Feasibility of Reduced Iodine Loads for Vascular Assessment Prior to Transcatheter Aortic Valve Implantation (TAVI) Using Spectral Detector CT
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sample
2.2. DLCT Image Acquisition Protocol
2.3. Contrast Media Injection Protocols
2.4. Image Reconstruction
2.5. Image Analysis
2.6. Statistics
2.7. Ethical Approval and Patient Consent
3. Results
3.1. Patient Characteristics
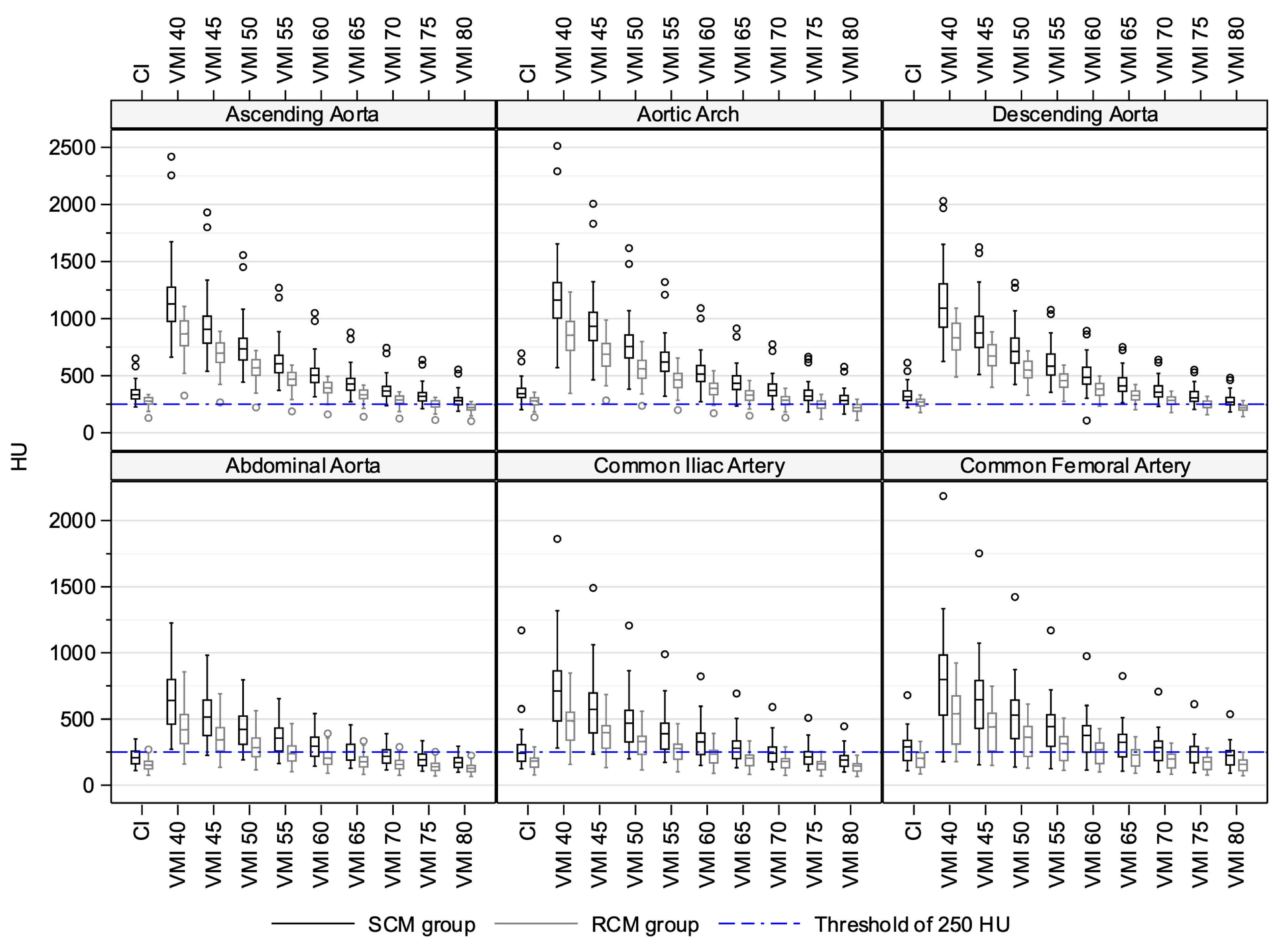
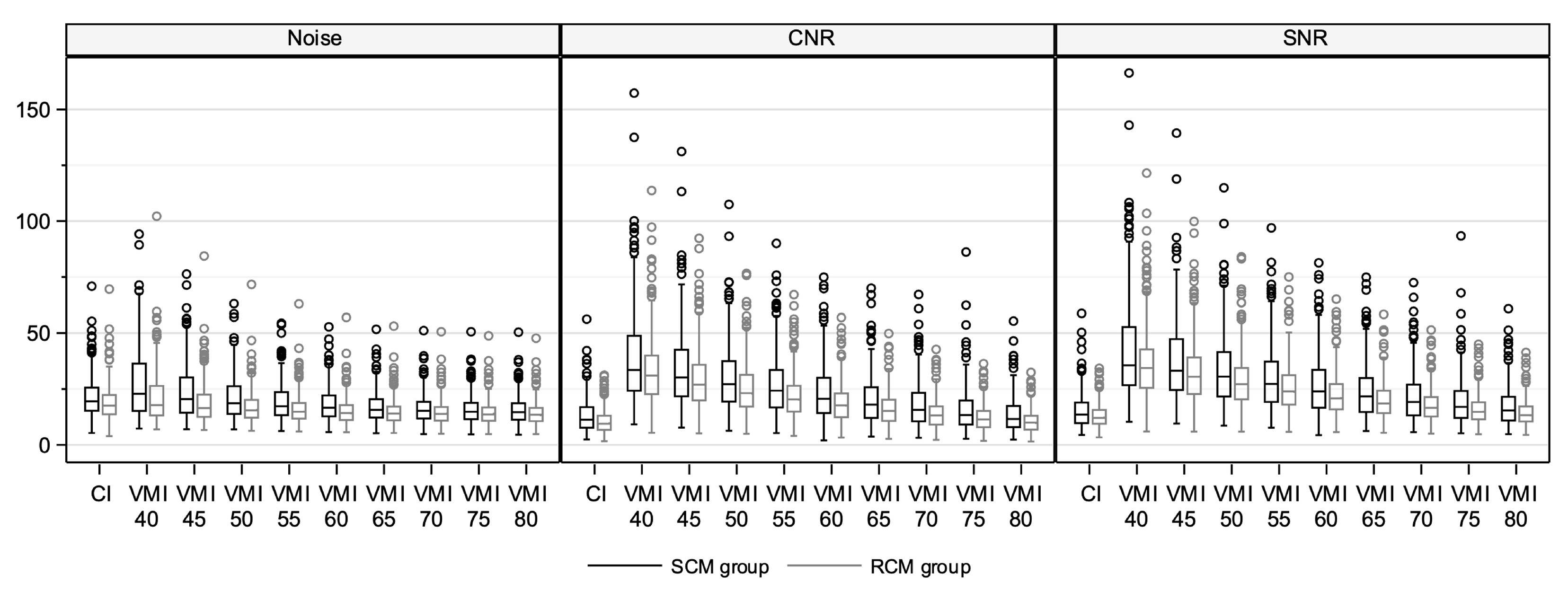
3.2. Quantitative Image Quality Assessment in Conventional Images
3.3. Quantitative Image Quality Assessment in Virtual Monoenergetic Images
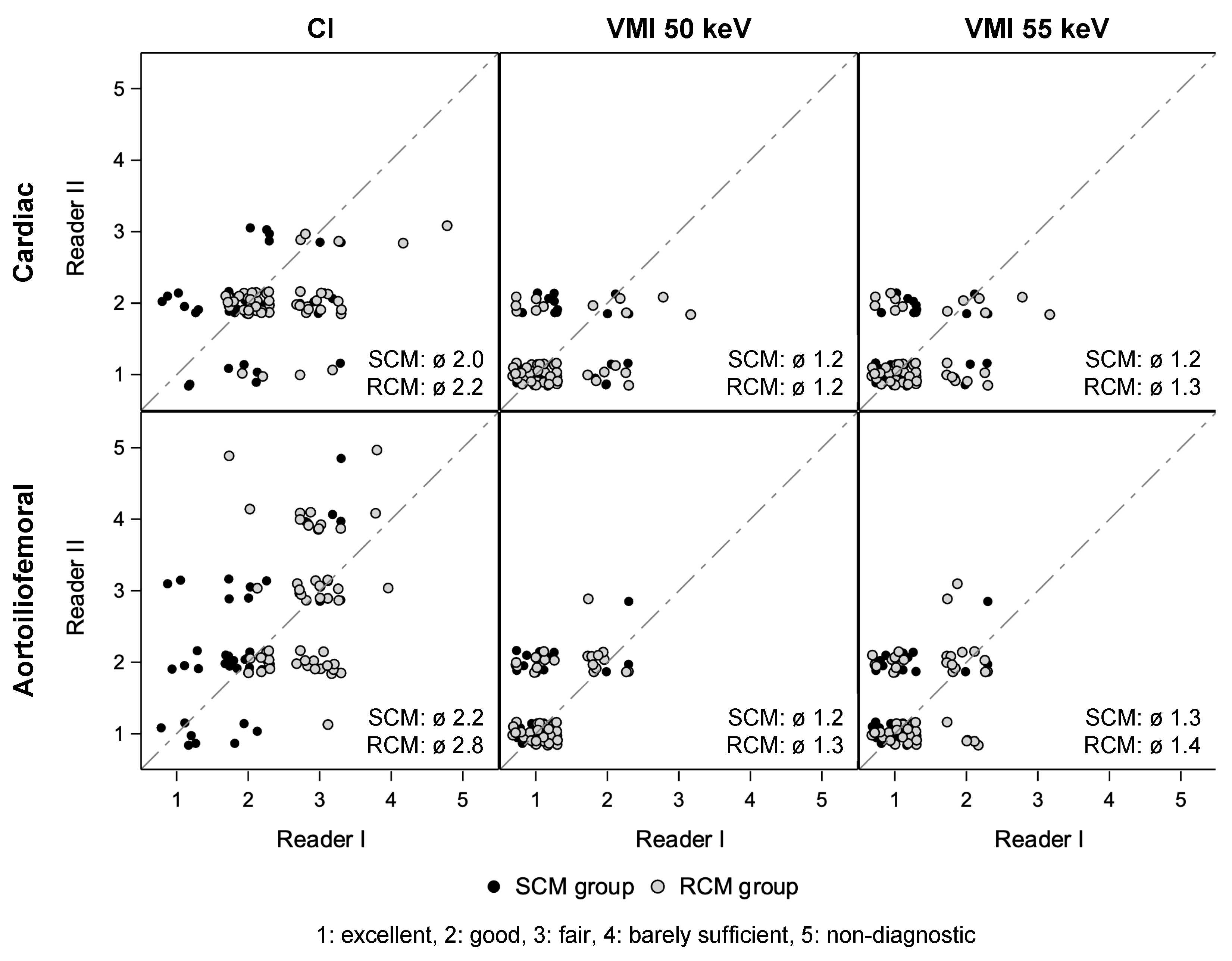
3.4. Qualitative Image Quality Assessment
3.5. Radiation Dose
3.6. Renal Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | conventional images |
| CNR | contrast-to-noise ratio |
| CT | computed tomography |
| DLCT | dual-layer detector CT |
| eGFR | estimated glomerular filtration rate |
| FOV | field of view |
| GFR | glomerular filtration rate |
| SD | standard deviation |
| SNR | signal-to-noise ratio |
| TAVI | transcatheter aortic valve implantation |
| TAVR | transcatheter aortic valve replacement |
| VMI | virtual monoenergetic (monochromatic) images |
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Norgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2019, 13, 1–20. [Google Scholar] [CrossRef]
- Rudzinski, P.N.; Leipsic, J.A.; Schoepf, U.J.; Dudek, D.; Schwarz, F.; Andreas, M.; Zlahoda-Huzior, A.; Thilo, C.; Renker, M.; Burt, J.R.; et al. CT in Transcatheter-delivered Treatment of Valvular Heart Disease. Radiology 2022, 304, 4–17. [Google Scholar] [CrossRef]
- Becker, C.R.; Davidson, C.; Lameire, N.; McCullough, P.A.; Stacul, F.; Tumlin, J.; Adam, A.; CIN Consensus Working Panel. High-risk situations and procedures. Am. J. Cardiol. 2006, 98, 37K–41K. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hayashida, K.; Mouillet, G.; Chevalier, B.; Meguro, K.; Watanabe, Y.; Dubois-Rande, J.L.; Morice, M.C.; Lefevre, T.; Teiger, E. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2013, 6, 479–486. [Google Scholar] [CrossRef]
- Geisler, D.; Rudzinski, P.N.; Hasan, W.; Andreas, M.; Hasimbegovic, E.; Adlbrecht, C.; Winkler, B.; Weiss, G.; Strouhal, A.; Delle-Karth, G.; et al. Identifying Patients without a Survival Benefit following Transfemoral and Transapical Transcatheter Aortic Valve Replacement. J. Clin. Med. 2021, 10, 4911. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.; Turek, J.; Mihl, C.; Reinartz, S.D.; Gohmann, R.F.; Nijssen, E.C.; Kats, S.; van Ommen, V.G.; Kietselaer, B.L.; Wildberger, J.E.; et al. Low contrast media volume in pre-TAVI CT examinations. Eur. Radiol. 2016, 26, 2426–2435. [Google Scholar] [CrossRef]
- Alaiti, M.A.; Attizzani, G.F.; Fares, A.; Eck, B.; Van Hedent, S.; Patel, S.; Cavallo, A.U.; Fahmi, R.; Levi, J.; Ichibori, Y.; et al. Contrast-Sparing Imaging Utilizing Spectral Detector CT for Transcatheter Aortic Valve Replacement Procedure Planning. Structural. Heart 2020, 4, 195–203. [Google Scholar] [CrossRef]
- Cavallo, A.U.; Patterson, A.J.; Thomas, R.; Alaiti, M.A.; Attizzani, G.F.; Laukamp, K.; Grosse Hokamp, N.; Bezerra, H.; Gilkeson, R.; Rajagopalan, S. Low dose contrast CT for transcatheter aortic valve replacement assessment: Results from the prospective SPECTACULAR study (spectral CT assessment prior to TAVR). J. Cardiovasc. Comput. Tomogr. 2020, 14, 68–74. [Google Scholar] [CrossRef]
- Rassouli, N.; Etesami, M.; Dhanantwari, A.; Rajiah, P. Detector-based spectral CT with a novel dual-layer technology: Principles and applications. Insights Imaging 2017, 8, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Mangold, D.; Salatzki, J.; Riffel, J.; Kauczor, H.U.; Weber, T.F. Dual-Layer Spectral CTA for TAVI Planning Using a Split-Phase Protocol and Low-keV Virtual Monoenergetic Images: Improved Image Quality in Comparison with Single-Phase Conventional CTA. RoFo Fortschritte Geb. Rontgenstrahlen Nukl. 2021, 194, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Mata-Mbemba, D.; Labani, A.; El Ghannudi, S.; Jeung, M.Y.; Ohlmann, P.; Roy, C.; Ohana, M. 320-row CT transcatheter aortic valve replacement planning with a single reduced contrast media bolus injection. PLoS ONE 2018, 13, e0204145. [Google Scholar] [CrossRef] [PubMed]
- Tsang, D.S.; Merchant, T.E.; Merchant, S.E.; Smith, H.; Yagil, Y.; Hua, C.H. Quantifying potential reduction in contrast dose with monoenergetic images synthesized from dual-layer detector spectral CT. Br. J. Radiol. 2017, 90, 20170290. [Google Scholar] [CrossRef] [PubMed]
- Contrast Media Safety Committee of the European Society of Urogenital Radiology. ESUR Guidelines on Contrast Agents (Version 10.0). 2018. Available online: http://www.esur.org/esur-guidelines/ (accessed on 17 January 2024).
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Euringer, W.; Baumann, T.; Reinohl, J.; Schlensak, C.; Langer, M.; Pache, G. Combined assessment of aortic root anatomy and aortoiliac vasculature with dual-source CT as a screening tool in patients evaluated for transcatheter aortic valve implantation. AJR Am. J. Roentgenol. 2010, 195, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Leipsic, J.; Wood, D.; Manders, D.; Nietlispach, F.; Masson, J.B.; Mayo, J.; Al-Bugami, S.; Webb, J.G. The evolving role of MDCT in transcatheter aortic valve replacement: A radiologists’ perspective. AJR Am. J. Roentgenol. 2009, 193, W214–W219. [Google Scholar] [CrossRef]
- Wuest, W.; Anders, K.; Schuhbaeck, A.; May, M.S.; Gauss, S.; Marwan, M.; Arnold, M.; Ensminger, S.; Muschiol, G.; Daniel, W.G.; et al. Dual source multidetector CT-angiography before Transcatheter Aortic Valve Implantation (TAVI) using a high-pitch spiral acquisition mode. Eur. Radiol. 2012, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Abbara, S.; Ghoshhajra, B.B. Ultra-low contrast computed tomographic angiography (CTA) with 20-mL total dose for transcatheter aortic valve implantation (TAVI) planning. J. Comput. Assist. Tomogr. 2014, 38, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Jurencak, T.; Turek, J.; Kietselaer, B.L.; Mihl, C.; Kok, M.; van Ommen, V.G.; van Garsse, L.A.; Nijssen, E.C.; Wildberger, J.E.; Das, M. MDCT evaluation of aortic root and aortic valve prior to TAVI. What is the optimal imaging time point in the cardiac cycle? Eur. Radiol. 2015, 25, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Higashigaito, K.; Mergen, V.; Eberhard, M.; Jungblut, L.; Hebeisen, M.; Ratzer, S.; Zanini, B.; Kobe, A.; Martini, K.; Euler, A.; et al. CT Angiography of the Aorta Using Photon-counting Detector CT with Reduced Contrast Media Volume. Radiol. Cardiothorac. Imaging 2023, 5, e220140. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.G.; De Cecco, C.N.; Schoepf, U.J.; Katzberg, R. Contrast-induced acute kidney injury: Definition, epidemiology, and outcome. BioMed Res. Int. 2014, 2014, 859328. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.A.; Newhouse, J.H. Risk of nephropathy after intravenous administration of contrast material: A critical literature analysis. Radiology 2006, 239, 392–397. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Bida, J.P.; Carter, R.E.; Fleming, C.J.; Misra, S.; Williamson, E.E.; Kallmes, D.F. Intravenous contrast material-induced nephropathy: Causal or coincident phenomenon? Radiology 2013, 267, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, F.; Pignatelli, A.; Cafaro, A.; Stabile, E.; Salemme, L.; Cioppa, A.; Pucciarelli, A.; Spione, F.; Loizzi, F.; De Cillis, E.; et al. Impact of contrast medium osmolality on the risk of acute kidney injury after transcatheter aortic valve implantation: Insights from the Magna Graecia TAVI registry. Int. J. Cardiol. 2021, 329, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Arana, E.; Marti-Bonmati, L.; Tobarra, E.; Sierra, C. Cost reduction in abdominal CT by weight-adjusted dose. Eur. J. Radiol. 2009, 70, 507–511. [Google Scholar] [CrossRef]
- Routhier, J.; Piazzo, K.; Sodickson, A. Contrast and cost savings by implementation of a multidose bulk IV contrast delivery system. J. Am. Coll Radiol. 2011, 8, 265–270. [Google Scholar] [CrossRef]
- Tu, L.H.; Miller, J.E.; Forman, H.P. The Critical Shortage of Iodinated Contrast Material-Will Value Prevail? N. Engl. J. Med. 2022, 387, 491–493. [Google Scholar] [CrossRef] [PubMed]


| Parameter | SCM | RCM |
|---|---|---|
| Sample size (n) | 50 | 50 |
| Gender (female) | 12 (24%) | 22 (44%) |
| Age (years) | 80 ± 6 | 83 ± 7 |
| Height (cm) | 171 ± 8 | 168 ± 7 |
| Weight (kg) | 77 ± 15 | 73 ± 16 |
| BMI (kg/m2) | 25 (18–40) | 25 (18–45) |
| <23 (n) | 11 | 17 |
| 23–30 (n) | 30 | 25 |
| >30 (n) | 9 | 8 |
| Parameter | Cardiac | Aortoiliofemoral |
|---|---|---|
| Acquisition | ||
| Mode | Low-pitch spiral | High-pitch spiral |
| ECG gating | Retrospective | – |
| Direction | Out (caudocranial) | In (craniocaudal) |
| Beam collimation (mm) | 64 × 0.625 | |
| Peak tube voltage (kVp) | 120 | |
| Dose modulation | On (“Cardiac”) | On (“3D Modulation”) |
| Tube current (mAsref) | 600 | 130 |
| Rotation time (s) | 0.27 | 0.4 |
| Pitch | 0.2 | 1.234 |
| Delay (s) | 6 following bolus tracking (≥110 HU) | 26 following bolus tracking (≥110 HU) |
| Reconstruction | ||
| Cardiac phase | Best systolic, diastolic at 75% of R-R interval | – |
| Orientation | Axial | |
| Slice thickness (mm) | 0.67 | 3.0 |
| Increment (mm) | 0.335 | 1.5 |
| Algorithm | IMR Level 1 | |
| Kernel | Cardiac Routine | Routine |
| Series | Parameter Vessel Segment | SCM | RCM | p Value |
|---|---|---|---|---|
| Attenuation (HU) | ||||
| Ascending aorta | 333 (226–651) | 277 (130–335) | <0.001 | |
| Aortic arch | 342 (203–696) | 279 (136–355) | <0.001 | |
| Descending aorta | 316 (221–614) | 269 (177–331) | <0.001 | |
| Abdominal aorta | 208 (110–350) | 152 (75–270) | <0.001 | |
| Common iliac artery | 242 (124–1170) | 183 (77–290) | <0.001 | |
| Common femoral artery | 290 (109–679) | 203 (84–331) | <0.001 | |
| Contrast-to-noise ratio | ||||
| Ascending aorta | 11 (4–30) | 10 (4–19) | 0.66 | |
| Aortic arch | 14 (6–42) | 12 (4–29) | 0.08 | |
| CI | Descending aorta | 10 (4–28) | 9 (3–18) | 0.63 |
| Abdominal aorta | 8 (2–20) | 5 (2–12) | 0.001 | |
| Common iliac artery | 12 (3–56) | 9 (2–24) | 0.01 | |
| Common femoral artery | 20 (4–38) | 12 (4–31) | 0.003 | |
| Signal-to-noise ratio | ||||
| Ascending aorta | 13 (5–33) | 13 (5–23) | 0.88 | |
| Aortic arch | 16 (7–46) | 15 (6–34) | 0.20 | |
| Descending aorta | 12 (5–32) | 12 (3–22) | 0.97 | |
| Abdominal aorta | 10 (5–24) | 9 (4–16) | 0.002 | |
| Common iliac artery | 15 (5–59) | 12 (5–29) | 0.03 | |
| Common femoral artery | 19 (4–50) | 14 (5–33) | 0.03 | |
| Attenuation (HU) | ||||
| Ascending aorta | 735 (443–1556) | 568 (222–721) | <0.001 | |
| VMI 50 keV | Aortic arch | 755 (382–1617) | 561 (236–799) | <0.001 |
| Descending aorta | 712 (422–1314) | 549 (329–717) | <0.001 | |
| Abdominal aorta | 422 (191–795) | 284 (116–563) | <0.001 | |
| Common iliac artery | 469 (199–1207) | 330 (115–560) | <0.001 | |
| Common femoral artery | 530 (137–1423) | 363 (128–612) | <0.001 | |
| Attenuation (HU) | ||||
| Ascending aorta | 605 (371–1269) | 469 (187–593) | <0.001 | |
| VMI 55 keV | Aortic arch | 619 (319–1320) | 463 (199–654) | <0.001 |
| Descending aorta | 583 (354–1076) | 456 (275–592) | <0.001 | |
| Abdominal aorta | 356 (164–652) | 239 (101–466) | <0.001 | |
| Common iliac artery | 389 (172–989) | 278 (101–465) | <0.001 | |
| Common femoral artery | 443 (124–1170) | 314 (112–508) | <0.001 |
| Group | Vessel Segment | Median Diff. VMI 50 keV to CI (HU) | p Value | Median Diff. VMI 55 keV to CI (HU) | p Value |
|---|---|---|---|---|---|
| Ascending aorta | +407 | <0.001 | +276 | <0.001 | |
| Aortic arch | +402 | <0.001 | +268 | <0.001 | |
| SCM | Descending aorta | +396 | <0.001 | +269 | <0.001 |
| Abdominal aorta | +215 | <0.001 | +147 | <0.001 | |
| Common iliac artery | +227 | <0.001 | +150 | <0.001 | |
| Common femoral artery | +250 | <0.001 | +161 | <0.001 | |
| Ascending aorta | +287 | <0.001 | +184 | <0.001 | |
| Aortic arch | +274 | <0.001 | +176 | <0.001 | |
| RCM | Descending aorta | +284 | <0.001 | +190 | <0.001 |
| Abdominal aorta | +132 | <0.001 | +87 | <0.001 | |
| Common iliac artery | +148 | <0.001 | +96 | <0.001 | |
| Common femoral artery | +157 | <0.001 | +103 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuppert, C.; Salatzki, J.; André, F.; Riffel, J.; Mangold, D.L.; Melzig, C.; Hagar, M.T.; Kauczor, H.-U.; Weber, T.F.; Rengier, F.; et al. Feasibility of Reduced Iodine Loads for Vascular Assessment Prior to Transcatheter Aortic Valve Implantation (TAVI) Using Spectral Detector CT. Diagnostics 2024, 14, 879. https://doi.org/10.3390/diagnostics14090879
Schuppert C, Salatzki J, André F, Riffel J, Mangold DL, Melzig C, Hagar MT, Kauczor H-U, Weber TF, Rengier F, et al. Feasibility of Reduced Iodine Loads for Vascular Assessment Prior to Transcatheter Aortic Valve Implantation (TAVI) Using Spectral Detector CT. Diagnostics. 2024; 14(9):879. https://doi.org/10.3390/diagnostics14090879
Chicago/Turabian StyleSchuppert, Christopher, Janek Salatzki, Florian André, Johannes Riffel, David L. Mangold, Claudius Melzig, Muhammad Taha Hagar, Hans-Ulrich Kauczor, Tim F. Weber, Fabian Rengier, and et al. 2024. "Feasibility of Reduced Iodine Loads for Vascular Assessment Prior to Transcatheter Aortic Valve Implantation (TAVI) Using Spectral Detector CT" Diagnostics 14, no. 9: 879. https://doi.org/10.3390/diagnostics14090879






