Changes in Vitellogenin (Vg) and Stress Protein (HSP 70) in Honey Bee (Apis mellifera anatoliaca) Groups under Different Diets Linked with Physico-Chemical, Antioxidant and Fatty and Amino Acid Profiles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
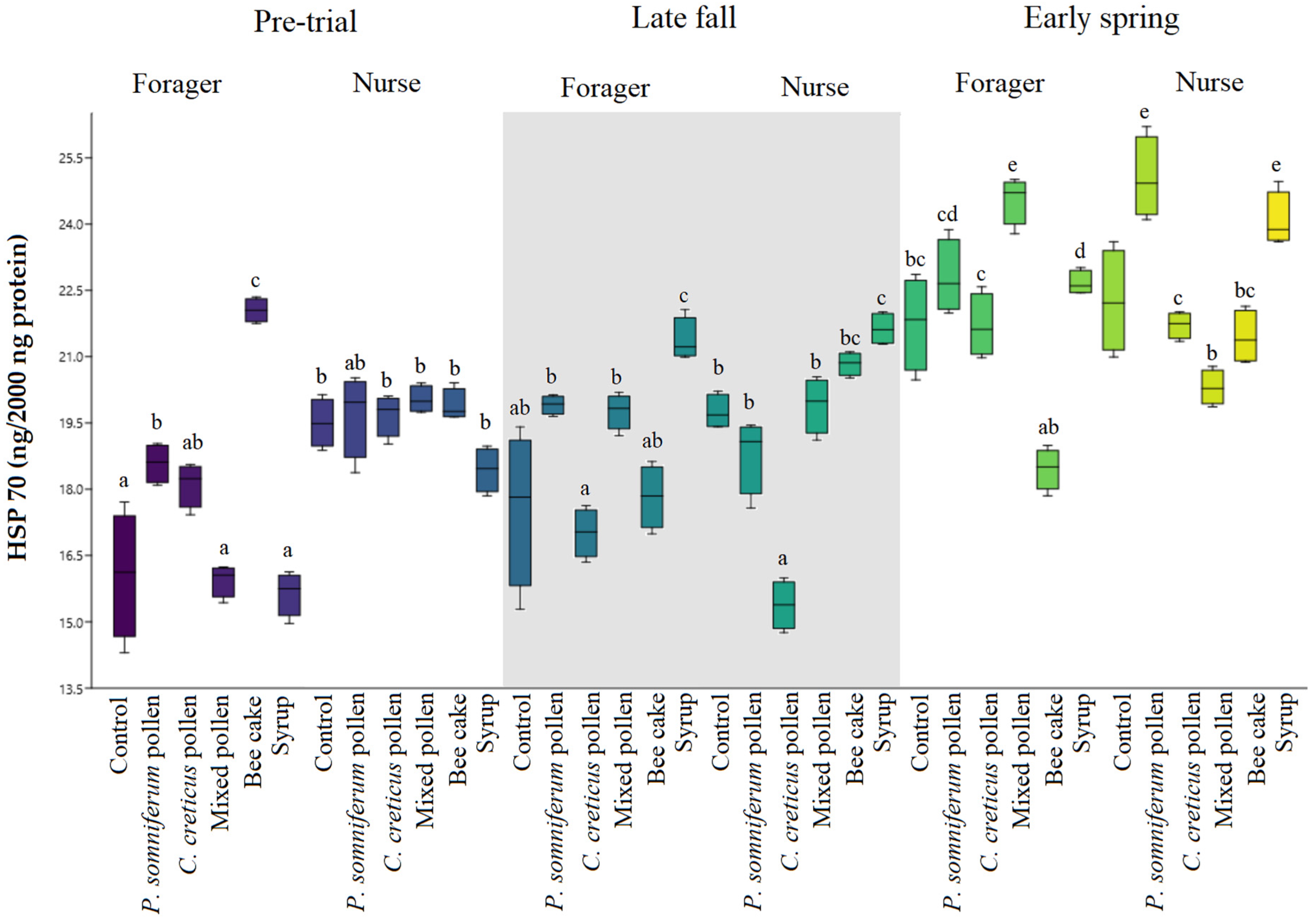
2.1. Stress Protein Analysis (HSP 70)
2.2. Vg Protein Analysis
2.3. Essential Amino Acids
2.4. Fatty Acids
2.5. Physico-Chemical Composition and Antioxidant Activity
2.6. Statistical Analysis
3. Results
3.1. Fatty Acids
3.2. Amino Acid Profiles
3.3. The General Condition of Colonies before and after Overwintering
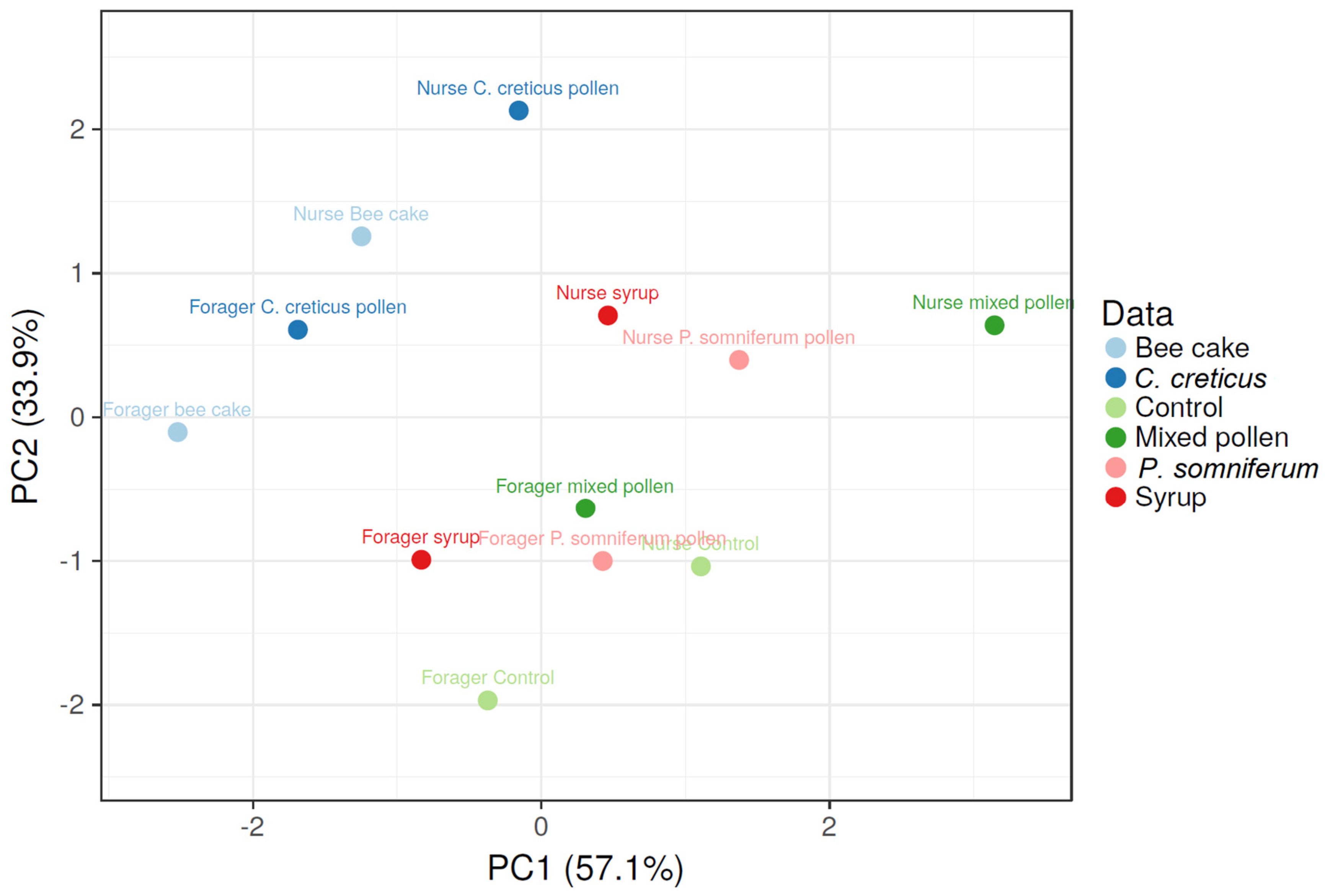
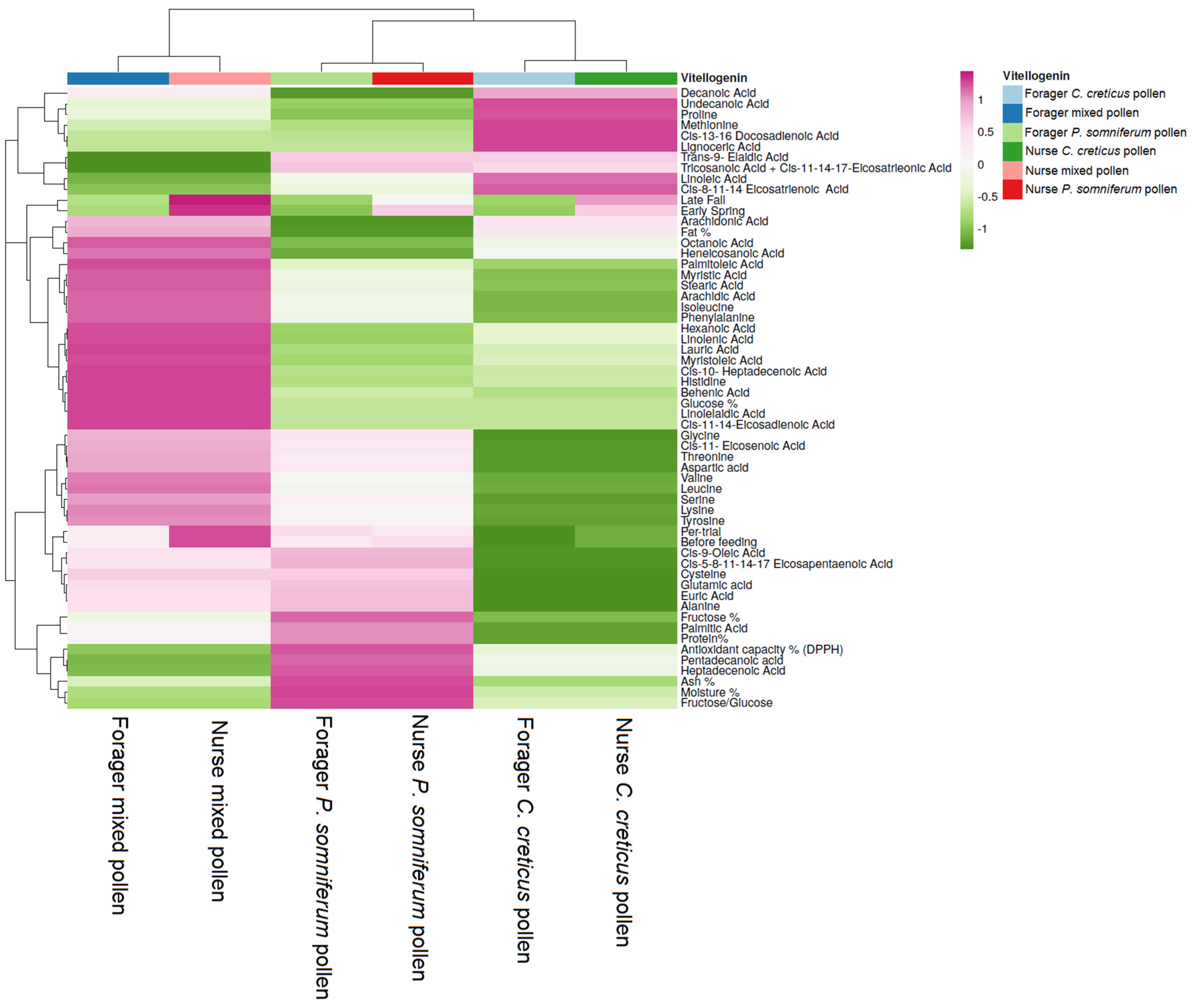
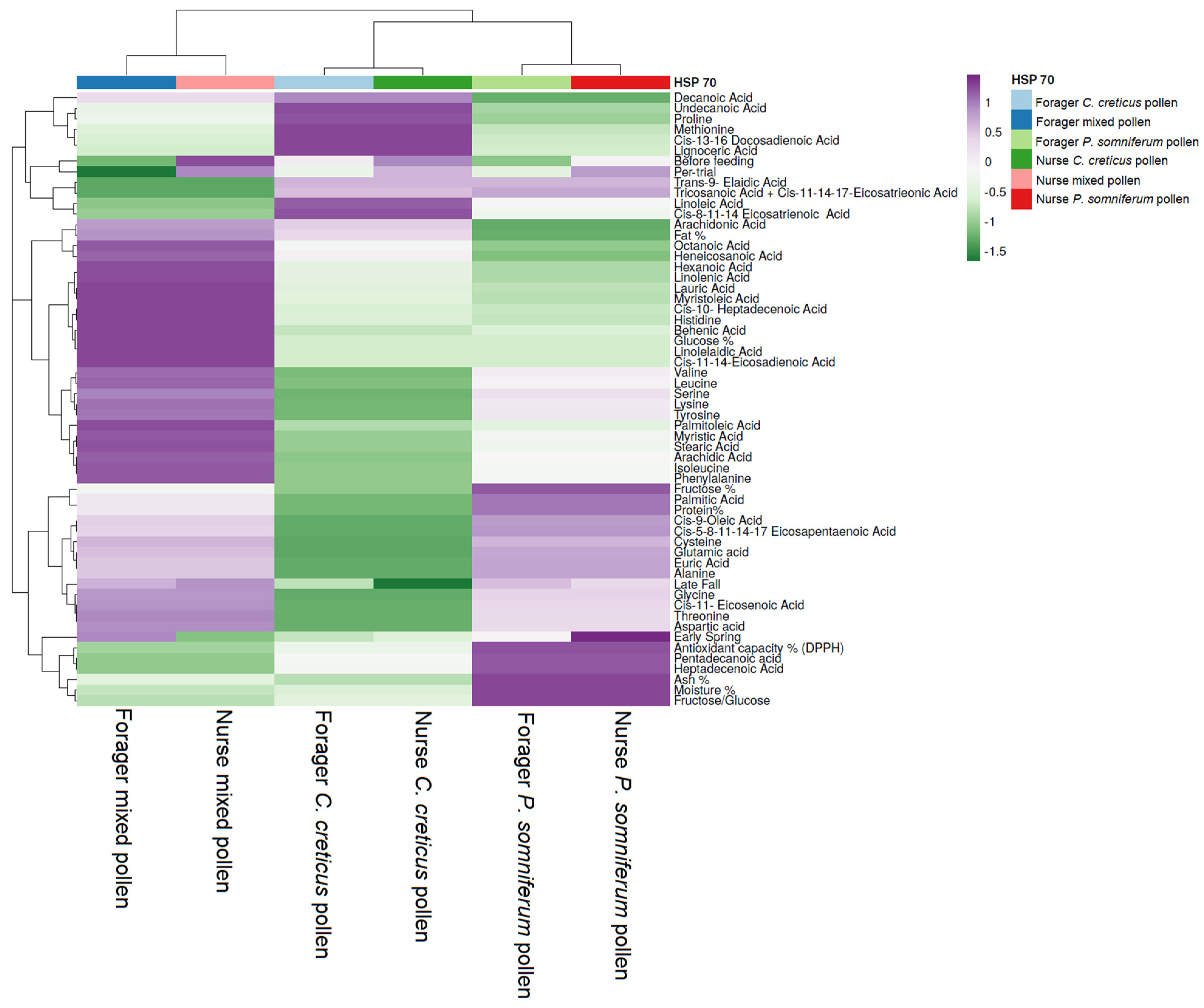
3.4. Relationship between Feeding Patterns and Vg and HSP 70 Values according to Fatty Acid and Amino Acid Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef] [PubMed]
- De Vere, N.; Jones, L.E.; Gilmore, T.; Moscrop, J.; Lowe, A.; Smith, D.; Hegarty, M.J.; Creer, S.; Ford, C.R.J.S.R. Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Sci. Rep. 2017, 7, 42838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Topal, E.; Cakici, N.; Margaoan, R.; Takma, C.; Guney, F.; Kosoglu, M.; Cornea-Cipcigan, M.; Atmaca, H. Annual Development Performance of Fixed Honeybee Colonies Linked with Chemical and Mineral Profile of Bee Collected Pollen. Chem. Biodivers. 2022, 19, e202200468. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional composition of honey bee food stores vary with floral composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Crailsheim, K.; Schneider, L.H.W.; Hrassnigg, N.; Buhlmann, G.; Brosch, U.; Gmeinbauer, R.; Schoffmann, B. Pollen Consumption and Utilization in Worker Honeybees (Apis mellifera carnica):Dependence on Individual Age and Function. J. Insect Physiol. 1992, 38, 409–419. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional Physiology and Ecology of Honey Bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef]
- Qu, N.; Jiang, J.; Sun, L.; Lai, C.; Sun, L.; Wu, X. Proteomic characterization of royal jelly proteins in Chinese (Apis cerana cerana) and European (Apis mellifera) honeybees. Biochemistry 2008, 73, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Crailsheim, K. The protein balance of the honey bee worker. Apidologie 1990, 21, 417–429. [Google Scholar] [CrossRef]
- Topal, E.; Saner, G.; Yücel, B.; Strant, M.; Engin, Ü.Ç.E.Ş.; Olgun, T.; Şengül, Z. An Evaluation on Beekeepers’ Health and Some Risk Factors in Beekeeping Farms: “A Case of Izmir-Turkey”. Tarım Ekon. Derg. 2019, 25, 149–159. [Google Scholar]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P.; vanEngelsdorp, D. Drivers of colony losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Buchler, R.; Berg, S.; Ritter, W.; Muhlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhao, H.; Liu, Z.; Wang, H.; Xu, B.; Guo, X. The Wisdom of Honeybee Defenses Against Environmental Stresses. Front. Microbiol. 2018, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Cabirol, A.; Devaud, J.M.; Barron, A.B.; Lihoreau, M. Why Bees Are So Vulnerable to Environmental Stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Guo, D.; Li, H.; Liu, Q.; Xu, B.; Guo, X. Response mechanisms to heat stress in bees. Apidologie 2021, 52, 388–399. [Google Scholar] [CrossRef]
- Bordier, C.; Klein, S.; Le Conte, Y.; Barron, A.B.; Alaux, C. Stress decreases pollen foraging performance in honeybees. J. Exp. Biol. 2018, 221, jeb171470. [Google Scholar] [CrossRef] [Green Version]
- Jandt, J.M.; Bengston, S.; Pinter-Wollman, N.; Pruitt, J.N.; Raine, N.E.; Dornhaus, A.; Sih, A. Behavioural syndromes and social insects: Personality at multiple levels. Biol. Rev. 2014, 89, 48–67. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.; Bartholmai, M.; Schiller, J.H.; Wiggerich, B.; Manolov, M. Micro-drone for the characterization and self-optimizing search of hazardous gaseous substance sources: A new approach to determine wind speed and direction. In Proceedings of the 2010 IEEE International Workshop on Robotic and Sensors Environments, Phoenix, AZ, USA, 15–16 October 2010; pp. 1–6. [Google Scholar]
- Bryden, J.; Gill, R.J.; Mitton, R.A.A.; Raine, N.E.; Jansen, V.A.A. Chronic sublethal stress causes bee colony failure. Ecol. Lett. 2013, 16, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, E.D.; Hartfelder, K. The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes. Arthropod Struct. Dev. 2004, 33, 431–442. [Google Scholar] [CrossRef]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam Gro, V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Velarde Rodrigo, A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes Kimberly, A.; Robinson Gene, E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amdam, G.V.; Fennern, E.; Havukainen, H. Vitellogenin in Honey Bee Behavior and Lifespan. In Honeybee Neurobiology and Behavior: A Tribute to Randolf Menzel; Galizia, C.G., Eisenhardt, D., Giurfa, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 17–29. [Google Scholar]
- Salmela, H.; Sundström, L. Vitellogenin in inflammation and immunity in social insects. Inflamm. Cell Signal. 2018, 5, 1–7. [Google Scholar]
- Bitondi, M.M.G.; Simões, Z.L.P. The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 1996, 35, 27–36. [Google Scholar] [CrossRef]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E., Jr.; Amdam, G.V. The Gene vitellogenin Has Multiple Coordinating Effects on Social Organization. PLOS Biol. 2007, 5, e62. [Google Scholar] [CrossRef] [PubMed]
- Antonio, D.S.M.; Guidugli-Lazzarini, K.R.; Do Nascimento, A.M.; Simões, Z.L.P.; Hartfelder, K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften 2008, 95, 953–961. [Google Scholar] [CrossRef]
- Azevedo, D.O.; Zanuncio, J.C.; Delabie, J.H.C.; Serrão, J.E. Temporal variation of vitellogenin synthesis in Ectatomma tuberculatum (Formicidae: Ectatomminae) workers. J. Insect Physiol. 2011, 57, 972–977. [Google Scholar] [CrossRef] [Green Version]
- Münch, D.; Amdam, G.V. The curious case of aging plasticity in honey bees. FEBS Lett. 2010, 584, 2496–2503. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Messan, M.; Page, R.E.; Kang, Y. Effects of vitellogenin in age polyethism and population dynamics of honeybees. Ecol. Model. 2018, 388, 88–107. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic Acid Exerts a Neuroprotective Effect Against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef] [Green Version]
- Gunes, N.; Aydın, L.; Belenli, D.; Hranitz, J.M.; Mengilig, S.; Selova, S. Stress responses of honey bees to organic acid and essential oil treatments against varroa mites. J. Apic. Res. 2017, 56, 175–181. [Google Scholar] [CrossRef]
- Sen Sarma, M.; Whitfield, C.W.; Robinson, G.E. Species differences in brain gene expression profiles associated with adult behavioral maturation in honey bees. BMC Genom. 2007, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Roberts, S.P.; Elekonich, M.M. Effect of age and oxidative stress on Hsp70 expression in the honey bee, Apis mellifera. In Proceedings of the IUSSI 2006 Congress, Washington, DC, USA, 30July–5 August 2006. [Google Scholar]
- Hranitz, J.M.; Abramson, C.I.; Carter, R.P. Ethanol increases HSP70 concentrations in honeybee (Apis mellifera L.) brain tissue. Alcohol 2010, 44, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Bobiș, O.; Bonta, V.; Dulf, F.V.; Mărgăoan, R.; Cornea-Cipcigan, M.; Campos, M.G. Botanical origin approach for a better understanding of chemical and nutritional composition of beebread as an important value-added food supplement. LWT 2021, 142, 111068. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analyses, Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Arlington, VA, USA, 2000. [Google Scholar]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Özkök, A.; Keskin, Ş.; Mayda, N.; Urcan, A.C.; Cornea-Cipcigan, M. Bee collected pollen as a value-added product rich in bioactive compounds and unsaturated fatty acids: A comparative study from Turkey and Romania. LWT 2021, 149, 111925. [Google Scholar] [CrossRef]
- Topal, E.; Mărgăoan, R.; Bay, V.; Takma, Ç.; Yücel, B.; Oskay, D.; Düz, G.; Acar, S.; Kösoğlu, M. The Effect of Supplementary Feeding with Different Pollens in Autumn on Colony Development under Natural Environment and In Vitro Lifespan of Honey Bees. Insects 2022, 13, 588. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Rivera, R.; Carroll, M.; Chambers, M.; Hidalgo, G.; de Jong, E.W. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie 2016, 47, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Yeninar, H.; Akyol, E.; Yörük, A. Effects of Additive Feeding with Pollen and Water on Some Characteristics of Honeybee Colonies and Pine Honey Production. Turk. J. Agric.-Food Sci. Technol. 2015, 3, 948–951. [Google Scholar] [CrossRef] [Green Version]
- Quesada-Béjar, V.; Contreras-Garduño, J.; Calvillo, L.K.A.; García, E.C. Survival, Body Condition, and Immune System of Apis mellifera liguistica Fed Avocado, Maize, and Polyfloral Pollen Diet. Neotrop. Èntomol. 2022, 51, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Harwood, G.; Amdam, G. Vitellogenin in the honey bee midgut. Apidologie 2021, 52, 837–847. [Google Scholar] [CrossRef]
- Johnson, B.R. Organization of work in the honeybee: A compromise between division of labour and behavioural flexibility. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, R. Fatty Acid Composition of Pollen and the Effect of Two Dominant Fatty Acids (Linoleic and Oleic) in Pollen and Flour Diets on Longevity and Nutritional Composition of Honey Bees (Apis mellifera). Ph.D. Thesis, School of Biological Sciences and Biotechnology, Murdoch University, Dell, Bernard, 2006. [Google Scholar]
- Vanderplanck, M.; Moerman, R.; Rasmont, P.; Lognay, G.; Wathelet, B.; Wattiez, R.; Michez, D. How Does Pollen Chemistry Impact Development and Feeding Behaviour of Polylectic Bees? PLoS ONE 2014, 9, e86209. [Google Scholar] [CrossRef]
- Hendriksma, H.P.; Pachow, C.D.; Nieh, J.C. Effects of essential amino acid supplementation to promote honey bee gland and muscle development in cages and colonies. J. Insect Physiol. 2019, 117, 103906. [Google Scholar] [CrossRef] [Green Version]
- Muth, F.; Breslow, P.R.; Masek, P.; Leonard, A.S. A pollen fatty acid enhances learning and survival in bumblebees. Behav. Ecol. Sociobiol. 2018, 29, 1371–1379. [Google Scholar] [CrossRef]
- Zarchin, S.; Dag, A.; Salomon, M.; Hendriksma, H.P.; Shafir, S. Honey bees dance faster for pollen that complements colony essential fatty acid deficiency. Behav. Ecol. Sociobiol. 2017, 71, 172. [Google Scholar] [CrossRef]
- Hendriksma, H.P.; Shafir, S. Honey bee foragers balance colony nutritional deficiencies. Behav. Ecol. Sociobiol. 2016, 70, 509–517. [Google Scholar] [CrossRef]
- Gage, S.L.; Calle, S.; Jacobson, N.; Carroll, M.; DeGrandi-Hoffman, G. Pollen Alters Amino Acid Levels in the Honey Bee Brain and This Relationship Changes With Age and Parasitic Stress. Front. Neurosci. 2020, 14, 231. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen Protein: Lipid Macronutrient Ratios May Guide Broad Patterns of Bee Species Floral Preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.M.; Welchert, A.C.; Carroll, M.; Shafir, S.; Smith, B.H.; Corby-Harris, V. Unbalanced fatty acid diets impair discrimination ability of honey bee workers to damaged and healthy brood odors. J. Exp. Biol. 2022, 225, jeb244103. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Williams, S.T.; Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 2022, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Awmack, C.S.; Murray, D.A.; Williams, I.H. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol. Entomol. 2003, 28, 622–627. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Assessment of physico-chemical properties, fatty acid, amino acid and mineral profile of bee pollen from India with a multivariate perspective. J. Food Nutr. Res. 2018, 57, 328–340. [Google Scholar]
- Bonoan, R.E.; Gonzalez, J.; Starks, P.T. The perils of forcing a generalist to be a specialist: Lack of dietary essential amino acids impacts honey bee pollen foraging and colony growth. J. Apic. Res. 2020, 59, 95–103. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeon, H.; Jung, C. Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ. 2020, 44, 4. [Google Scholar] [CrossRef] [Green Version]
- Amdam, G.; Ihle, K.; Page, R. Regulation of Honeybee Worker (Apis mellifera) Life Histories by Vitellogenin; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Switanek, M.; Crailsheim, K.; Truhetz, H.; Brodschneider, R. Modelling seasonal effects of temperature and precipitation on honey bee winter mortality in a temperate climate. Sci. Total Environ. 2017, 579, 1581–1587. [Google Scholar] [CrossRef]
- Vicens, N.; Bosch, J. Weather-Dependent Pollinator Activity in an Apple Orchard, with Special Reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 2000, 29, 413–420. [Google Scholar] [CrossRef]
- Szabo, T.I. Effect of Weather Factors on Honeybee Flight Activity and Colony Weight Gain. J. Apic. Res. 1980, 19, 164–171. [Google Scholar] [CrossRef]
- Abou-Shaara, H. The foraging behaviour of honey bees, Apis mellifera: A review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Park, H.G.; Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.Y.; Wan, H.; Li, J.; Jin, B.R. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018, 85, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Salmela, H.; Stark, T.; Stucki, D.; Fuchs, S.; Freitak, D.; Dey, A.; Kent, C.F.; Zayed, A.; Dhaygude, K.; Hokkanen, H.; et al. Ancient Duplications Have Led to Functional Divergence of Vitellogenin-Like Genes Potentially Involved in Inflammation and Oxidative Stress in Honey Bees. Genome Biol. Evol. 2016, 8, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Stabentheiner, A.; Kovac, H.; Mandl, M.; Käfer, H. Coping with the cold and fighting the heat: Thermal homeostasis of a superorganism, the honeybee colony. J. Comp. Physiol. A 2021, 207, 337–351. [Google Scholar] [CrossRef]
- Li, Z.; Ma, R.; Wang, L.; Wang, Y.; Qin, Q.; Chen, L.; Dang, X.; Zhou, Z. Starvation stress affects iron metabolism in honeybee Apis mellifera. Biologia 2022, 77, 2133–2148. [Google Scholar] [CrossRef]
- Dussaubat, C.; Maisonnasse, A.; Belzunces, L.P.; Brunet, J.-L.; Kretzschmar, A. Preliminary report of honeybee physiological changes pre- and post-hybrid lavender season in high and low weight gain colonies. Apidologie 2021, 52, 463–472. [Google Scholar] [CrossRef]
- Jones, J.C.; Helliwell, P.; Beekman, M.; Maleszka, R.; Oldroyd, B.P. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A 2005, 191, 1121–1129. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.C.; Pereira, E.M.; Sereia, M.J.; Lima, É.G.; Silva, B.G.; Toledo, V.A.; Ruvolo-Takasusuki, M.C. Expression of MRJP3 and HSP70 mRNA Levels in Apis mellifera L. Workers after Dietary Supplementation with Proteins, Prebiotics, and Probiotics. Insects 2022, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Morammazi, S.; Shokrollahi, B. The pattern of HSP70 gene expression, flight activity and temperature in Apis mellifera meda colonies. J. Therm. Biol. 2020, 91, 102647. [Google Scholar] [CrossRef]
- Mogren, C.L.; Margotta, J.; Danka, R.G.; Healy, K. Supplemental carbohydrates influence abiotic stress resistance in honey bees. J. Apic. Res. 2018, 57, 682–689. [Google Scholar] [CrossRef]
- Amini, M.B.; Sadeghi, A.A.; Shawrang, P.; Chamani, M.; Aminafshar, M. Nano-selenium and nano-zinc oxide supplementation in syrup on laying area, population size and HSP gene expression of honey bees in hot climate. Acta Sci. Anim. Sci. 2020, 43, e48574. [Google Scholar] [CrossRef]


| Fatty Acids | Commercial Bee Cake | C. creticus Pollen | Mixed Pollen | P. somniferum Pollen |
|---|---|---|---|---|
| Myristic Acid | nd | 1.7 ± 0.1 | 7.5 ± 0.3 | 3.8 ± 0.6 |
| Hexanoic Acid | nd | 2.3 ± 0.1 | 6.6 ± 0.2 | 1.1 ± 0.5 |
| Octanoic Acid | nd | 1.2 ± 0.0 | 2.5 ± 0.5 | 0.4 ± 0.1 |
| Decanoic Acid | nd | 8.5 ± 0.3 | 6.2 ± 0.2 | 0.7 ± 0.2 |
| Undecanoic Acid | nd | 0.4 ± 0.0 | 0.1 ± 0.0 | nd |
| Lauric Acid | nd | 1.7 ± 0.2 | 5.0 ± 0.5 | 1.2 ± 0.2 |
| Tridecanoic Acid | nd | nd | nd | nd |
| Myristoleic Acid | nd | 1.5 ± 0.1 | 4.9 ± 0.6 | 0.8 ± 0.1 |
| Pentadecanoic acid | nd | 0.8 ± 0.0 | 0.6 ± 0.1 | 1.1 ± 0.3 |
| Palmitic Acid | nd | 204.4 ± 5.7 | 293.3 ± 8.8 | 355.5 ± 15.3 |
| Palmitoleic Acid | nd | 0.9 ± 0.3 | 6.9 ± 2.1 | 2.1 ± 0.1 |
| Heptadecenoic Acid | nd | 1.2 ± 0.1 | 0.9 ± 0.2 | 1.7 ± 0.3 |
| Cis-10-Heptadecenoic Acid | nd | 0.5 ± 0.0 | 5.5 ± 0.1 | 0.2 ± 0.1 |
| Stearic Acid | nd | 7.7 ± 1.1 | 25.9 ± 0.6 | 13.8 ± 0.5 |
| Trans-9-Elaidic Acid | nd | 0.6 ± 0.2 | 0.1 ± 0.0 | 0.6 ± 0.1 |
| Cis-9-Oleic Acid | nd | 36.7 ± 5.5 | 91.5 ± 2.7 | 102.8 ± 10.9 |
| Linolelaidic Acid | nd | 1.0 ± 0.1 | 1.5 ± 1.1 | 1.0 ± 0.2 |
| Linoleic Acid | nd | 352.1 ± 8.9 | 203.3 ± 11.3 | 267.8 ± 13.6 |
| Arachidic Acid | nd | 0.4 ± 0.0 | 1.1 ± 0.2 | 0.7 ± 0.2 |
| Cis-11-Eicosenoic Acid | nd | 0.9 ± 0.0 | 3.7 ± 1.1 | 3.0 ± 0.1 |
| Linolenic Acid | nd | 7.3 ± 0.2 | 9.7 ± 2.1 | 6.6 ± 0.3 |
| Heneicosanoic Acid | nd | 1.1 ± 0.0 | 1.6 ± 0.4 | 0.6 ± 0.0 |
| Cis-11-14-Eicosadienoic Acid | nd | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| Behenic Acid | nd | 0.3 ± 0.0 | 1.5 ± 0.1 | 0.4 ± 0.2 |
| Cis-8-11-14-Eicosatrienoic Acid | nd | 0.1 ± 0.0 | 0.01 ± 0.0 | 0.04 ± 0.0 |
| Euric Acid | nd | 0.6 ± 0.1 | 3.6 ± 0.2 | 4.0 ± 0.1 |
| Tricosanoic Acid + Cis-11-14-17-Eicosatrieonic Acid | nd | 4.1 ± 0.3 | 1.7 ± 0.0 | 4.3 ± |
| Arachidonic Acid | nd | 5.1 ± 0.2 | 6.3 ± 0.1 | nd |
| Cis-13-16-Docosadienoic Acid | nd | 17.2 ± 0.6 | 0.5 ± 0.0 | 0.1 ± 0.0 |
| Lignoceric Acid | nd | 1.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Cis-5-8-11-14-17-Eicosapentaenoic Acid | nd | 1.9 ± 0.1 | 3.4 ± 0.0 | 3.8 ± 0.1 |
| Total | - | 663.6 | 695.9 | 778.3 |
| Commercial Bee Cake | C. creticus Pollen | Mixed Pollen | P. somniferum Pollen | |
|---|---|---|---|---|
| Alanine | nd | 8.1 ± 2.2 | 9.5 ± 0.6 | 9.7 ± 0.4 |
| Glycine | nd | 4.8 ± 1.2 | 7.2 ± 0.5 | 6.7 ± 0.7 |
| Valine | nd | 4.2 ± 0.9 | 5.9 ± 1.1 | 5.1 ± 0.6 |
| Leucine | nd | 10.6 ± 2.4 | 16.2 ± 3.3 | 13.3 ± 1.7 |
| Isoleucine | nd | 3.9 ± 2.2 | 5.9 ± 0.5 | 4.7 ± 0.5 |
| Threonine | nd | 1.6 ± 0.6 | 3.0 ± 0.7 | 2.6 ± 0.4 |
| Serine | nd | 2.5 ± 0.3 | 4.8 ± 2.9 | 4.0 ± 0.6 |
| Proline | nd | 33.9 ± 11.3 | 25.7 ± 5.1 | 21.9 ± 0.9 |
| Aspartic acid | nd | 9.7 ± 1.4 | 16.6 ± 4.4 | 14.7 ± 3.3 |
| Methionine | nd | 2.4 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 |
| Hydroxyproline | nd | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Glutamic acid | 0.1 | 15.1 ± 0.5 | 20.3 ± 2.3 | 20.8 ± 4.3 |
| Phenylalanine | nd | 7.1 ± 0.3 | 11.4 ± 0.5 | 8.8 ± 2.3 |
| Lysine | 0.1 | 21.3 ± 0.6 | 31.9 ± 1.6 | 27.3 ± 5.1 |
| Histidine | nd | 6.8 ± 0.5 | 9.7 ± 0.4 | 6.5 ± 2.2 |
| Hydroxylysine | nd | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Tyrosine | nd | 3.1 ± 0.3 | 6.0 ± 0.5 | 4.8 ± 2.1 |
| Cysteine | nd | 1.8 ± 1.1 | 1.9 ± 0.2 | 1.9 ± 0.7 |
| Total | 0.2 | 137.4 | 178.1 | 154.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarioğlu-Bozkurt, A.; Topal, E.; Güneş, N.; Üçeş, E.; Cornea-Cipcigan, M.; Coşkun, İ.; Cuibus, L.; Mărgăoan, R. Changes in Vitellogenin (Vg) and Stress Protein (HSP 70) in Honey Bee (Apis mellifera anatoliaca) Groups under Different Diets Linked with Physico-Chemical, Antioxidant and Fatty and Amino Acid Profiles. Insects 2022, 13, 985. https://doi.org/10.3390/insects13110985
Sarioğlu-Bozkurt A, Topal E, Güneş N, Üçeş E, Cornea-Cipcigan M, Coşkun İ, Cuibus L, Mărgăoan R. Changes in Vitellogenin (Vg) and Stress Protein (HSP 70) in Honey Bee (Apis mellifera anatoliaca) Groups under Different Diets Linked with Physico-Chemical, Antioxidant and Fatty and Amino Acid Profiles. Insects. 2022; 13(11):985. https://doi.org/10.3390/insects13110985
Chicago/Turabian StyleSarioğlu-Bozkurt, Aybike, Erkan Topal, Nazmiye Güneş, Engin Üçeş, Mihaiela Cornea-Cipcigan, İlknur Coşkun, Lucian Cuibus, and Rodica Mărgăoan. 2022. "Changes in Vitellogenin (Vg) and Stress Protein (HSP 70) in Honey Bee (Apis mellifera anatoliaca) Groups under Different Diets Linked with Physico-Chemical, Antioxidant and Fatty and Amino Acid Profiles" Insects 13, no. 11: 985. https://doi.org/10.3390/insects13110985







