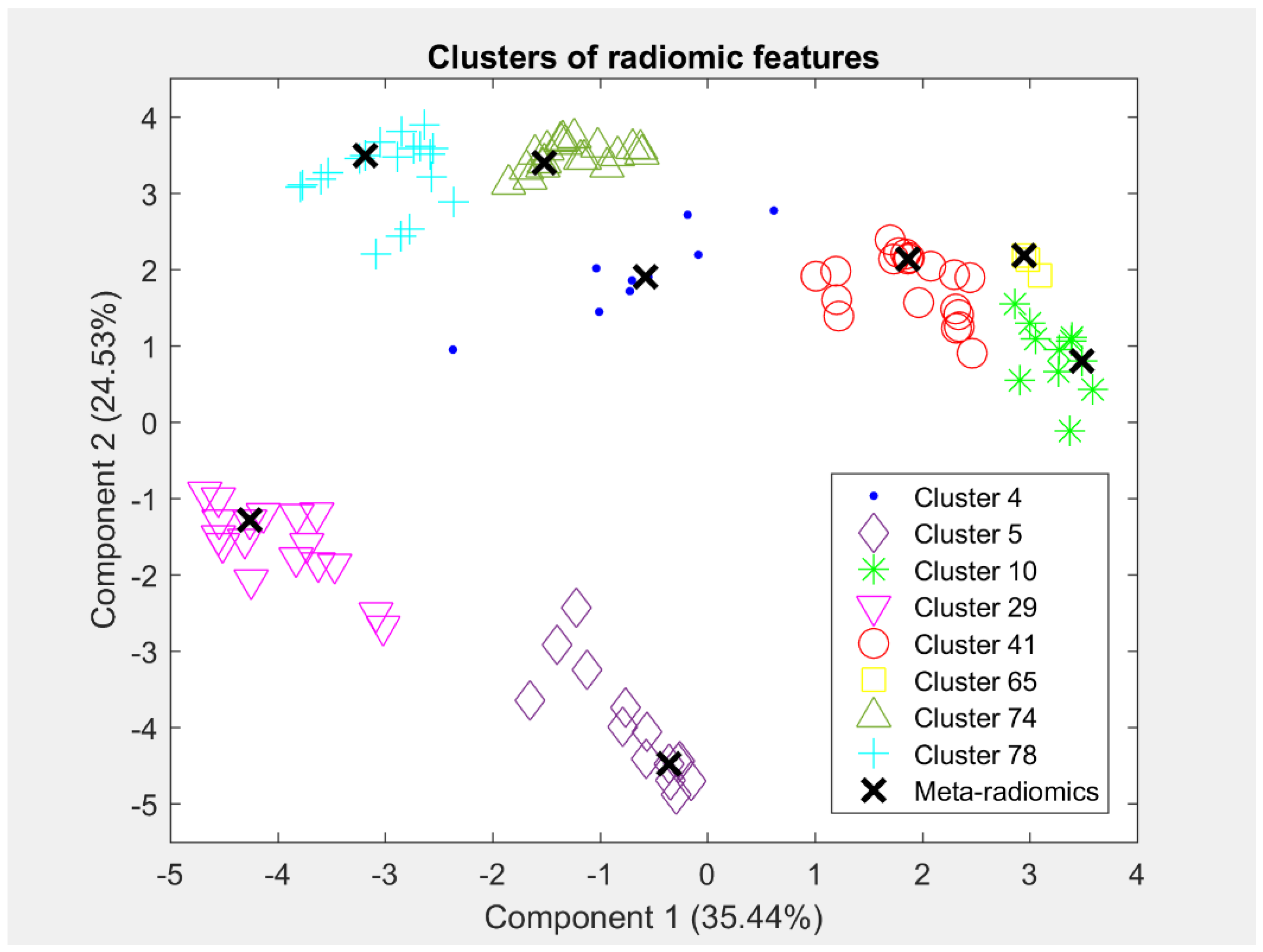
3.1. Meta-Radiomics Signature
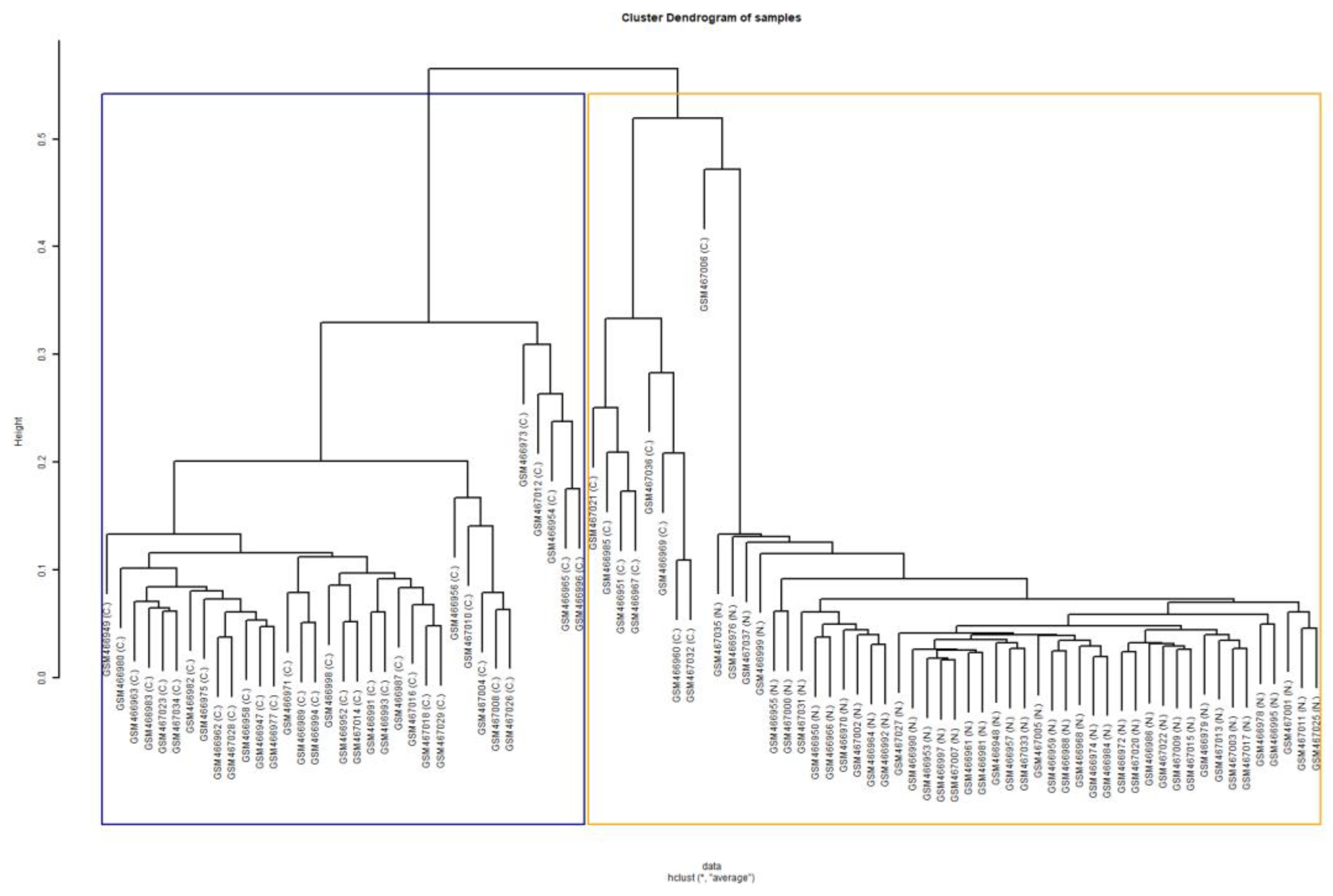
3.2. Transcriptomics Signature
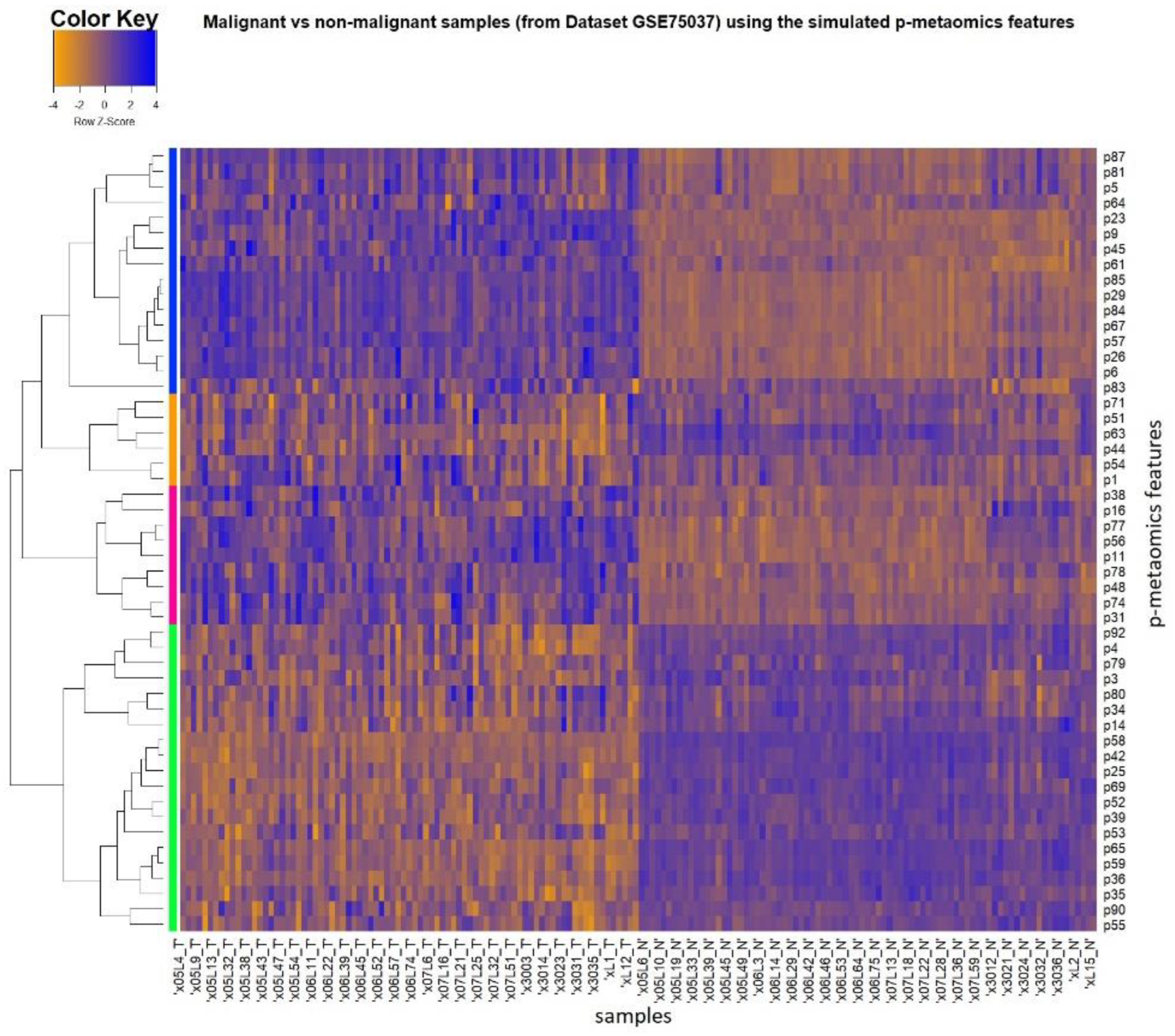
3.3. p-Metaomics Signature
3.4. Evaluation of the Discrimination Ability of p-Metaomics Models
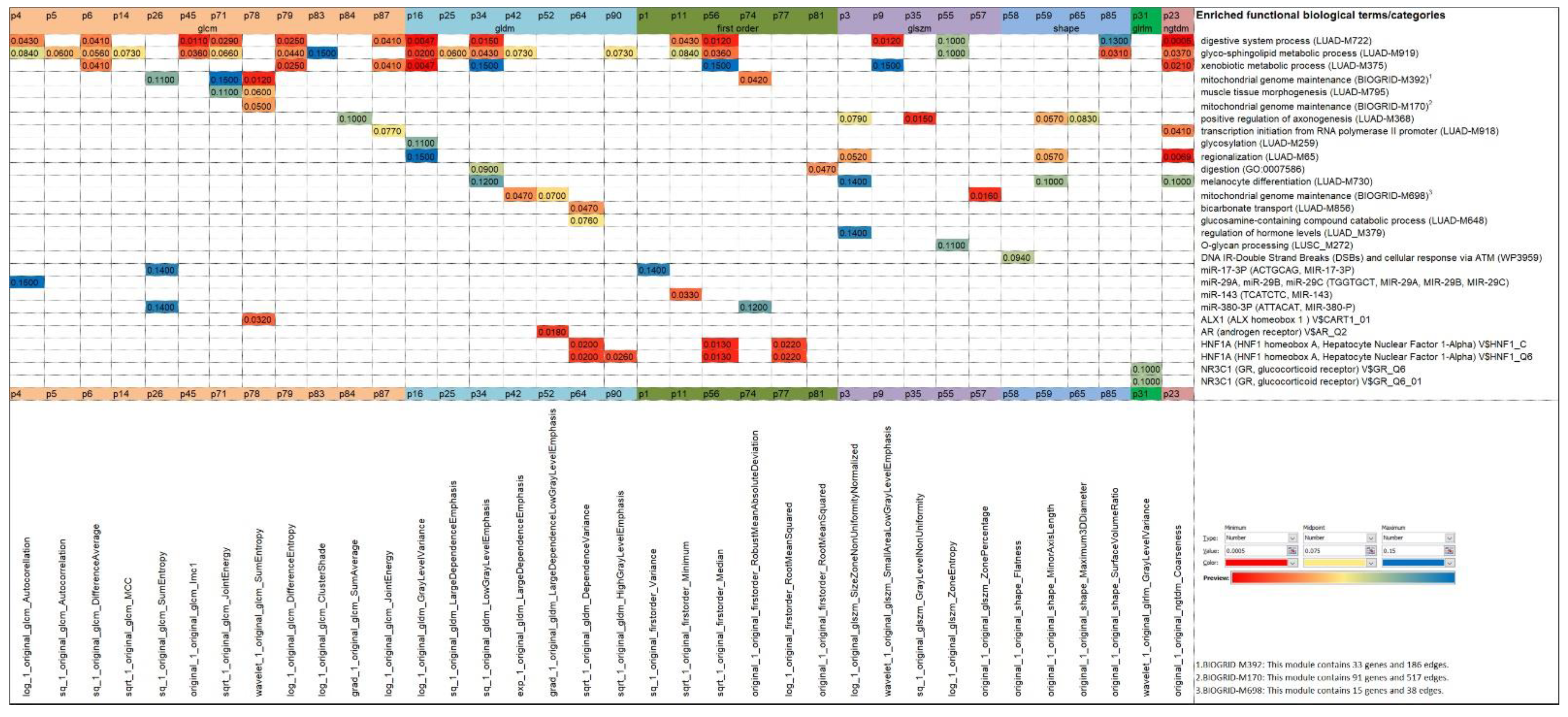
3.5. Enrichment Analysis of Radiotranscriptomics Models
3.5.1. Overrepresentation Analysis on DEGs
3.5.2. Biological Interpretation of Radiomics via the p-Metaomics Models
3.5.3. Evaluation of Significant Genes in Independent Lung Cancer Datasets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining Molecular and Imaging Metrics in Cancer: Radiogenomics. Insights Imaging 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Panth, K.M.; Leijenaar, R.T.H.; Carvalho, S.; Lieuwes, N.G.; Yaromina, A.; Dubois, L.; Lambin, P. Is There a Causal Relationship between Genetic Changes and Radiomics-Based Image Features? An in Vivo Preclinical Experiment with Doxycycline Inducible GADD34 Tumor Cells. Radiother. Oncol. 2015, 116, 462–466. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Thrall, J.H. Moreton Lecture: Imaging in the Age of Precision Medicine. J. Am. Coll. Radiol. 2015, 12, 1106–1111. [Google Scholar] [CrossRef]
- Trivizakis, E.; Papadakis, G.Z.; Souglakos, I.; Papanikolaou, N.; Koumakis, L.; Spandidos, D.A.; Tsatsakis, A.; Karantanas, A.H.; Marias, K. Artificial Intelligence Radiogenomics for Advancing Precision and Effectiveness in Oncologic Care (Review). Int. J. Oncol. 2020, 57, 43–53. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging Imaging and Genomics. Abdom. Radiol. 2019, 44, 1960–1984. [Google Scholar] [CrossRef]
- Li, X.; Yin, G.; Zhang, Y.; Dai, D.; Liu, J.; Chen, P.; Zhu, L.; Ma, W.; Xu, W. Predictive Power of a Radiomic Signature Based on 18F-FDG PET/CT Images for EGFR Mutational Status in NSCLC. Front. Oncol. 2019, 9, 1062. [Google Scholar] [CrossRef]
- Le, N.Q.K.; Kha, Q.H.; Nguyen, V.H.; Chen, Y.C.; Cheng, S.J.; Chen, C.Y. Machine Learning-Based Radiomics Signatures for Egfr and Kras Mutations Prediction in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9254. [Google Scholar] [CrossRef]
- Shiri, I.; Maleki, H.; Hajianfar, G.; Abdollahi, H.; Ashrafinia, S.; Hatt, M.; Zaidi, H.; Oveisi, M.; Rahmim, A. Next-Generation Radiogenomics Sequencing for Prediction of EGFR and KRAS Mutation Status in NSCLC Patients Using Multimodal Imaging and Machine Learning Algorithms. Mol. Imaging Biol. 2020, 22, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Bonfante, M.; Zurek, E.; Cherezov, D.; Goldgof, D.; Hall, L.; Schabath, M. A Radiogenomics Ensemble to Predict EGFR and KRAS Mutations in NSCLC. Tomography 2021, 7, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, G.; Pereira, T.; Dias, C.; Freitas, C.; Hespanhol, V.; Costa, J.L.; Cunha, A.; Oliveira, H.P. Identifying Relationships between Imaging Phenotypes and Lung Cancer-Related Mutation Status: EGFR and KRAS. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, O.; Echegaray, S.; Khuong, A.; Hoang, C.D.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Guo, H.H.; Lau, C.; Plevritis, S.K.; et al. Predictive Radiogenomics Modeling of EGFR Mutation Status in Lung Cancer. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Nair, V.S.; Gevaert, O.; Davidzon, G.; Napel, S.; Graves, E.E.; Hoang, C.D.; Shrager, J.B.; Quon, A.; Rubin, D.L.; Plevritis, S.K. Prognostic PET 18F-FDG Uptake Imaging Features Are Associated with Major Oncogenomic Alterations in Patients with Resected Non-Small Cell Lung Cancer. Cancer Res. 2012, 72, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, J.; Cha, H.; Park, W.Y.; Ahn, J.S.; Ahn, M.J.; Park, K.; Park, Y.J.; Choi, J.Y.; Lee, K.H.; et al. Metabolic Radiogenomics in Lung Cancer: Associations between FDG PET Image Features and Oncogenic Signaling Pathway Alterations. Sci. Rep. 2020, 10, 13231. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, O.; Leung, A.N.; Quon, A.; Rubin, D.L.; Napel, S.; Xu, J.; Hoang, C.D.; Xu, Y.; Plevritis, S.K. Identifying Prognostic Imaging Biomarkers by Leveraging Public Gene Expression Microarray Data. Radiology 2012, 264, 387–396. [Google Scholar] [CrossRef]
- Zhou, M.; Leung, A.; Echegaray, S.; Gentles, A.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Plevritis, S.K.; Rubin, D.L.; Napel, S.; et al. Non-Small Cell Lung Cancer Radiogenomics Map Identifies Relationships between Molecular and Imaging Phenotypes with Prognostic Implications. Radiology 2018, 286, 307–315. [Google Scholar] [CrossRef]
- Smedley, N.F.; Aberle, D.R.; Hsu, W. Identifying Transcription Patterns of Histology and Radiomics Features in NSCLC with Neural Networks. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Sandy, N.; Plevritis, S.K. NSCLC Radiogenomics: Initial Stanford Study of 26 Cases. Cancer Imaging Arch. 2014. [Google Scholar] [CrossRef]
- Bakr, S.; Gevaert, O.; Echegaray, S.; Ayers, K.; Zhou, M.; Shafiq, M.; Zheng, H.; Benson, J.A.; Zhang, W.; Leung, A.N.C.; et al. Data Descriptor: A Radiogenomic Dataset of Non-Small Cell Lung Cancer. Sci. Data 2018, 5, 180202. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.; Rodriguez-Canales, J.; Behrens, C.; Thompson, D.M.; Botros, I.W.; Tang, H.; Xie, Y.; Rekhtman, N.; Travis, W.D.; Wistuba, I.I.; et al. An Expression Signature as an Aid to the Histologic Classification of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 4880–4889. [Google Scholar] [CrossRef]
- Morrow, J.D.; Zhou, X.; Lao, T.; Jiang, Z.; Demeo, D.L.; Cho, M.H.; Qiu, W.; Cloonan, S.; Pinto-Plata, V.; Celli, B.; et al. Functional Interactors of Three Genome-Wide Association Study Genes Are Differentially Expressed in Severe Chronic Obstructive Pulmonary Disease Lung Tissue. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Sanchez-Palencia, A.; Gomez-Morales, M.; Gomez-Capilla, J.A.; Pedraza, V.; Boyero, L.; Rosell, R.; Fárez-Vidal, M.E. Gene Expression Profiling Reveals Novel Biomarkers in Nonsmall Cell Lung Cancer. Int. J. Cancer 2011, 129, 355–364. [Google Scholar] [CrossRef]
- Wei, T.Y.W.; Juan, C.C.; Hisa, J.Y.; Su, L.J.; Lee, Y.C.G.; Chou, H.Y.; Chen, J.M.M.; Wu, Y.C.; Chiu, S.C.; Hsu, C.P.; et al. Protein Arginine Methyltransferase 5 Is a Potential Oncoprotein That Upregulates G1 Cyclins/Cyclin-Dependent Kinases and the Phosphoinositide 3-Kinase/AKT Signaling Cascade. Cancer Sci. 2012, 103, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.W.; Hsia, J.Y.; Chiu, S.C.; Su, L.J.; Juan, C.C.; Lee, Y.C.G.; Chen, J.M.M.; Chou, H.Y.; Huang, J.Y.; Huang, H.M.; et al. Methylosome Protein 50 Promotes Androgen- and Estrogen-Independent Tumorigenesis. Cell. Signal. 2014, 26, 2940–2950. [Google Scholar] [CrossRef]
- Rousseaux, S.; Debernardi, A.; Jacquiau, B.; Vitte, A.L.; Vesin, A.; Nagy-Mignotte, H.; Moro-Sibilot, D.; Brichon, P.Y.; Lantuejoul, S.; Hainaut, P.; et al. Ectopic Activation of Germline and Placental Genes Identifies Aggressive Metastasis-Prone Lung Cancers. Sci. Transl. Med. 2013, 5, 186ra66. [Google Scholar] [CrossRef]
- Seo, J.S.; Ju, Y.S.; Lee, W.C.; Shin, J.Y.; Lee, J.K.; Bleazard, T.; Lee, J.; Jung, Y.J.; Kim, J.O.; Shin, J.Y.; et al. The Transcriptional Landscape and Mutational Profile of Lung Adenocarcinoma. Genome Res. 2012, 22, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, M. Ckmeans.1d.Dp: Optimal k-Means Clustering in One Dimension by Dynamic Programming. R J. 2011, 3, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Bolshakova, N.; Azuaje, F. Cluster Validation Techniques for Genome Expression Data. Signal Process. 2003, 83, 825–833. [Google Scholar] [CrossRef]
- Ristevski, B.; Loshkovska, S.; Dzeroski, S.; Slavkov, I. A Comparison of Validation Indices for Evaluation of Clustering Results of DNA Microarray Data. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 587–591. [Google Scholar] [CrossRef]
- Davies, D.L.; Bouldin, D.W. A Cluster Separation Measure. IEEE Trans. Pattern Anal. Mach. Intell. 1979, PAMI-1, 224–227. [Google Scholar] [CrossRef]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the Widespread and Critical Impact of Batch Effects in High-Throughput Data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance Analysis of Microarrays Applied to the Ionizing Radiation Response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef]
- Damle, M.T.; Kshirsagar, M. Role of Permutations in Significance Analysis of Microarray and Clustering of Significant Microarray Gene List. Int. J. Comput. Sci. Issues 2012, 9, 342–344. [Google Scholar]
- Gevaert, O.; Mitchell, L.A.; Achrol, A.S.; Xu, J.; Echegaray, S.; Steinberg, G.K.; Cheshier, S.H.; Napel, S.; Zaharchuk, G.; Plevritis, S.K. Glioblastoma Multiforme: Exploratory Radiogenomic Analysis by Using Quantitative Image Features. Radiology 2014, 273, 168–174. [Google Scholar] [CrossRef]
- Datta, S.; Datta, S. Methods for Evaluating Clustering Algorithms for Gene Expression Data Using a Reference Set of Functional Classes. BMC Bioinform. 2006, 7, 397. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Ogutu, J.O.; Schulz-Streeck, T.; Piepho, H.P. Genomic Selection Using Regularized Linear Regression Models: Ridge Regression. BMC Proc. BioMed Cent. 2012, 6, S10. [Google Scholar]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, A.R.; Torrano, V.; Martín-Martín, N.; Caro-Maldonado, A.; Camacho, L.; Hermanova, I.; Guruceaga, E.; Lorenzo-Martín, L.F.; Caloto, R.; Gomis, R.R.; et al. Cancertool: A Visualization and Representation Interface to Exploit Cancer Datasets. Cancer Res. 2018, 78, 6320–6328. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.F.; Klimowicz, A.C.; Otsuka, S.; Elegbede, A.A.; Petrillo, S.K.; Williamson, T.; Williamson, C.T.; Konno, M.; Lees-Miller, S.P.; Hao, D.; et al. Loss of Tumour-Specific ATM Protein Expression Is an Independent Prognostic Factor in Early Resected NSCLC. Oncotarget 2017, 8, 38326–38336. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Prakash, C. Xenobiotic-Metabolizing Enzymes in Human Lung. Curr. Drug Metab. 2006, 7, 939–948. [Google Scholar] [CrossRef]
- Cordat, E.; Casey, J.R. Bicarbonate Transport in Cell Physiology and Disease. Biochem. J. 2009, 417, 423–439. [Google Scholar] [CrossRef]
- Alka, K.; Casey, J.R. Bicarbonate Transport in Health and Disease. IUBMB Life 2014, 66, 596–615. [Google Scholar] [CrossRef]
- Villicaña, C.; Cruz, G.; Zurita, M. The Basal Transcription Machinery as a Target for Cancer Therapy. Cancer Cell Int. 2014, 14, 18. [Google Scholar] [CrossRef]
- Wang, W.; Han, T.; Tong, W.; Zhao, J.; Qiu, X. Overexpression of GPR35 Confers Drug Resistance in NSCLC Cells by β-Arrestin/Akt Signaling. OncoTargets Ther. 2018, 11, 6249–6257. [Google Scholar] [CrossRef]
- Sorenson, G.D.; Cate, C.C.; Pettengill, O.S. Regulation of Hormone Production in Small Cell Carcinoma of the Lung. In Peptide Hormones in Lung Cancer; Recent Results Cancer Research; Havemann, K., Sorenson, G., Gropp, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; Volume 99, pp. 143–156. [Google Scholar]
- Fuentes, N.; Silva Rodriguez, M.; Silveyra, P. Role of Sex Hormones in Lung Cancer. Exp. Biol. Med. 2021, 246, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Han, P.; Wang, T.; Ren, H.; Gao, L.; Shi, P.; Zhang, S.; Yang, A.; Li, Z.; Chen, M. Stage-Associated Differences in the Serum N- and O-Glycan Profiles of Patients with Non-Small Cell Lung Cancer. Clin. Proteom. 2019, 16, 20. [Google Scholar] [CrossRef]
- Zhuo, D.; Li, X.; Guan, F. Biological Roles of Aberrantly Expressed Glycosphingolipids and Related Enzymes in Human Cancer Development and Progression. Front. Physiol. 2018, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Ohmi, Y.; Ohkawa, Y.; Bhuiyan, R.H.; Zhang, P.; Tajima, O.; Hashimoto, N.; Hamamura, K.; Furukawa, K. New Era of Research on Cancer-Associated Glycosphingolipids. Cancer Sci. 2019, 110, 1544–1551. [Google Scholar] [CrossRef]
- Tata, P.R.; Chow, R.D.; Saladi, S.V.; Tata, A.; Konkimalla, A.; Bara, A.; Montoro, D.; Hariri, L.P.; Shih, A.R.; Mino-Kenudson, M.; et al. Developmental History Provides a Roadmap for the Emergence of Tumor Plasticity. Dev. Cell 2018, 44, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Yu, X.; Zhou, H.; Luo, Y.; Wang, W.; Wang, L. Clinical Application of Plasma Mitochondrial DNA Content in Patients with Lung Cancer. Oncol. Lett. 2018, 16, 7074–7081. [Google Scholar] [CrossRef]
- Michael, M.; Doherty, M. Tumoral Drug Metabolism: Overview and Its Implications for Cancer Therapy. J. Clin. Oncol. 2005, 23, 205–229. [Google Scholar] [CrossRef]
- Castell, J.; Donato, M.; Gómez-Lechón, M. Metabolism and Bioactivation of Toxicants in the Lung. The in Vitro Cellular Approach. Exp. Toxicol. Pathol. 2005, 57, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.F.; Kadonaga, J.T. The RNA Polymerase II Core Promoter: A Key Component in the Regulation of Gene Expression. Genes Dev. 2002, 16, 2583–2592. [Google Scholar] [CrossRef]
- Munkley, J.; Elliott, D.J. Hallmarks of Glycosylation in Cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Helman, E.; Naxerova, K.; Kohane, I.S. DNA Hypermethylation in Lung Cancer Is Targeted at Differentiation- Associated Genes. Oncogene 2012, 31, 1181–1188. [Google Scholar] [CrossRef]
- García-Lepe, U.O.; Bermúdez-Cruz, R.M. Mitochondrial Genome Maintenance: Damage and Repair Pathways. In DNA Repair-An Update; Mognato, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Mohd Khair, S.Z.N.; Abd Radzak, S.M.; Mohamed Yusoff, A.A. The Uprising of Mitochondrial DNA Biomarker in Cancer. Dis. Markers 2021, 2021, 7675269. [Google Scholar] [CrossRef] [PubMed]
- Fei, P. New Understanding of Fanconi Anemia Signaling Network upon Studying FANCD2. Am. J. Biomed. Sci. Res. 2020, 7, 124–126. [Google Scholar] [CrossRef]
- Croft, L.V.; Bolderson, E.; Adams, M.N.; El-Kamand, S.; Kariawasam, R.; Cubeddu, L.; Gamsjaeger, R.; Richard, D.J. Human Single-Stranded DNA Binding Protein 1 (HSSB1, OBFC2B), a Critical Component of the DNA Damage Response. Semin. Cell Dev. Biol. 2019, 86, 121–128. [Google Scholar] [CrossRef]
- Boucher, D.; Ashton, N.; Suraweera, A.; Burgess, J.; Bolderson, E.; Barr, M.; Gray, S.; Gately, K.; Adams, M.; Croft, L.; et al. Human Single-Stranded DNA Protein 1 (HSSB1): A Prognostic Factor and Target for Non-Small Cell Lung Cancer (NSCLC) Treatment. In Proceedings of the 17th Annual British Thoracic Oncology Group Conference, Dublin, Ireland, 23–25 January 2019; Volume 127. [Google Scholar]
- Yao, W.; Liu, Y.; Zhang, Z.; Li, G.; Xu, X.; Zou, K.; Xu, Y.; Zou, L. ALX1 Promotes Migration and Invasion of Lung Cancer Cells through Increasing Snail Expression. Int. J. Clin. Exp. Pathol. 2015, 8, 12129–12139. [Google Scholar]
- Chang, C.; Lee, S.O.; Yeh, S.; Chang, T.M. Androgen Receptor (AR) Differential Roles in Hormone-Related Tumors Including Prostate, Bladder, Kidney, Lung, Breast and Liver. Oncogene 2014, 33, 3225–3234. [Google Scholar] [CrossRef]
- Zhang, D.L.; Qu, L.W.; Ma, L.; Zhou, Y.C.; Wang, G.Z.; Zhao, X.C.; Zhang, C.; Zhang, Y.F.; Wang, M.; Zhang, M.Y.; et al. Genome-Wide Identification of Transcription Factors That Are Critical to Non-Small Cell Lung Cancer. Cancer Lett. 2018, 434, 132–143. [Google Scholar] [CrossRef]
- Hernández-Pedro, N.; Soca-Chafre, G.; Alaez-Versón, C.; Carrillo-Sánchez, K.; Avilés-Salas, A.; Vergara, E.; Arrieta, O. Mutational Profile by Targeted next Generation Sequencing of Non-Small Cell Lung Cancer in the Mexican Population. Salud Publica Mex. 2019, 61, 308–317. [Google Scholar] [CrossRef]
- Lu, Y.S.; Lien, H.C.; Yeh, P.Y.; Kuo, S.H.; Chang, W.C.; Kuo, M.L.; Cheng, A.L. Glucocorticoid Receptor Expression in Advanced Non-Small Cell Lung Cancer: Clinicopathological Correlation and in Vitro Effect of Glucocorticoid on Cell Growth and Chemosensitivity. Lung Cancer 2006, 53, 303–310. [Google Scholar] [CrossRef]
- Xia, H.; Sun, S.; Wang, B.; Wang, T.; Liang, C.; Li, G.; Huang, C.; Qi, D.; Chu, X. MiR-143 Inhibits NSCLC Cell Growth and Metastasis by Targeting Limk1. Int. J. Mol. Sci. 2014, 15, 11973–11983. [Google Scholar] [CrossRef]
- Asakura, K.; Kadota, T.; Matsuzaki, J.; Yoshida, Y.; Yamamoto, Y.; Nakagawa, K.; Takizawa, S.; Aoki, Y.; Nakamura, E.; Miura, J.; et al. A MiRNA-Based Diagnostic Model Predicts Resectable Lung Cancer in Humans with High Accuracy. Commun. Biol. 2020, 3, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, B.; Zhang, P.; Zhang, J.; Zhu, Z.; Liu, B.; Fan, R. MiR-380-3p Regulates Melanogenesis by Targeting SOX6 in Melanocytes from Alpacas (Vicugna pacos). BMC Genom. 2019, 20, 962. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A Systematic Review of MiR-29 in Cancer. Mol. Ther. Oncolytics 2018, 12, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Wei, J.S.; Ringnér, M.; Saal, L.H.; Ladanyi, M.; Westermann, F.; Berthold, F.; Schwab, M.; Antonescu, C.R.; Peterson, C. Classification and Diagnostic Prediction of Cancers Using Gene Expression Profiling and Artificial Neural Networks. Nat. Med. 2001, 7, 623–679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mohamed, A.S.R.; Lai, S.Y.; Yang, S.; Kanwar, A.; Wei, L.; Kamal, M.; Sengupta, S.; Elhalawani, H.; Skinner, H.; et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations Between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and The Cancer Imaging Archive. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Burnside, E.S.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Whitman, G.J.; Sutton, E.J.; Net, J.M.; et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology 2016, 281, 382–391. [Google Scholar] [CrossRef]
- Hectors, S.; Cherny, M.; Yadav, K.; Beksaç, A.; Thulasidass, H.; Lewis, S.; Davicioni, E.; Wang, P.; Tewari, A.; Taouli, B. Radiomics Features Measured with Multiparametric Magnetic Resonance Imaging Predict Prostate Cancer Aggressiveness. J. Urol. 2019, 202, 498–505. [Google Scholar] [CrossRef]
- Katrib, A.; Hsu, W.; Bui, A.; Xing, Y. “Radiotranscriptomics”: A Synergy of Imaging and Transcriptomics in Clinical Assessment. Quant. Biol. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Smedley, N.F.; Aberle, D.R.; Hsu, W. Using Deep Neural Networks and Interpretability Methods to Identify Gene Expression Patterns That Predict Radiomic Features and Histology in Non-Small Cell Lung Cancer. J. Med. Imaging 2021, 8, 031906. [Google Scholar] [CrossRef]
- Yip, S.S.F.; Liu, Y.; Parmar, C.; Li, Q.; Liu, S.; Qu, F.; Ye, Z.; Gillies, R.J.; Aerts, H.J.W.L. Associations between Radiologist-Defined Semantic and Automatically Computed Radiomic Features in Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Hu, X.; Zhao, X.; Kong, X.; Meng, Y.M.; Chen, Y.; Su, L.; Jiang, X.; Qiu, X.; Huang, C.; et al. A Circular Network of Coregulated Sphingolipids Dictates Lung Cancer Growth and Progression. EBioMedicine 2021, 66, 103301. [Google Scholar] [CrossRef] [PubMed]
- Kashatus, D.F. The Regulation of Tumor Cell Physiology by Mitochondrial Dynamics. Biochem. Biophys. Res. Commun. 2018, 500, 9–16. [Google Scholar] [CrossRef] [PubMed]




|
Datasets (GEO Accession) |
Demographics and Clinical Characteristics | Radiomic Features | Datasets Utilization and Methodology |
|---|---|---|---|
| Dataset 1 (GSE28827) Samples 24 NSCLC samples No. of Genes 24371 Authors Nair et al. (2012) | Age, y (range) 66.6 (46–84) Gender(female) 6 Histology 19 LUAD/5 LUSC Stage (4) (0) (5) IA1/(3) IA2/(6) IB (3) IIB/(3) IIIA CT emphysema 8 |
749
|
|
| Dataset 2 (GSE75037) Samples 83 NSCLC samples 83 non-malignant lung samples No. of Genes 19227 Authors Girard et al. (2016) | Age, y (range) 68.1 (39–90) Gender(female) 59 Histology 83 LUAD Stage (24) IA/(26) IB (3) IIA/(17) IIB (1) III/(9) IIIA/(1) IIIB (2) IV CT emphysema n/a | - |
|
| Dataset 3 (GSE76925) Samples 40 normal lung samples No. of Genes 17130 Authors Morrow et al. (2017) | Age, y (range) 65.7 (42–86) Gender(female) 25 CT emphysema 18 | - |
|
| Dataset 4 (GSE18842) Samples 44 NSCLC samples 44 non-malignant lung samples Authors Sanchez–Palencia et al. (2011) | Age, y (range) n/a Gender (female) n/a Histology 12 LUAD/32 LUSC Stage (6) IA/(32) IB (1) IIA/(2) IIB (2) IIIA/(1) IIIB CT emphysema n/a | - |
|
| Dataset 5 (GSE27262) Samples 25 NSCLC samples 25 non-malignant lung samples Authors Wei et al. (2012) | Age, y (range) 58.1 (34–77) Gender(female) n/a Histology 25 LUAD Stage (7) IA/(18) IB CT emphysema n/a | - |
|
| Dataset 6 (GSE30219) Samples 143 NSCLC samples 14 normal lung samples Authors Rousseaux et al. (2013) | Age, y (range) 62.3 (44–84) Gender(female) 24 Histology 85 LUAD/58 LUSC Stage (117) IA/(12) IB (2) IIA/(7) IIB (3) IIIA/(2) IIIB CT emphysema n/a | - |
|
| Dataset 7 (GSE40419-RNA-Seq) Samples 87 NSCLC samples 77 non-malignant lung samples Authors Seo et al. (2012) | Age, y (range) 63.8 (38–85) Gender(female) 34 Histology 87 LUAD Stage (31) IA/(24) IB (5) IIA/(8) IIB (10) IIIA/(3) IIIB (4) IV (2) n/a CT emphysema n/a | - |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dovrou, A.; Bei, E.; Sfakianakis, S.; Marias, K.; Papanikolaou, N.; Zervakis, M. Synergies of Radiomics and Transcriptomics in Lung Cancer Diagnosis: A Pilot Study. Diagnostics 2023, 13, 738. https://doi.org/10.3390/diagnostics13040738
Dovrou A, Bei E, Sfakianakis S, Marias K, Papanikolaou N, Zervakis M. Synergies of Radiomics and Transcriptomics in Lung Cancer Diagnosis: A Pilot Study. Diagnostics. 2023; 13(4):738. https://doi.org/10.3390/diagnostics13040738
Chicago/Turabian StyleDovrou, Aikaterini, Ekaterini Bei, Stelios Sfakianakis, Kostas Marias, Nickolas Papanikolaou, and Michalis Zervakis. 2023. "Synergies of Radiomics and Transcriptomics in Lung Cancer Diagnosis: A Pilot Study" Diagnostics 13, no. 4: 738. https://doi.org/10.3390/diagnostics13040738
APA StyleDovrou, A., Bei, E., Sfakianakis, S., Marias, K., Papanikolaou, N., & Zervakis, M. (2023). Synergies of Radiomics and Transcriptomics in Lung Cancer Diagnosis: A Pilot Study. Diagnostics, 13(4), 738. https://doi.org/10.3390/diagnostics13040738











