Characteristics of Gorilla-Specific Lactobacillus Isolated from Captive and Wild Gorillas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth
2.2. Phylogenic Analysis of 16S rRNA Gene
2.3. Phenotypic Characteristics
2.4. Antimicrobial Activity Assay
2.5. Antibiotic Resistance Profile
2.6. Ethics
3. Results
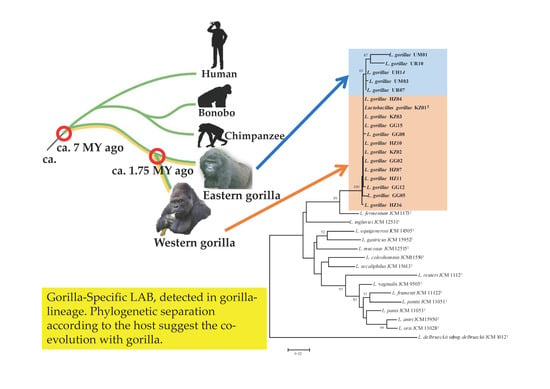
3.1. Phylogenetic Analysis of 16S rRNA Gene
3.2. Phenotypic Characteristics
3.3. Antimicrobial Activity Assay
3.4. Antibiotic Resistance Profile
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hooper, L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, M.; Taylor, T.D. The human intestinal microbiome: A new frontier of human biology. DNA Res. 2009, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J. Infectious history. Science 2000, 288, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Miwa, T.; Taniguchi, H.; Nagano, T.; Shimamura, K.; Tanaka, T.; Kumagai, H. Binding specificity of Lactobacillus to glycolipids. Biochem. Biophys. Res. Commun. 1996, 228, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Conway, S.; Aminov, R. Commensal gut bacteria: Mechanisms of immune modulation. Trends Immunol. 2005, 26, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Kinoshita, H.; Kawai, Y.; Kitazawa, H.; Miura, K.; Shiiba, K.; Horii, A.; Kimura, K.; Taketomo, N.; Oda, M.; et al. Lactobacilli binding human A-antigen expressed in intestinal mucosa. Res. Microbiol. 2006, 157, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Fink, L.N.; Zeuthen, L.H.; Christensen, H.R.; Morandi, B.; Frøkiær, H.; Ferlazzo, G. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. Int. Immunol. 2007, 19, 1319–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riboulet-Bisson, E.; Sturme, M.H.; Jeffery, I.B.; O’Donnell, M.M.; Neville, B.A.; Forde, B.M.; Claesson, M.J.; Harris, H.; Gardiner, G.E.; Casey, P.G.; et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS ONE 2012, 7, e31113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuoka, T.; Kaneuchi, C. Ecology of the bifidobacteria. Am. J. Clin. Nutr. 1977, 30, 1799–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, A.; Endo, F.Y.; Dicks, M.T.L. Diversity of Lactobacillus and Bifidobacterium in feces of herbivores, omnivores and carnivores. Anaerobe 2010, 16, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Lamendella, R.; Santo Domingo, J.W.; Kelty, C.; Oerther, D.B. Bifidobacteria in feces and environmental waters. Appl. Environ. Microbiol. 2008, 73, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.P.; Reichatdt, P.B.; Clausen, T.P.; Provenza, F.D.; Kuropat, P.J. Woody plant–mammal interactions. In Ecological and Evolutionary Processes; Rosenthal, G.A., Berenbaum, M.R., Eds.; Elsevier: London, UK, 1992; pp. 343–370. [Google Scholar]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlik, O.I.; Musilova, L.; Ridl, J.; Hroudova, M.; Vlcek, C.; Koubek, J.; Holeckova, M.; Mackova, M.; Macek, T. Plant secondary metabolite-induced shifts in bacterial community structure and degradative ability in contaminated soil. Appl. Microbiol. Biotechnol. 2013, 97, 9245–9256. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.J.; Mayberry, W.R.; McSweeney, C.S.; Stahl, D.A. Synergistes jonesii, gen. nov., sp. nov.: A rumen bacterium that degrades toxic pyridinediols. Syst. Appl. Microbiol. 1992, 15, 522–529. [Google Scholar] [CrossRef]
- Osawa, R.; Walsh, P.T.; Cork, J.S. Metabolism of tannin-protein complex by facultatively anaerobic bacteria isolated from koala feces. Biodegradation 1993, 4, 91–99. [Google Scholar] [CrossRef]
- Tsuchida, S.; Murata, K.; Ohkuma, M.; Ushida, K. Isolation of Streptococcus gallolyticus with very high degradability of condensed tannins from feces of the wild Japanese rock ptarmigans on Mt. Tateyama. J. Gen. Appl. Microbiol. 2017, 63, 195–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, S.; Ohara, Y.; Kuramochi, K.; Murata, K.; Ushida, K. Effective degradation of phenolic glycoside rhododendron and its aglycone rhododendron by feces of wild Japanese rock ptarmigans. J. Zoo Wildl. Med. 2017, 22, 41–45. [Google Scholar] [CrossRef]
- Tsuchida, S.; Kitahara, M.; Nguema, P.P.M.; Norimitsu, S.; Fujita, S.; Yamagiwa, J.; Ngomanda, A.; Ohkuma, M.; Ushida, K. Lactobacillus gorillae sp. nov. isolated from the faeces of captive and wild western lowland gorillas (Gorilla gorilla gorilla). Int. J. Syst. Evol. Microbiol. 2014, 64, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Takahashi, S.; Nguema, P.P.M.; Fujita, S.; Kitahara, M.; Yamagiwa, J.; Ngomanda, A.; Ohkuma, M.; Ushida, K. Bifidobacterium moukalabense sp. nov. isolated from the faeces of wild west lowland gorilla (Gorilla gorilla gorilla) in Gabon. Int. J. Syst. Evol. Microbiol. 2014, 64, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Ushida, K. Succinate accumulation in pig large intestine during antibiotic-associated diarrhea and the constitution of succinate-producing flora. J. Gen. Appl. Microbiol. 2002, 48, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J.; Clustal, W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Onda, T.; Yanagida, F.; Tsuji, M.; Ogino, S.; Shinohara, T. Isolation and characterization of the Lactic acid bacterial strain GM005 producing a antibacterial substance from miso-paste product. Food Sci. Technol. Res. 1999, 5, 247–250. [Google Scholar] [CrossRef]
- Scally, A.; Dutheil, J.Y.; Hillier, L.W.; Jordan, G.E.; Goodhead, I.; Herrero, J.; Hobolth, A.; Lappalainen, T.; Mailund, T.; Marques-Bonet, T.; et al. Insight into hominid evolution from the gorilla genome sequence. Nature 2012, 483, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Kato, N.; Liu, C.; Matsumiya, Y.; Kato, H.; Watanabe, K. Rapid identification 11 human intestinal Lactobacillus species by multiplex PCR assays using group-and species-specific primers derived from the 16S–23S rRNA intergenic specer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 2000, 187, 167–173. [Google Scholar] [CrossRef]
- Gaqneux, P.; Wills, C.; Gerloff, U.; Tautz, D.; Morin, P.A.; Boesch, C.; Fruth, B.; Hohmann, G.; Ryder, O.A.; Woodruff, D.S. Mitochondrial sequences show diverse evolutionaly histories of African hominoids. Proc. Natl. Acad. Sci. USA 1999, 96, 5077–5082. [Google Scholar] [CrossRef]
- Stewart, C.B.; Disotell, T.R. Primate evolution-in and out of Africa. Curr. Biol. 1998, 8, 582–588. [Google Scholar] [CrossRef]
- Kundaković, T.; Ćirić, A.; Stanojković, T.; Soković, M.; Kovačević, N. Cytotoxicity and antimicrobial activity of Pyrus pyraster Burgsd. and Pyrus spinosa Forssk (Rosaceae). Afr. J. Microbiol. Res. 2014, 8, 511–518. [Google Scholar]
- Jones, R.J.; Lowry, J.B. Australian goats detoxify the goitrogen 3-hydroxy-4(1H) pyridone (DHP) after rumen infusion from an Indonesian goat. Experientia 1984, 40, 1435–1436. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Nakashima, Y.; Tsuchida, S.; Nguema, P.P.; Ando, C.; Ushida, K.; Yamagiwa, J. Decaying toxic wood as sodium supplement for herbivorous mammals in Gabon. J. Vet. Med. Sci. 2015, 77, 1247–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magliocca, F.; Gautier-Hion, A. Mineral content as a basis for food selection by western lowland gorillas in the Dzanga National Park, Central Africa Republic. J. Trop. Ecol. 1998, 14, 829–839. [Google Scholar]
- Tsuchida, S.; Nezuo, M.; Tsukahara, M.; Ogura, Y.; Hayashi, T.; Ushida, K. Draft genome sequence of Lactobacillus gorillae Strain KZ01T, isolated from a Western Lowland Gorilla. Genome Announc. 2015, 3, e01196-15. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Sanchez, B.G.; de Los Reyes-Gavilan, C.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef] [PubMed]


| Strain ID | Animal Species | Captive or Wild |
|---|---|---|
| KZ01T (JCM 19575T) | Western lowland gorilla | Captive |
| KZ02 (JCM 19576) | Western lowland gorilla | Captive |
| KZ03 (JCM 19577) | Western lowland gorilla | Captive |
| HZ04 | Western lowland gorilla | Captive |
| HZ07 | Western lowland gorilla | Captive |
| HZ10 | Western lowland gorilla | Captive |
| HZ11 | Western lowland gorilla | Captive |
| HZ16 | Western lowland gorilla | Captive |
| GG02 (JCM 19574) | Western lowland gorilla | Wild |
| GG05 | Western lowland gorilla | Wild |
| GG08 | Western lowland gorilla | Wild |
| GG12 | Western lowland gorilla | Wild |
| GG15 | Western lowland gorilla | Wild |
| UM01 | Mountain gorilla | Wild |
| UM03 | Mountain gorilla | Wild |
| UR07 | Mountain gorilla | Wild |
| UR10 | Mountain gorilla | Wild |
| UH14 | Mountain gorilla | Wild |
| Animal Species | Western Lowland Gorilla | Mountain Gorilla | Prevalent in Human | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated from | Captive Individuals | Wild Individuals | Fermented Food | ||||||||||||||||
| Characteristic | KZ01T | KZ02 | KZ03 | HZ04 | HZ07 | HZ10 | HZ11 | HZ16 | GG02 | GG05 | GG08 | GG12 | GG15 | UM01 | UM03 | UR07 | UR10 | UH14 | L. fermentum JCM 1173T |
| Acid production from (API 50CH): | |||||||||||||||||||
| D-xylose | − | − | + | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | − |
| Arbutin | − | − | w | − | − | w | − | + | w | w | w | w | w | + | w | w | + | w | − |
| Esculin | + | − | + | + | + | + | + | + | + | + | + | + | + | w | + | + | + | w | − |
| Salicin | − | − | − | − | − | − | − | − | w | w | w | w | w | + | + | w | + | + | − |
| Cellobiose | − | − | w | − | w | w | - | + | w | w | w | w | w | + | + | + | + | + | − |
| Lactose | − | − | + | − | − | + | - | + | w | w | w | + | w | + | + | + | + | + | + |
| Trehalose | − | − | + | − | − | + | - | + | + | + | + | + | + | + | + | + | + | + | − |
| Gluconate | − | − | + | − | − | w | - | w | w | w | w | w | + | + | + | + | + | + | w |
| 2-keto-gluconate | − | − | − | − | − | - | - | - | - | - | - | - | - | + | + | + | + | + | − |
| API ZYM results: | |||||||||||||||||||
| Cystine arylamidase | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + |
| Naphthol-AS-BI-phosphohydrolase | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| α-galactosidase | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − |
| α-glucosidase | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| Growth in Nacl | |||||||||||||||||||
| 6.5% | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − |
| 8% | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| Animal Species | Western Lowland Gorilla | Mountain Gorilla | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated from | Captive Individuals | Wild Individuals | ||||||||||||||||
| Characteristic | KZ01T | KZ02 | KZ03 | HZ04 | HZ07 | HZ10 | HZ11 | HZ16 | GG02 | GG05 | GG08 | GG12 | GG15 | UM01 | UM03 | UR07 | UR10 | UH14 |
| Antibiotics: | ||||||||||||||||||
| Imipenem (10 µg/disk) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Cefotaxime (30 µg/disk) | I | R | S | I | I | I | R | S | S | S | S | S | S | S | S | S | S | S |
| Ofloxacin (5 µg/disk) | R | R | R | R | R | R | R | R | S | S | S | S | S | R | R | R | R | R |
| Amoxicillin (25 µg/disk) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Gentamicin (10 µg/disk) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Lincomycin (2 µg/disk) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Tetracycline (30 µg/disk) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Erythromycin (15 µg/disk) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchida, S.; Kakooza, S.; Mbehang Nguema, P.P.; Wampande, E.M.; Ushida, K. Characteristics of Gorilla-Specific Lactobacillus Isolated from Captive and Wild Gorillas. Microorganisms 2018, 6, 86. https://doi.org/10.3390/microorganisms6030086
Tsuchida S, Kakooza S, Mbehang Nguema PP, Wampande EM, Ushida K. Characteristics of Gorilla-Specific Lactobacillus Isolated from Captive and Wild Gorillas. Microorganisms. 2018; 6(3):86. https://doi.org/10.3390/microorganisms6030086
Chicago/Turabian StyleTsuchida, Sayaka, Steven Kakooza, Pierre Philippe Mbehang Nguema, Eddie M. Wampande, and Kazunari Ushida. 2018. "Characteristics of Gorilla-Specific Lactobacillus Isolated from Captive and Wild Gorillas" Microorganisms 6, no. 3: 86. https://doi.org/10.3390/microorganisms6030086