Non-Crop Host Sampling Yields Insights into Small-Scale Population Dynamics of Drosophila suzukii (Matsumura)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Seasonal Host Use
3.2. Trapping
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Panizzi, A.R. Wild hosts of pentatomids: Ecological significance and role in their pest status on crops. Annu. Rev. Entomol. 1997, 42, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.G.; Storer, N.P. Life systems of polyphagous arthropod pests in temporally unstable cropping systems. Annu. Rev. Entomol. 2000, 45, 467–493. [Google Scholar] [CrossRef] [PubMed]
- Aluja, M.; Mangan, R.L. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 2008, 53, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Rwomushana, I.; Ekesi, S.; Gordon, I.; Ogol, C.K. Host plants and host plant preference studies for Bactrocera invadens (Diptera: Tephritidae) in Kenya, a new invasive fruit fly species in Africa. Ann. Entomol. Soc. Am. 2008, 101, 331–340. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying host potentials: Indexing postharvest fresh fruits for spotted wing drosophila, Drosophila suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef] [PubMed]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Sarto, V.; Sorribas, R. Drosophila suzukii (Matsumura, 1931) nueva amenaza para las producciones agrícolas. Phytoma Esp. 2011, 234, 1–6. (In Spanish) [Google Scholar]
- SWD Impacts 2013. Available online: http://swd.ces.ncsu.edu/working-group-activities/swd-impacts-2013/ (accessed on 26 November 2017).
- Lee, J.C.; Dreves, A.J.; Cave, A.M.; Kawai, S.; Isaacs, R.; Miller, J.C.; Van Timmeren, S.; Bruck, D.J. Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 2015, 108, 117–129. [Google Scholar] [CrossRef]
- Kenis, M.; Tonina, L.; Eschen, R.; van der Sluis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-crop plants used as hosts by Drosophila suzukii. J. Pest Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Klick, J.; Yang, W.Q.; Walton, V.M.; Dalton, D.T.; Hagler, J.R.; Dreves, A.J.; Lee, J.C.; Bruck, D.J. Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J. Appl. Entomol. 2016, 140, 37–46. [Google Scholar] [CrossRef]
- Evans, R.K.; Toews, M.D.; Sial, A.A. Diel periodicity of Drosophila suzukii (Diptera: Drosophilidae) under field conditions. PLoS ONE 2017, 12, e0171718. [Google Scholar] [CrossRef] [PubMed]
- Swoboda Bhattarai, K.A. Determining Factors that Affect Host Use by the Invasive Vinegar Fly Drosophila suzukii (Diptera: Drosophilidae). Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2017. [Google Scholar]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Stacconi, M.V.; Kaur, R.; Mazzoni, V.; Ometto, L.; Grassi, A.; Gottardello, A.; Rota-Stabelli, O.; Anfora, G. Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii. J. Pest Sci. 2016, 89, 689–700. [Google Scholar] [CrossRef]
- Mitsui, H.; Beppu, K.; Kimura, M.T. Seasonal life cycles and resource uses of flower-and fruit-feeding drosophilid flies (Diptera: Drosophilidae) in central Japan. Entomol. Sci. 2010, 13, 60–67. [Google Scholar] [CrossRef]
- Tochen, S.; Walton, V.M.; Lee, J.C. Impact of floral feeding on adult Drosophila suzukii. J. Pest Sci. 2016, 89, 793–802. [Google Scholar] [CrossRef]
- Stephens, A.R.; Asplen, M.K.; Hutchison, W.D.; Venette, R.C. Cold hardiness of winter-acclimated Drosophila suzukii (Diptera: Drosophilidae) adults. Environ. Entomol. 2015, 44, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Shearer, P.W.; West, J.D.; Walton, V.M.; Brown, P.H.; Svetec, N.; Chiu, J.C. Seasonal cues induce phenotypic plasticity of Drosophila suzukii to enhance winter survival. BMC Ecol. 2016, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, A.K.; Loeb, G.M. Developmental acclimation of Drosophila suzukii (Diptera: Drosophilidae) and its effect on diapause and winter stress tolerance. Environ. Entomol. 2016, 45, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Arnó, J.; Solà, M.; Riudavets, J.; Gabarra, R. Population dynamics, non-crop hosts, and fruit susceptibility of Drosophila suzukii. J. Pest Sci. 2016, 89, 713–723. [Google Scholar] [CrossRef]
- Poyet, M.; Eslin, P.; Héraude, M.; Le Roux, V.; Prévost, G.; Gibert, P.; Chabrerie, O. Invasive host for invasive pest: When the Asiatic cherry fly (Drosophila suzukii) meets the American black cherry (Prunus serotina) in Europe. Agric. For. Entomol. 2014, 16, 251–259. [Google Scholar] [CrossRef]
- Swoboda-Bhattarai, K.A.; Burrack, H.J. Drosophila suzukii infestation in ripe and ripening caneberries. In Proceedings of the XI International Rubus and Ribes Symposium, Asheville, NC, USA, 21–24 June 2015; Volume 1133, pp. 419–430. [Google Scholar]
- Gleason, H.; Cronquist, A. Manual of Vascular Plants of Northeastern United States and Adjacent Canada, 2nd ed.; The New York Botanical Garden Press: Bronx, NY, USA, 1991. [Google Scholar]
- Burrack, H.J.; Asplen, M.; Bahder, L.; Collins, J.; Drummond, F.A.; Guédot, C.; Isaacs, R.; Johnson, D.; Blanton, A.; Lee, J.C.; et al. Multistate comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2015, 44, 704–712. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 20 September 2017).
- Tochen, S.; Dalton, D.T.; Wiman, N.G.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, F.; Pansa, M.G.; Ingegno, B.L.; Tavella, L.; Alma, A. Monitoring of the exotic fly Drosophila suzukii in stone, pome and soft fruit orchards in NW Italy. J. Asia-Pac. Entomol. 2015, 18, 321–329. [Google Scholar] [CrossRef]
- Wang, X.G.; Stewart, T.J.; Biondi, A.; Chavez, B.A.; Ingels, C.; Caprile, J.; Grant, J.A.; Walton, V.M.; Daane, K.M. Population dynamics and ecology of Drosophila suzukii. J. Pest Sci. 2016, 89, 701–712. [Google Scholar] [CrossRef]
- U.S. Climate Data. Available online: https://www.usclimatedata.com/climate/geneva/new-york/united-states/usny0548 (accessed on 17 November 2017).
- Hardin, J.A.; Kraus, D.A.; Burrack, H.J. Diet quality mitigates intraspecific larval competition in Drosophila suzukii. Entomol. Exp. Appl. 2015, 156, 59–65. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Swoboda-Bhattarai, K.A.; Burrack, H.J. Ovipositional preference, fidelity, and fitness of Drosophila suzukii. J. Pest Sci. 2016, 89, 761–769. [Google Scholar] [CrossRef]
- Rodriguez, D.J. A model of population dynamics for the fruit fly Drosophila melanogaster with density dependence in more than one life stage and delayed density effects. J. Anim. Ecol. 1989, 58, 349–365. [Google Scholar] [CrossRef]
- Poyet, M.; Le Roux, V.; Gibert, P.; Meirland, A.; Prévost, G.; Eslin, P.; Chabrerie, O. The wide potential trophic niche of the asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE 2015, 10, e0142785. [Google Scholar] [CrossRef] [PubMed]
- Pelton, E.; Gratton, C.; Isaacs, R.; Van Timmeren, S.; Blanton, A.; Guédot, C. Earlier activity of Drosophila suzukii. J. Pest Sci. 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Coyne, J.A.; Boussy, I.A.; Prout, T.; Bryant, S.H.; Jones, J.S.; Moore, J.A. Long-distance migration of Drosophila. Am. Nat. 1982, 119, 589–595. [Google Scholar] [CrossRef]
- Coyne, J.A.; Bryant, S.H.; Turelli, M. Long-distance migration of Drosophila. 2. Presence in desolate sites and dispersal near a desert oasis. Am. Nat. 1987, 129, 847–861. [Google Scholar] [CrossRef]
- Coyne, J.A.; Milstead, B. Long-distance migration of Drosophila. 3. Dispersal of D. melanogaster alleles from a Maryland orchard. Am. Nat. 1987, 130, 70–82. [Google Scholar] [CrossRef]
- Biota of North America Program. Available online: http://www.bonap.org/ (accessed on 4 October 2017).
- Carrière, Y.; Ellers-Kirk, C.; Hartfield, K.; Larocque, G.; Degain, B.; Dutilleul, P.; Dennehy, T.J.; Marsh, S.E.; Crowder, D.W.; Li, X.; et al. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 775–780. [Google Scholar]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. Lond. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, L.G.; Seymour, C.L.; Veldtman, R.; Nicolson, S.W. Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J. Appl. Ecol. 2010, 47, 810–820. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Beers, E.H.; Van Steenwyk, R.A.; Shearer, P.W.; Coates, W.W.; Grant, J.A. Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag. Sci. 2011, 67, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, S.; Isaacs, R. Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
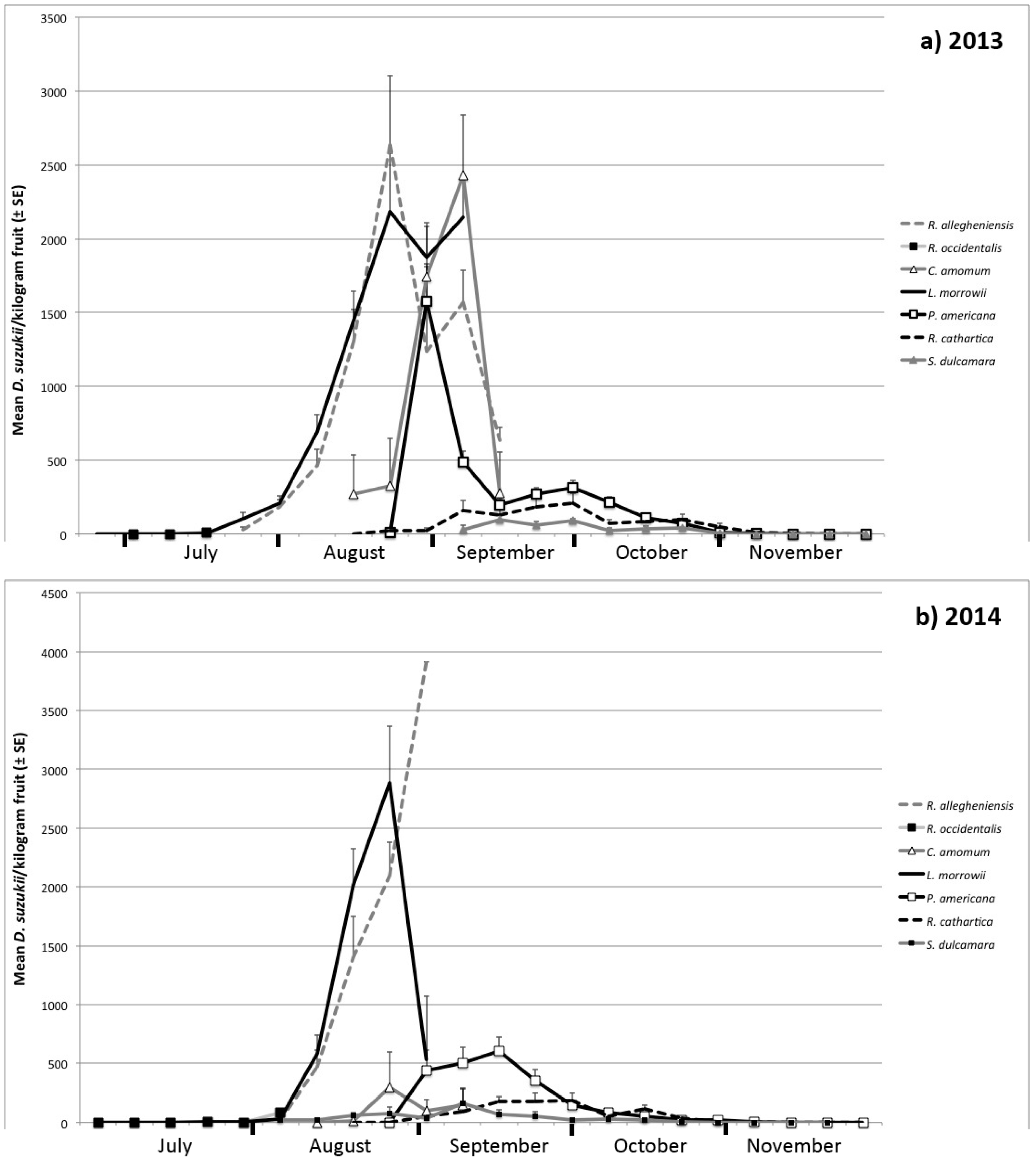
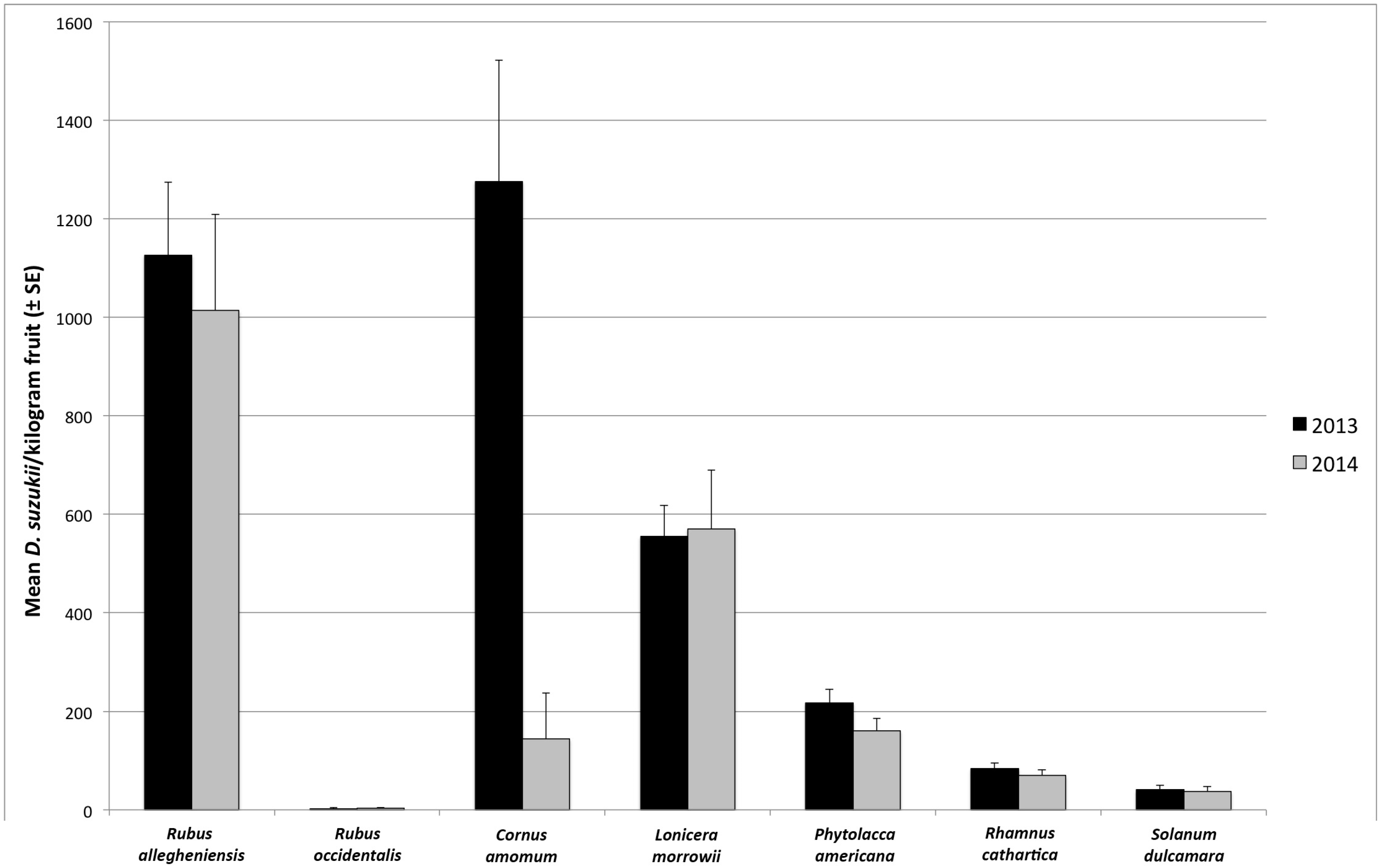
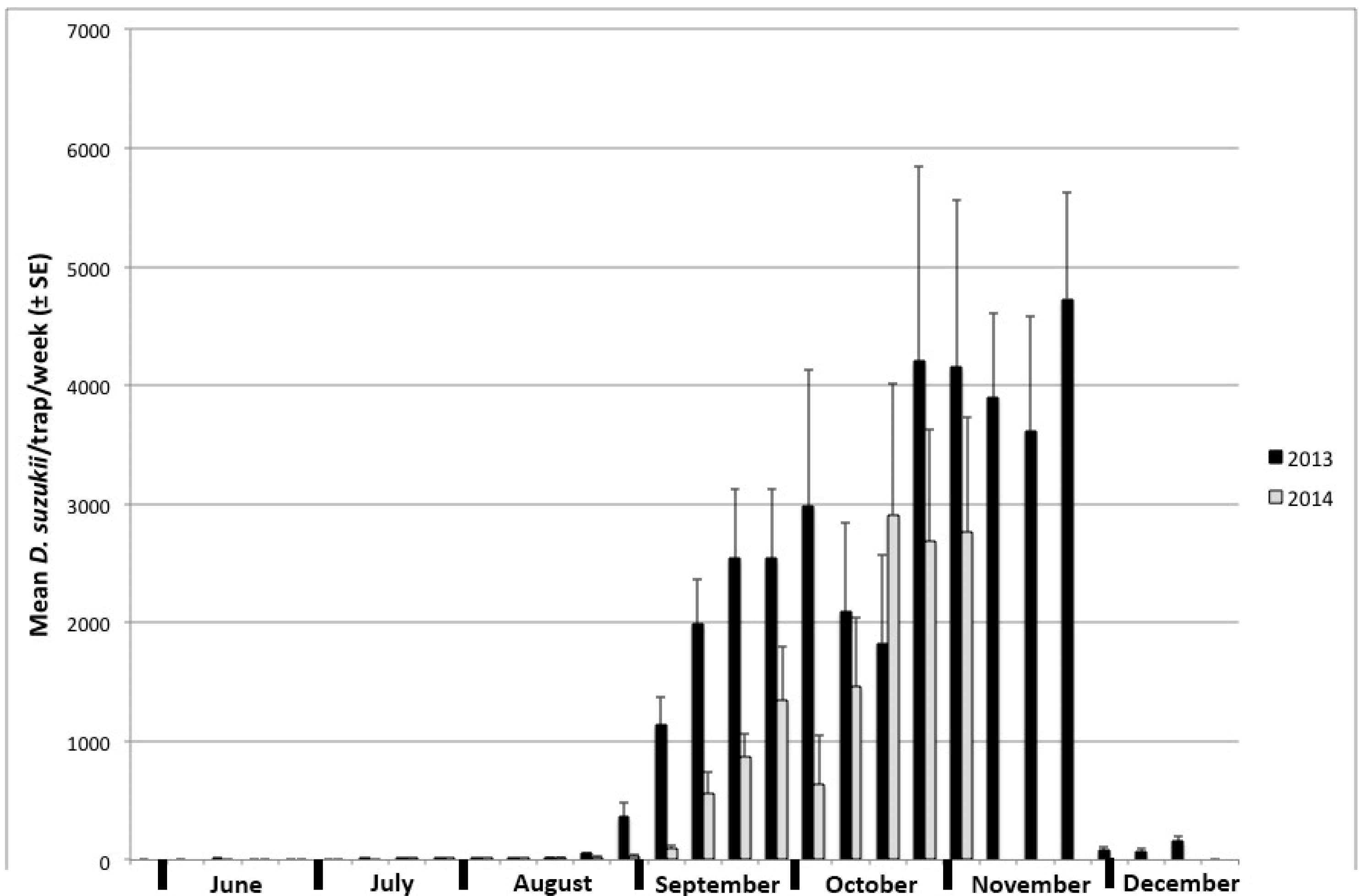
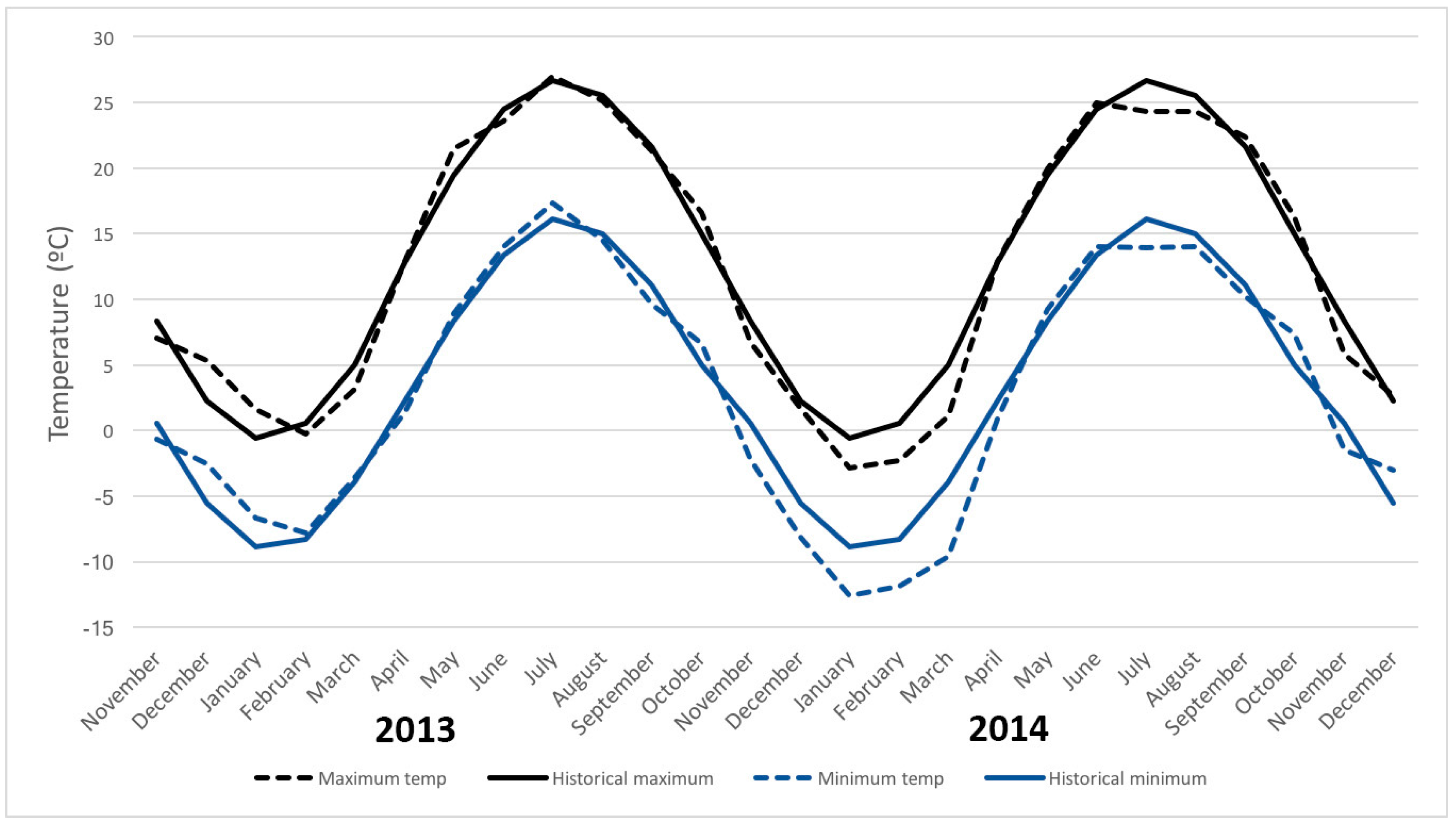

| Month | Host Species | Number of Samples | Percent of Samples Infested | Infestation Rate ± SE (Flies/kg Fruit) | |||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | ||
| June | L. morrowii | 15 | -- | 0 | -- | 0 ± 0 | -- |
| July | R. allegheniensis | 3 | -- | 66.7 | -- | 28 ± 18 | -- |
| R. occidentalis | 46 | 46 | 6.5 | 2.2 | 2.4 ± 1.5 | 1.1 ± 1.1 | |
| L. morrowii | 141 | 50 | 23.4 | 6 | 47 ± 13 | 1.4 ± 0.8 | |
| August | R. allegheniensis | 73 | 32 | 91.7 | 84.4 | 1167 ± 161 | 923 ± 177 |
| R. occidentalis | -- | 1 | -- | 100 | -- | 84 | |
| C. amomum | 17 | 15 | 64.7 | 13.3 | 984 ± 268 | 163 ± 158 | |
| L. morrowii | 99 | 38 | 85.9 | 84.2 | 1313 ± 126 | 1322 ± 231 | |
| P. americana | 17 | 2 | 76.5 | 0 | 1208 ± 256 | 0 ± 0 | |
| R. cathartica | 25 | 6 | 28 | 0 | 23 ± 12 | 0 ± 0 | |
| S. dulcamara | -- | 20 | -- | 40 | -- | 55 ± 24 | |
| September | R. allegheniensis | 5 | 1 | 100 | 100 | 1198 ± 259 | 3913 |
| C. amomum | 8 | 12 | 87.5 | 25 | 1895 ± 466 | 120 ± 78 | |
| L. morrowii | 3 | 2 | 100 | 50 | 2145 ± 270 | 536 ± 536 | |
| P. americana | 59 | 35 | 89.8 | 97.1 | 308 ± 34 | 479 ± 62 | |
| R. cathartica | 60 | 40 | 63.3 | 67.5 | 157 ± 33 | 135 ± 29 | |
| S. dulcamara | 11 | 32 | 72.7 | 37.5 | 64 ± 18 | 81 ± 32 | |
| October | P. americana | 108 | 50 | 74.1 | 58 | 151 ± 18 | 65 ± 13 |
| R. cathartica | 82 | 61 | 68.3 | 45.9 | 105 ± 19 | 77 ± 17 | |
| S. dulcamara | 17 | 41 | 52.9 | 12.2 | 37 ± 11 | 15 ± 7 | |
| November | P. americana | 70 | 38 | 5.7 | 2.6 | 2 ± 1.1 | 0.8 ± 0.8 |
| R. cathartica | 61 | 38 | 13.1 | 2.6 | 8 ± 4 | 0.7 ± 0.7 | |
| S. dulcamara | 5 | 23 | 0 | 0 | 0 ± 0 | 0 ± 0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsensohn, J.E.; Loeb, G.M. Non-Crop Host Sampling Yields Insights into Small-Scale Population Dynamics of Drosophila suzukii (Matsumura). Insects 2018, 9, 5. https://doi.org/10.3390/insects9010005
Elsensohn JE, Loeb GM. Non-Crop Host Sampling Yields Insights into Small-Scale Population Dynamics of Drosophila suzukii (Matsumura). Insects. 2018; 9(1):5. https://doi.org/10.3390/insects9010005
Chicago/Turabian StyleElsensohn, Johanna E., and Gregory M. Loeb. 2018. "Non-Crop Host Sampling Yields Insights into Small-Scale Population Dynamics of Drosophila suzukii (Matsumura)" Insects 9, no. 1: 5. https://doi.org/10.3390/insects9010005
APA StyleElsensohn, J. E., & Loeb, G. M. (2018). Non-Crop Host Sampling Yields Insights into Small-Scale Population Dynamics of Drosophila suzukii (Matsumura). Insects, 9(1), 5. https://doi.org/10.3390/insects9010005





