Prey-Mediated Effects of Drought on the Consumption Rates of Coccinellid Predators of Elatobium abietinum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drought Treatments
2.2. Plant Material
2.2.1. Material for Petri Dish Arena Cuttings
2.2.2. Material for E. abietinum Cultures
2.3. Insect Cultures
2.3.1. Aphids
2.3.2. Coccinellids
2.4. Consumption in a Petri Dish
2.4.1. Adult Consumption
2.4.2. Larval Consumption
2.5. Consumption on Host Plant Material
2.6. Statistical Analysis
3. Results
3.1. Effects on Adult Coccinellids
3.1.1. Aphidecta obliterata Adults
3.1.2. Adalia bipunctata Adults
3.2. Effects on 1st Instar Coccinellid Larvae
3.2.1. Aphidecta obliterata Larvae
3.2.2. Adalia bipunctata Larvae
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Pellissier, L.; Defossez, E.; Jactel, H.; Kunstler, G. Climate-driven change in plant-insect interactions along elevation gradients. Funct. Ecol. 2014, 28, 46–54. [Google Scholar] [CrossRef]
- Pritchard, J.; Griffiths, B.; Hunt, E.J. Can the plant-mediated impacts on aphids of elevated CO2 and drought be predicted? Glob. Chang. Biol. 2007, 13, 1616–1629. [Google Scholar] [CrossRef]
- Hale, B.K.; Bale, J.S.; Pritchard, J.; Masters, G.J. Effects of host plant drought stress on the performance of the bird cherry-oat aphid, Rhopalosiphum padi (L.): A mechanistic analysis. Ecol. Entomol. 2003, 28, 666–677. [Google Scholar] [CrossRef]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Mody, K.; Eichenberger, D.; Dorn, S. Stress magnitude matters: Different intensities of pulsed water stress produce non-monotonic resistance responses of host plants to insect herbivores. Ecol. Entomol. 2009, 34, 133–143. [Google Scholar] [CrossRef]
- Romo, C.M.; Tylianakis, J.M. Elevated temperature and drought interact to reduce parasitoid effectiveness in suppressing hosts. PLoS ONE 2013, 8, e58136. [Google Scholar] [CrossRef] [PubMed]
- McCluney, K.E.; Sabo, J.L. Water availability directly determines per capita consumption at two trophic levels. Ecology 2009, 90, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- McCluney, K.E.; Sabo, J.L. Animal water balance drives top-down effects in a riparian forest—Implications for terrestrial trophic cascades. Proc. R. Soc. B 2016. [Google Scholar] [CrossRef] [PubMed]
- McCluney, K.E.; Sabo, J.L. Sensitivity and tolerance of riparian arthropod communities to altered water resources along a drying river. PLoS ONE 2014, 9, e109276. [Google Scholar] [CrossRef] [PubMed]
- Koricheva, J.; Larsson, S.; Haukioja, E. Insect performance on experimentally stressed woody plants: A meta-analysis. Ann. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Banfield-Zanin, J.A.; Leather, S.R. Frequency and intensity of drought stress alters the population size and dynamics of Elatobium abietinum on Sitka spruce. Ann. Appl. Biol. 2014, 165, 260–269. [Google Scholar] [CrossRef]
- Banfield-Zanin, J.A.; Leather, S.R. Season and drought stress mediate growth and weight of the green spruce aphid on Sitka spruce. Agric. For. Entomol. 2015, 17, 48–56. [Google Scholar] [CrossRef]
- Banfield-Zanin, J.A.; Leather, S.R. Drought intensity and frequency have contrasting effects on development time and survival of the green spruce aphid. Agric. For. Entomol. 2015, 17, 309–316. [Google Scholar] [CrossRef]
- Banfield-Zanin, J.A.; Leather, S.R. Reproduction of an arboreal aphid pest, Elatobium abietinum, is altered under drought stress. J. Appl. Entomol. 2015, 139, 302–313. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhang, X.S.; Huang, Z.Y. Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J. Plant Ecol. 2010, 3, 79–87. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Influences of species, latitudes, and methodologies on estimates of phenological response to global warming. Glob. Chang. Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Binzer, A.; Guill, C.; Brose, U.; Rall, B.C. The dynamics of food chains under climate change and nutrient enrichment. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, C.T.; Lewis, O.T. Effects of climate-warming on host-parasitoid interactions. Ecol. Entomol. 2013, 38, 209–218. [Google Scholar] [CrossRef]
- Voigt, W.; Perner, J.; Davis, A.J.; Eggers, T.; Schumacher, J.; Bährmann, R.; Fabian, B.; Heinrich, W.; Köhler, G.; Lichter, D.; et al. Trophic levels are differentially sensitive to climate. Ecology 2003, 84, 2444–2453. [Google Scholar] [CrossRef]
- Staley, J.T.; Girling, R.D.; Stewart-Jones, A.; Poppy, G.M.; Leather, S.R.; Wright, D.J. Organic and conventional fertilizer effects on a tritrophic interaction: Parasitism, performance and preference of Cotesia vestalis. J. Appl. Entomol. 2011, 135, 658–665. [Google Scholar] [CrossRef]
- Banfield-Zanin, J.A.; Rossiter, J.T.; Wright, D.J.; Leather, S.R.; Staley, J.T. Predator mortality depends on whether its prey feeds on organic or conventionally fertilised plants. Biol. Control 2012, 63, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Caballero-López, B.; Blanco-Moreno, J.M.; Pérez-Hidalgo, N.; Michelena-Saval, J.M.; Pujade-Villar, J.; Guerrieri, E.; Sánchez-Espigares, J.A.; Sans, F.X. Weeds, aphids, and specialist parasitoids and predators benefit differently from organic and conventional cropping of winter cereals. J. Pest Sci. 2012, 85, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Timms, J.E.L.; Leather, S.R. Ladybird egg cluster size: Relationships between species, oviposition substrate and cannibalism. Bull. Entomol. Res. 2007, 97, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Timms, J.E.; Oliver, T.H.; Straw, N.A.; Leather, S.R. The effects of host plant on the coccinellid functional response: Is the conifer specialist Aphidecta obliterata (L.) (Coleoptera: Coccinellidae) better adapted to spruce than the generalist Adalia bipunctata (L.) (Coleoptera: Coccinellidae)? Biol. Control 2008, 47, 273–281. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Leather, S.R. Nitrogen fertiliser affects the functional response and prey consumption of Harmonia axyridis (Coleoptera: Coccinellidae) feeding on cereal aphids. Ann. Appl. Biol. 2012, 160, 6–15. [Google Scholar] [CrossRef]
- Pons, X.; Tatchell, G.M. Drought stress and cereal aphid performance. Ann. Appl. Biol. 1995, 126, 19–31. [Google Scholar] [CrossRef]
- Aslam, T.J.; Johnson, S.N.; Karley, A.J. Plant-mediated effects of drought on aphid population structure and parasitoid attack. J. Appl. Entomol. 2013, 137, 136–145. [Google Scholar] [CrossRef]
- Crute, S.; Day, K.R. Chapter 29. Understanding the impact of natural enemies on spruce aphid populations through simulation modelling. In Population Dynamics of Forest Insects; Intercept Ltd.: Andover, UK, 1990; pp. 329–337. [Google Scholar]
- Day, K.R.; Kidd, N.A.C. Chapter 4. Green spruce aphid population dynamics: Effects of climate, weather and regulation. In The Green Spruce Aphid in Western Europe: Ecology, Status, Impacts and Prospects for Management; Forestry Commission: Edinburgh, UK, 1998; pp. 41–52. [Google Scholar]
- Day, K.R.; Ayres, M.P.; Harrington, R.; Kidd, N.A.C. Interannual dynamics of aerial and arboreal green spruce aphid populations. Popul. Ecol. 2010, 52, 317–327. [Google Scholar] [CrossRef]
- Straw, N.A. Climate change and the impact of green spruce aphid, Elatobium abietinum (Walker), in the UK. Scott. For. 1995, 49, 134–145. [Google Scholar]
- Austarå, O.; Carter, C.; Eilenburg, J.; Halldórsson, G.; Harding, S. Chapter 5. A conspectus of potential natural enemies found in association with the green spruce aphid in north-west European spruce plantations. In The Green Spruce Aphid in Western Europe: Ecology, Status, Impacts and Prospects for Management; Forestry Commission: Edinburgh, UK, 1998; pp. 53–60. [Google Scholar]
- Timms, J.E.L. Factors Affecting Natural Control of the Green Spruce Aphid, Elatobium abietinum (Walker). Ph.D. Thesis, Imperial College London, London, UK, 2004. [Google Scholar]
- Parry, W.H. A comparison of Aphidecta obliteratae (L.) (Col., Coccinellidae) populations feeding on Elatobium abietinum (Walker) and on Adelges cooleyi (Gillette). J. Appl. Entomol. 1992, 114, 280–288. [Google Scholar] [CrossRef]
- Murphy, J.M.; Sexton, D.M.H.; Jenkins, G.J.; Boorman, P.M.; Booth, B.B.B.; Brown, C.C.; Clark, R.T.; Collins, M.; Harris, G.R.; Kendon, E.J.; et al. UK Climate Projections Science Report: UKCP09; Met Office Hadley Centre: Exeter, UK, 2009.
- Johnson, S.N.; Staley, J.T.; McLeod, F.A.L.; Hartley, S.E. Plant-mediated effects of soil invertebrates and summer drought on above-ground multitrophic interactions. J. Ecol. 2011, 99, 57–65. [Google Scholar] [CrossRef]
- Samuel, C.J.A.; Fletcher, A.M.; Lines, R. Choice of Sitka Spruce Seed Origins for Use in British Forests; Forestry Commission: Edinburgh, UK, 2007.
- Gardiner, B.; Leban, J.M.; Auty, D.; Simpson, H. Models for predicting wood density on British-grown Sitka spruce. Forestry 2011, 84, 119–132. [Google Scholar] [CrossRef]
- Straw, N.A.; Fielding, N.J.; Green, G.; Price, J. Defolspruce and growth loss in young Sitka spruce following repeated attack by the green spruce aphid, Elatobium abietinum (Walker). For. Ecol. Manag. 2005, 213, 349–368. [Google Scholar] [CrossRef]
- Sarah, G.; Ray, D. Potential Impacts of Drought and Disease on Forestry in Scotland. In Research Note FCRN004: Forest Research (Forestry Commission); HMSO: London, UK, 2009. [Google Scholar]
- Jarvis, N.J.; Mullins, C.E. Modelling the effects of drought on the growth of Sitka spruce in Scotland. Forestry 1987, 60, 13–30. [Google Scholar] [CrossRef]
- Hodek, I. Biology of Coccinellidae; Academia: Prague, Czech Republic, 1973. [Google Scholar]
- Leather, S.R.; Owuor, A. The influence of natural enemies and migration on spring populations of the green spruce aphid, Elatobium abietinum Walker (Hom., Aphididae). J. Appl. Entomol. 1996, 120, 529–536. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B. Lme4: Linear Mixed-Effects Models Using S4 Classes. r package version 0.999999-0 ed. 2012. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evolut. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J. The R Book, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Evans, H.; Straw, N.A.; Watt, A.D. Chapter 8. Climate Change: Implications for Insect Pests. In Climate Change: Impacts on UK Forests; Forestry Commission: Edinburgh, UK, 2002; pp. 99–118. [Google Scholar]
- Heijari, J.; Nerg, A.M.; Holopainen, J.K.; Kainulainen, P. Wood borer performance and wood characteristics of drought-stressed Scots pine seedlings. Entomol. Exp. Appl. 2010, 137, 105–110. [Google Scholar] [CrossRef]
- Major, E.J. Chapter 8. Water Stress in Sitka Spruce and its Effect on the Green Spruce Aphid Elatobium abietinum. In Population Dynamics of Forest Insects; Intercept Ltd.: Andover, UK, 1990; pp. 85–93. [Google Scholar]
- Branco, M.; Pereira, J.S.; Mateus, E.; Tavares, C.; Paiva, M.R. Water stress affects Tomicus destruens host pine preference and performance during the shoot feeding phase. Ann. For. Sci. 2010. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Ulrichs, C.; Mewis, I. Water stress alters aphid-induced glucosinolate response in Brassica oleracea var. italica differently. Chemoecology 2011, 21, 235–242. [Google Scholar] [CrossRef]
- Hu, B.; Simon, J.; Rennenberg, H. Drought and air warming affect the species-specific levels of stress-related foliar metabolites of three oak species on acidic and calcareous soil. Tree Physiol. 2013, 33, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Cregg, B.M.; Zhang, J.W. Physiology and morphology of Pinus sylvestris seedlings from diverse sources under cyclic drought stress. For. Ecol. Manag. 2001, 154, 131–139. [Google Scholar] [CrossRef]
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salguero, R.; Navaro-Cerrillo, R.M.; Camarero, J.J.; Fernández-Cancio, A. Selective drought-induced decline of pine species in southeastern Spain. Clim. Chang. 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Gessler, A. Global climate change and tree nutrition: Influence of water availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.D.R.; Pinho, B.X.; Meyer, S.T.; Wirth, R.; Leal, I.R. Drought stress drives intraspecific choice of food plants by Atta leaf-cutting ants. Entomol. Exp. Appl. 2012, 144, 209–215. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Battaglia, M.; Pinkard, E.A. Counting the costs of multiple stressors: Is the whole greater than the sum of the parts? Tree Physiol. 2013, 33, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Lamb, K.P.; Booth, C.O. Responses of Aphis fabae Scop. to water shortage in host plants in pots. Entomol. Exp. Appl. 1958, 1, 274–291. [Google Scholar] [CrossRef]
- McVean, R.I.K.; Dixon, A.F.G. The effect of plant drought-stress on populations of the pea aphid Acyrthosiphon pisum. Ecol. Entomol. 2001, 26, 440–443. [Google Scholar] [CrossRef]
- Simpson, K.L.S.; Jackson, G.E.; Grace, J. The response of aphids to plant water stress—The case of Myzus persicae and Brassica oleracea var capitata. Entomol. Exp. Appl. 2012, 142, 191–202. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Ulrichs, C.; Mewis, I. Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomol. Exp. Appl. 2010, 137, 229–236. [Google Scholar]
- Gutbrodt, B.; Mody, K.; Dorn, S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos 2011, 120, 1732–1740. [Google Scholar] [CrossRef]
- Staley, J.T.; Mortimer, S.R.; Morecroft, M.D.; Brown, V.K.; Masters, G.J. Summer drought alters plant-mediated competition between foliar- and root-feeding insects. Glob. Chang. Biol. 2007, 13, 866–877. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Factors limiting the effectiveness of the coccinellid beetle, Adalia bipunctata (L.), as a predator of the sycamore aphid, Drepanosiphum platanoides (Schr.). J. Anim. Ecol. 1970, 39, 739–751. [Google Scholar] [CrossRef]
- Paiva, M.R.; Mateus, E.; Santos, M.H.; Branco, M.R. Pine volatiles mediate host selection for oviposition by Thaumetopoea pityocampa (Lep., Notodontidae). J. Appl. Entomol. 2011, 135, 195–203. [Google Scholar] [CrossRef]
- Uefune, M.; Kugimiya, S.; Sano, K.; Takabayashi, J. Herbivore-induced plant volatiles enhance the ability of parasitic wasps to find hosts on a plant. J. Appl. Entomol. 2012, 136, 133–138. [Google Scholar] [CrossRef]
- Moser, G.; Leuschner, C.; Hertal, D.; Hölscher, D.; Köhler, M.; Leitner, D.; Michalzik, B.; Prihastanti, E.; Tjitrosemito, S.; Schwendenmann, L. Response of cocoa trees (Theobroma cacao) to a 13-month desiccation period in Sulawesi, Indonesia. Agrofor. Syst. 2010, 79, 171–187. [Google Scholar] [CrossRef]
- Hassell, M.P.; Lawton, J.H.; Beddington, J.R. Sigmoid functional responses by invertebrate predators and parasitoids. J. Anim. Ecol. 1977, 46, 249–262. [Google Scholar] [CrossRef]
- Everson, P. The relative activity and functional response of Phytoseiulus persimilis (Acarina: Phytoseiidae) and Tetranychus urticae (Acarina: Tetranychidae): The effect of temperature. Can. Entomol. 1980, 112, 17–24. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Number 44 in Studies in Biology. In Biology of Aphids; Edward Arnold Ltd.: London, UK, 1973. [Google Scholar]
- Day, K.R.; Docherty, M.; Leather, S.R.; Kidd, N.A.C. The role of generalist insect predators and pathogens in suppressing green spruce aphid populations through direct mortality and mediation of aphid dropping behaviour. Biol. Control 2006, 38, 233–246. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Insect Predator-Prey Dynamics: Ladybird Beetles and Biological Control; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Johns, C.V.; Beaumont, L.J.; Hughes, L. Effects of elevated CO2 and temperature on development and consumption rates of Octotoma championi and O. scabripennis feeding on Lantana camara. Entomol. Exp. Appl. 2003, 108, 169–178. [Google Scholar] [CrossRef]
- Dyer, L.; Richards, L.A.; Short, S.A.; Dodson, D.C. Effects of CO2 and temperature on tritrophic interactions. PLoS ONE 2013, 8, e62528. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Norby, R.J.; Lincoln, D.E. Effects of elevated CO2 and temperature-grown red and sugar maple on gypsy moth performance. Glob. Chang. Biol. 2000, 6, 685–695. [Google Scholar] [CrossRef]
- Lindroth, R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010, 36, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Darling, E.S.; Cote, I.M. Quantifying the evidence for ecological synergies. Ecol. Lett. 2008, 11, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, W.I.; Vicca, S.; Dijkstra, F.A.; Hagedorn, F.; Hovenden, M.J.; Larsen, K.S.; Morgan, J.A.; Volder, A.; Beier, C.; Dukes, J.S.; et al. Simple additive effects are rare: A quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Chang. Biol. 2012, 18, 2681–2693. [Google Scholar] [CrossRef] [PubMed]

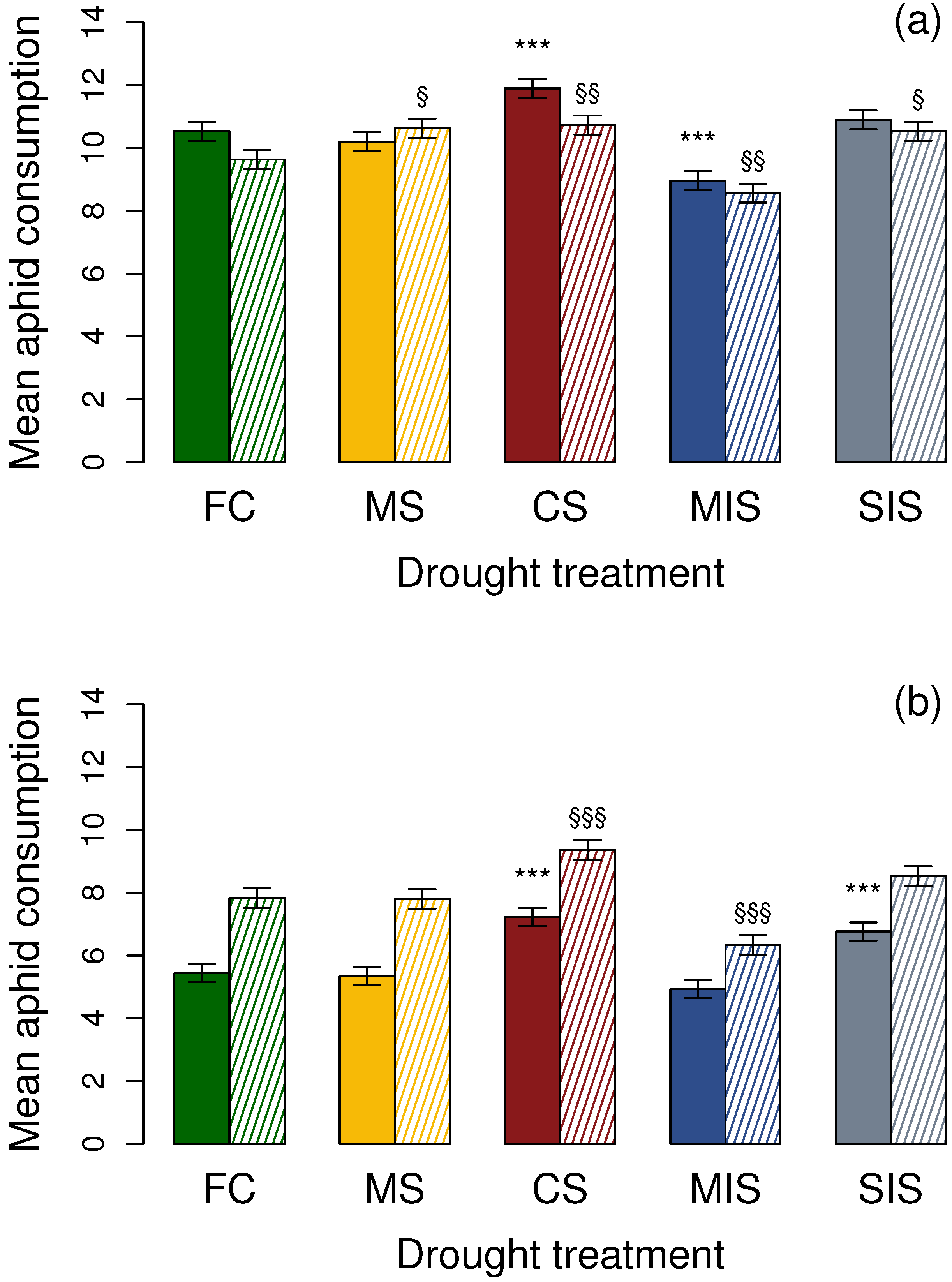
| A. obliterata | A. bipunctata | |||
|---|---|---|---|---|
| Drought | with Host Plant | without Host Plant | with Host Plant | without Host Plant |
| ± SE | ± SE | ± SE | ± SE | |
| FC | 26.67 ± 1.01 | 29.75 ± 0.91 | 45.90 ± 1.23 | 54.01 ± 0.97 |
| MS | 30.21 ± 1.15 | 32.76 ± 1.08 | 47.64 ± 1.26 | 58.58 ± 0.85 |
| CS | 35.43 ± 1.20 | 36.62 ± 0.95 | 54.02 ± 1.18 | 63.66 ± 1.14 |
| MIS | 24.02 ± 1.26 | 30.51 ± 1.15 | 46.02 ± 1.08 | 53.32 ± 1.04 |
| SIS | 34.54 ± 1.35 | 35.66 ± 1.19 | 50.77 ± 1.31 | 60.26 ± 1.36 |
| Drought | A. obliterata | A. bipunctata | ||
|---|---|---|---|---|
| with Host Plant | without Host Plant | with Host Plant | without Host Plant | |
| ± SE | ± SE | ± SE | ± SE | |
| FC | 10.46 ± 0.22 | 9.58 ± 0.19 | 5.34 ± 0.18 | 7.74 ± 0.21 |
| MS | 10.15 ± 0.18 | 10.57 ± 0.21 | 5.23 ± 0.20 | 7.68 ± 0.26 |
| CS | 11.83 ± 0.25 | 10.66 ± 0.24 | 7.09 ± 0.26 | 9.31 ± 0.18 |
| MIS | 8.94 ± 0.14 | 8.48 ± 0.23 | 4.88 ± 0.14 | 6.19 ± 0.25 |
| SIS | 10.80 ± 0.28 | 10.48 ± 0.20 | 6.66 ± 0.22 | 8.46 ± 0.21 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banfield-Zanin, J.A.; Leather, S.R. Prey-Mediated Effects of Drought on the Consumption Rates of Coccinellid Predators of Elatobium abietinum. Insects 2016, 7, 49. https://doi.org/10.3390/insects7040049
Banfield-Zanin JA, Leather SR. Prey-Mediated Effects of Drought on the Consumption Rates of Coccinellid Predators of Elatobium abietinum. Insects. 2016; 7(4):49. https://doi.org/10.3390/insects7040049
Chicago/Turabian StyleBanfield-Zanin, Jennifer A., and Simon R. Leather. 2016. "Prey-Mediated Effects of Drought on the Consumption Rates of Coccinellid Predators of Elatobium abietinum" Insects 7, no. 4: 49. https://doi.org/10.3390/insects7040049






