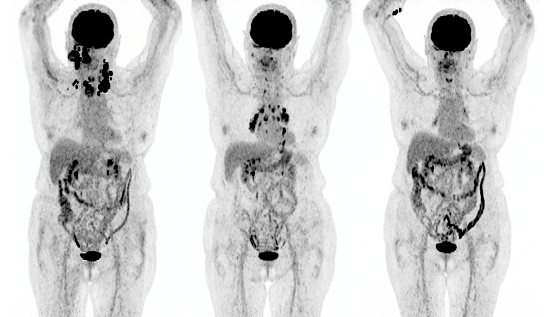
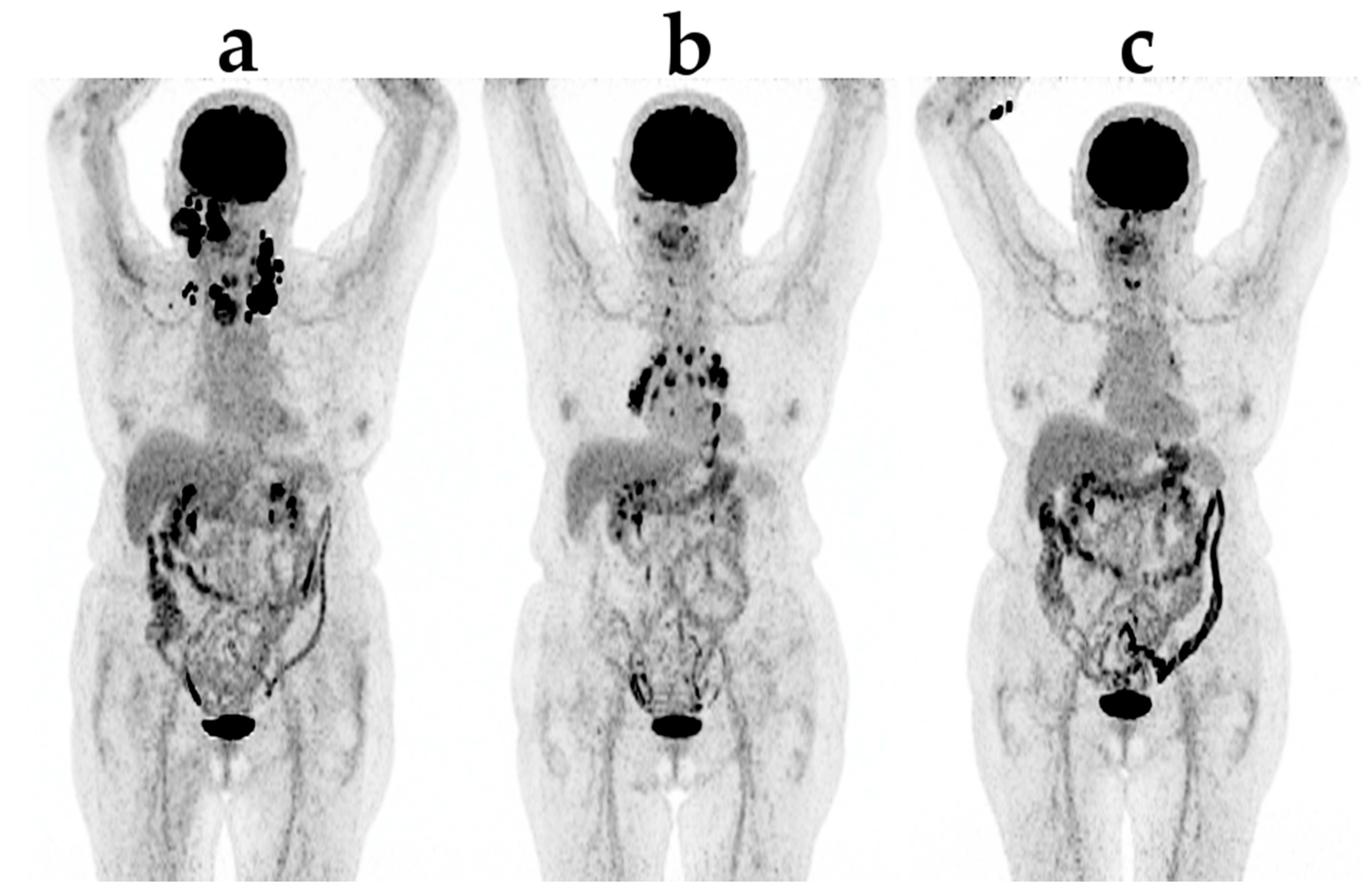
Nuclear Molecular Imaging Strategies in Immune Checkpoint Inhibitor Therapy
Abstract
:1. Introduction
2. Immune Checkpoint Therapy
3. Patterns of Response
Immune-Related Response Criteria (irRC)
4. FDG PET/CT
Pseudoprogression
5. New Imaging Biomarkers
5.1. Imaging PD-L1 (Programmed Cell Death Ligand 1)
5.2. 18F-Fluorothymidine PET
5.3. T-Cell Tracking
6. Discussion
7. Conclusions
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (keynote-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.-M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Grob, J.J.; Aamdal, S.; Bondarenko, I.; Robert, C.; Thomas, L.; Garbe, C.; Chiarion-Sileni, V.; Testori, A.; Chen, T.T.; et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Ann. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (keynote-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- O’Day, S.J.; Maio, M.; Chiarion-Sileni, V.; Gajewski, T.F.; Pehamberger, H.; Bondarenko, I.N.; Queirolo, P.; Lundgren, L.; Mikhailov, S.; Roman, L.; et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann. Oncol. 2010, 21, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T., Jr.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- Weber, J.S.; O’Day, S.; Urba, W.; Powderly, J.; Nichol, G.; Yellin, M.; Snively, J.; Hersh, E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Hwu, W.J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of immune-related response criteria and recist v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.R.; Jalil, J.; Economou, J.S.; Chmielowski, B.; Koya, R.C.; Mok, S.; Sazegar, H.; Seja, E.; Villanueva, A.; Gomez-Navarro, J.; et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin. Cancer Res. 2011, 17, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Oble, D.A.; Drappatz, J.; Velazquez, E.F.; Ramaiya, N.; Ramakrishna, N.; Day, A.L.; Kruse, A.; Mac Rae, S.; Hoos, A.; et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat. Clin. Pract. Oncol. 2008, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Urba, W.J.; Yellin, M.; Nichol, G.M.; Weber, J.; Hersh, E.M.; Tchekmedyian, S.; Hodi, F.S.; Weber, R.; O’Day, S. Kinetics of response to ipilimumab (MDX-010) in patients with stage III/IV melanoma. J. Clin. Oncol. 2007, 25, 8525. [Google Scholar]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a common language for tumor response to immunotherapy: Immune-related response criteria using unidimensional measurements. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3936–3943. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. Irecist: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Buhl, T.; Jurlander, J.; Buus, S.; Keiding, S.; D’Amore, F.; Boesen, A.M.; et al. Fdg-pet after two cycles of chemotherapy predicts treatment failure and progression-free survival in hodgkin lymphoma. Blood 2006, 107, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.; Chiang, S.; Johnson, G.; Schuchter, L.M.; Zhuang, H.; Alavi, A. Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? J. Nucl. Med. 2004, 45, 1323–1327. [Google Scholar] [PubMed]
- Reinhardt, M.J.; Joe, A.Y.; Jaeger, U.; Huber, A.; Matthies, A.; Bucerius, J.; Roedel, R.; Strunk, H.; Bieber, T.; Biersack, H.-J.; et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: Experience with 250 consecutive patients. J. Clin. Oncol. 2006, 24, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Dummer, R.; Steinert, H.C.; Conzett, K.B.; Schad, K.; Lago, M.P.; Soyka, J.D.; Veit-Haibach, P.; Seifert, B.; Kalff, V. Chemotherapy response assessment in stage IV melanoma patients—Comparison of 18F-FDG-PET/CT, CT, brain MRI, and tumormarker S-100B. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Larribere, L.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: Preliminary results of an ongoing study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Menzies, A.M.; Saunders, C.A.; Liniker, E.; Ramanujam, S.; Guminski, A.; Kefford, R.F.; Long, G.V.; Carlino, M.S. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment. Cell Melanoma Res. 2016, 29, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Breki, C.M.; Dimitrakopoulou-Strauss, A.; Hassel, J.; Theoharis, T.; Sachpekidis, C.; Pan, L.; Provata, A. Fractal and multifractal analysis of PET/CT images of metastatic melanoma before and after treatment with ipilimumab. EJNMMI Res. 2016, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Menda, Y.; Graham, M.; Milhem, M. F-18 FDG PET/CT evaluation of treatment response to ipilimumab therapy in advanced stage melanoma. J. Nucl. Med. 2015, 56, 1464. [Google Scholar]
- Fredrickson, J.; Callahan, J.; Funke, R.; Sanabria, S.; Weber, W.; de Crespigny, A.; Hicks, R. Utility of FDG-PET in immunotherapy: Results from a phase II study of NSCLC patients undergoing therapy with the PD-L1 inhibitor, atezolizumab (MPDL3280A). J. Nucl. Med. 2016, 57, 134. [Google Scholar]
- Abramson Cancer Center of the University of Pennsylvania. Imaging FDG Flare in Melanoma. Available online: https://ClinicalTrials.gov/show/NCT02791594 (accessed on 8 January 2017).
- Abramson Cancer Center of the University of Pennsylvania. Early FDG pet in melanoma. Available online: https://ClinicalTrials.gov/show/NCT02716077 (accessed on 8 January 2017).
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From recist to percist: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009, 50, 122s–150s. [Google Scholar] [CrossRef] [PubMed]
- Bacanovic, S.; Burger, I.A.; Stolzmann, P.; Hafner, J.; Huellner, M.W. Ipilimumab-induced adrenalitis: A possible pitfall in 18F-FDG-PET/CT. Clin. Nucl. Med. 2015, 40, e518–e519. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Hamid, O.; Ribas, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.; Hersey, P.; Weber, J.S.; Joseph, R.; et al. Abstract ct104: Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma(mel): Correlation of tumor pd-l1 expression with outcome. Cancer Res. 2014, 74, CT104. [Google Scholar] [CrossRef]
- Tsao, M.S.; Le Teuff, G.; Shepherd, F.A.; Landais, C.; Hainaut, P.; Filipits, M.; Pirker, R.; Le Chevalier, T.; Graziano, S.; Kratze, R.; et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Maute, R.L.; Gordon, S.R.; Mayer, A.T.; McCracken, M.N.; Natarajan, A.; Ring, N.G.; Kimura, R.; Tsai, J.M.; Manglik, A.; Kruse, A.C.; et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-pet imaging. Proc. Natl. Acad. Sci. USA 2015, 112, E6506–E6514. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Tannock, I.F. The distribution of the therapeutic monoclonal antibodies cetuximab and trastuzumab within solid tumors. BMC Cancer 2010, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Hettich, M.; Braun, F.; Bartholoma, M.D.; Schirmbeck, R.; Niedermann, G. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics 2016, 6, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lesniak, W.G.; Miller, M.S.; Lisok, A.; Sikorska, E.; Wharram, B.; Kumar, D.; Gabrielson, M.; Pomper, M.G.; Gabelli, S.B.; et al. Rapid PD-L1 detection in tumors with PET using a highly specific peptide. Biochem. Biophys. Res. Commun. 2017, 483, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Heskamp, S.; Hobo, W.; Molkenboer-Kuenen, J.D.; Olive, D.; Oyen, W.J.; Dolstra, H.; Boerman, O.C. Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies. Cancer Res. 2015, 75, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lesniak, W.G.; Gabrielson, M.; Lisok, A.; Wharram, B.; Sysa-Shah, P.; Azad, B.B.; Pomper, M.G.; Nimmagadda, S. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget 2016, 7, 10215–10227. [Google Scholar] [PubMed]
- Josefsson, A.; Nedrow, J.R.; Park, S.; Banerjee, S.R.; Rittenbach, A.; Jammes, F.; Tsui, B.; Sgouros, G. Imaging, biodistribution, and dosimetry of radionuclide-labeled PD-L1 antibody in an immunocompetent mouse model of breast cancer. Cancer Res. 2016, 76, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Benz, M.R.; Allen-Auerbach, M.S.; Radu, C.; Chmielowski, B.; Seja, E.; Williams, J.L.; Gomez-Navarro, J.; McCarthy, T.; Czernin, J. Imaging of CTLA4 blockade–induced cell replication with 18F-FLT pet in patients with advanced melanoma treated with tremelimumab. J. Nucl. Med. 2010, 51, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Tavaré, R.; McCracken, M.N.; Zettlitz, K.A.; Knowles, S.M.; Salazar, F.B.; Olafsen, T.; Witte, O.N.; Wu, A.M. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Tavare, R.; Escuin-Ordinas, H.; Mok, S.; McCracken, M.N.; Zettlitz, K.A.; Salazar, F.B.; Witte, O.N.; Ribas, A.; Wu, A.M. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 2016, 76, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Tjin, E.P.M.; Krebbers, G.; Meijlink, K.J.; van de Kasteele, W.; Rosenberg, E.H.; Sanders, J.; Nederlof, P.M.; van de Wiel, B.A.; Haanen, J.B.A.G.; Melief, C.J.M.; et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol. Res. 2014, 2, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti–PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- UMC Groningen. Il2 Imaging in Metastatic Melanoma. Available online: https://ClinicalTrials.gov/show/NCT02922283 (accessed on 15 March 2017).
- UMC Groningen. Mpdl3280a-imaging-ist-umcg. Available online: https://ClinicalTrials.gov/show/NCT02453984 (accessed on 15 March 2017).
- UMC Groningen. Mpdl3280a-treatment-ist-umcg. Available online: https://ClinicalTrials.gov/show/NCT02478099 (accessed on 15 March 2017).
- Graham, M.M.; Weber, W.A. Evaluation of the efficacy of targeted imaging agents. J. Nucl. Med. 2016, 57, 653–659. [Google Scholar] [CrossRef] [PubMed]

| Study | No. of Patients | Method of Response Assesment | Results |
|---|---|---|---|
| Sachpekidis et al. [32] | 22 | FDG PET/CT at baseline, after two cycles of ipilimumab and post-treatment. EORTC 2 criteria used for response classification | Early scan predictive of post-treatment response in 18 of 22 patients |
| Kong et al. [33] | 27 | FDG PET/CT after at least 12 months of treatment with pembrolizumab or nivolumab categorized as positive or negative for presence of metabolically active disease compared to response on CT at the time of the PET/CT scan | 43% of patients with residual disease on CT had negative PET scans |
| Breki et al. [34] | 31 | FDG PET/CT at baseline, after two cycles of ipilimumab and post-treatment. Fractal and multifractal analysis compared to visual image assesment by nuclear medicine physicians. Seven patients excluded in comparison because of hypermetabolic lesions not related to melanoma (such as irAEs 3) | Fractal analysis results match treatment outcome in 20 out of 24 cases |
| Zheng et al. (Abstract) [35] | 28 | Retrospective study. FDG PET/CT at baseline and after 2–4 cycles of ipilimumab treatment. Response assessed according to PERCIST 4 | Two-year survival rate 31% with PMD 5 and 73% with non-PMD |
| Fredrickson et al. (Abstract) [36] | 103 | Retrospective study. FDG PET/CT at baseline and after six weeks of atezolizumab treatment evaluated according to EORTC | Metabolic responders had higher overall response rate than non-responders (73.9% vs. 6.3%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guldbrandsen, K.F.; Hendel, H.W.; Langer, S.W.; Fischer, B.M. Nuclear Molecular Imaging Strategies in Immune Checkpoint Inhibitor Therapy. Diagnostics 2017, 7, 23. https://doi.org/10.3390/diagnostics7020023
Guldbrandsen KF, Hendel HW, Langer SW, Fischer BM. Nuclear Molecular Imaging Strategies in Immune Checkpoint Inhibitor Therapy. Diagnostics. 2017; 7(2):23. https://doi.org/10.3390/diagnostics7020023
Chicago/Turabian StyleGuldbrandsen, Kasper F., Helle W. Hendel, Seppo W. Langer, and Barbara M. Fischer. 2017. "Nuclear Molecular Imaging Strategies in Immune Checkpoint Inhibitor Therapy" Diagnostics 7, no. 2: 23. https://doi.org/10.3390/diagnostics7020023