Isotopic Composition of Spring Water in Greece: Spring Waters Isoscapes
Abstract
:1. Introduction
2. Data and Methods
3. Regional Climate
4. Results and Discussion
4.1. Spring Water
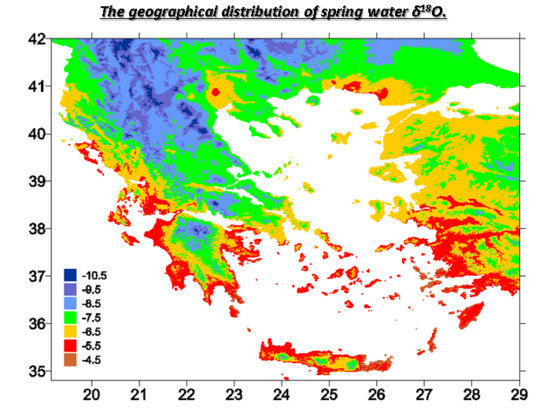
4.2. Geographical Distribution of δ18O Values in Precipitation and Spring Water
4.3. δ18O Values in Relation to the Salinity of Spring Water
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gat, J.; Carmi, I. Evolution of the isotopic composition of atmospheric waters in the Mediterranean Sea area. J. Geophys. Res. 1970, 75, 3039–3048. [Google Scholar] [CrossRef]
- Gat, J.; Klein, B.; Kushnir, Y.; Roether, W.; Wernli, H.; Yam, R.; Shemesh, A. Isotope composition of air moisture over the Mediterranean Sea: An index of the air–sea interaction pattern. Tellus B 2003, 55, 953–965. [Google Scholar] [CrossRef]
- Ambrose, S.H. Isotopic analysis of palaeodiets: Methodological and interpretive consideration. In Investigations of Ancient Human Tissue; Gordon & Breach Science Publishers: Langhorne, PA, USA, 1993; Volume 10, pp. 59–130. [Google Scholar]
- Garnsey, P. Food and Society in Classical Antiquity; Cambridge University Press: Cambridge, UK, 1999; ISBN 0521645883. [Google Scholar]
- Ambrose, S.H.; Norr, L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In Prehistoric Human Bone; Springer: New York, NY, USA, 1993; pp. 1–37. [Google Scholar]
- Bartelink, E.J.; Berg, G.E.; Beasley, M.M.; Chesson, L.A. Application of stable isotope forensics for predicting region of origin of human remains from past wars and conflicts. Ann. Anthropol. Pract. 2014, 38, 124–136. [Google Scholar] [CrossRef]
- Bartelink, E.; Berry, R.; Chesson, L. Stable isotopes and human provenancing. In Advances in Forensic Human Identification; CRC Press: Boca Raton, FL, USA, 2014; pp. 165–192. [Google Scholar]
- Ehleringer, J.R.; Bowen, G.J.; Chesson, L.A.; West, A.G.; Podlesak, D.W.; Cerling, T.E. Hydrogen and oxygen isotope ratios in human hair are related to geography. Proc. Natl. Acad. Sci. USA 2008, 105, 2788–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehleringer, J.R.; Thompson, A.H.; Podlesak, D.W.; Bowen, G.J.; Chesson, L.A.; Cerling, T.E.; Park, T.; Dostie, P.; Schwarcz, H. A framework for the incorporation of isotopes and isoscapes in geospatial forensic investigations. In Isoscapes; Springer: New York, NY, USA, 2010; pp. 357–387. [Google Scholar]
- Bencini, A.; Duchi, V.; Casatello, A.; Kolios, N.; Fytikas, M.; Sbaragli, L. Geochemical study of fluids on Lesbos island, Greece. Geothermics 2004, 33, 637–654. [Google Scholar] [CrossRef]
- Christodoulou, T.; Leontiadis, I.; Morfis, A.; Payne, B.; Tzimourtas, S. Isotope hydrology study of the Axios River plain in northern Greece. J. Hydrol. 1993, 146, 391–404. [Google Scholar] [CrossRef]
- Dimitriou, E.; Zacharias, I. Using state-of-the-art techniques to develop water management scenarios in a lake catchment. Hydrol. Res. 2007, 38, 79–97. [Google Scholar] [CrossRef]
- Dotsika, E. Utilisation du Geothermometre Isotopique Sulfate-Eau en Milieux de Haute Temperature Sous Influence Marine Potentielle: Les Systemes Geothermaux de Grece. Ph.D. Thesis, University Paris-Sud, Orsay, France, 1991; 184p. [Google Scholar]
- Dotsika, E.; Michelot, J.-L. Origine et températures en profondeur des eaux thermales d'Ikaria (Grèce). C. R. Acad. Sci. 1992, 315, 1261–1266. [Google Scholar]
- Dotsika, E.; Poutoukis, D.; Michelot, J.; Kloppmann, W. Stable isotope and chloride, boron study for tracing sources of boron contamination in groundwater: Boron contents in fresh and thermal water in different areas in Greece. Water Air Soil Pollut. 2006, 174, 19–32. [Google Scholar] [CrossRef]
- Dotsika, E.; Leontiadis, I.; Poutoukis, D.; Cioni, R.; Raco, B. Fluid geochemistry of the Chios geothermal area, Chios Island, Greece. J. Volcanol. Geotherm. Res. 2006, 154, 237–250. [Google Scholar] [CrossRef]
- Dotsika, E.; Poutoukis, D.; Michelot, J.; Raco, B. Natural tracers for identifying the origin of the thermal fluids emerging along the Aegean Volcanic arc (Greece): Evidence of Arc-Type Magmatic Water (ATMW) participation. J. Volcanol. Geotherm. Res. 2009, 179, 19–32. [Google Scholar] [CrossRef]
- Duriez, A.; Marlin, C.; Dotsika, E.; Massault, M.; Noret, A.; Morel, J. Geochemical evidence of seawater intrusion into a coastal geothermal field of central Greece: Example of the Thermopylae system. Environ. Geol. 2008, 54, 551–564. [Google Scholar] [CrossRef]
- Grassi, S.; Kolios, N.; Mussi, M.; Saradeas, A. Groundwater circulation in the Nea Kessani low-temperature geothermal field (NE Greece). Geothermics 1996, 25, 231–247. [Google Scholar] [CrossRef]
- Griffiths, S.J.; Street-Perrott, F.A.; Holmes, J.A.; Leng, M.J.; Tzedakis, C. Chemical and isotopic composition of modern water bodies in the Lake Kopais Basin, central Greece: Analogues for the interpretation of the lacustrine sedimentary sequence. Sediment. Geol. 2002, 148, 79–103. [Google Scholar] [CrossRef]
- Kallergis, G.; Leontiadis, I. Isotope hydrology study of the Kalamos Attikis and Assopos riverplain areas in Greece. J. Hydrol. 1983, 60, 209–225. [Google Scholar] [CrossRef]
- Kavouridis, T.; Kuris, D.; Leonis, C.; Liberopoulou, V.; Leontiadis, J.; Panichi, C.; La Ruffa, G.; Caprai, A. Isotope and chemical studies for a geothermal assessment of the island of Nisyros (Greece). Geothermics 1999, 28, 219–239. [Google Scholar] [CrossRef]
- Kelepertsis, A.; Alexakis, D.; Kita, I. Environmental geochemistry of soils and waters of Susaki area, Korinthos, Greece. Environ. Geochem. Health 2001, 23, 117–135. [Google Scholar] [CrossRef]
- La Ruffa, G.; Panichi, C.; Kavouridis, T.; Liberopoulou, V.; Leontiadis, J.; Caprai, A. Isotope and chemical assessment of geothermal potential of Kos Island, Greece. Geothermics 1999, 28, 205–217. [Google Scholar] [CrossRef]
- Leontiadis, J. Isotope Hydrology Study of Molai Area in Laconia; Internal Report, Demo 81/4; Democritos Nuclear Research Center: Athens, Greece, 1981. [Google Scholar]
- Leontiadis, I.; Payne, B.; Letsios, A.; Papagianni, N.; Kakarelis, D.; Chadjiagorakis, D. Isotope hydrology study of Kato Nevrokopi of Drama. In Proceedings of the Symposium on Isotope Hydrology, Vienna, Austria, 12–16 September 1983; pp. 193–206. [Google Scholar]
- Leontiadis, I.; Payne, B.; Christodoulou, T. Isotope hydrology of the Aghios Nikolaos area of Crete, Greece. J. Hydrol. 1988, 98, 121–132. [Google Scholar] [CrossRef]
- Leontiadis, I.; Stamos, A.; Manakos, A. Isotope hydrology study of the wider area of Kozani, Greece. In Proceedings of the 6th International Symposium on Water Tracing, Karlsruhe, Germany, 21–26 September 1992; pp. 233–240. [Google Scholar]
- Leontiadis, I.; Vergis, S.; Christodoulou, T. Isotope hydrology study of areas in Eastern Macedonia and Thrace, Northern Greece. J. Hydrol. 1996, 182, 1–17. [Google Scholar] [CrossRef]
- Leontiadis, I.; Nikolaou, E. Environmental isotopes in determining groundwater flow systems, northern part of Epirus, Greece. Hydrogeol. J. 1999, 7, 219–226. [Google Scholar] [CrossRef]
- Leontiadis, I.; Nikolaou, E.; Dotsika, E. Environmental isotopes in determining groundwater flow systems, Epirus, Greece. Bull. Geol. Soc. Greece 2006, 14, 47–70. [Google Scholar]
- Michelot, J.-L.; vet Dotsika, E.; Fytikas, M. A hydrochemical and isotopic study of thermal waters on Lesbos Island (Greece). Geothermics 1993, 22, 91–99. [Google Scholar] [CrossRef]
- Mitropoulos, P.; Kita, I. Geochemistry of oxygen and hydrogen isotopes in Greek regional waters. In Proceedings of the 4th Hydrogeological Congress; Commission of Greece: Athens, Greece, 1997; pp. 285–291. [Google Scholar]
- Payne, B.; Dimitroulas, D.; Leontiadis, I.; Kallergis, G.; Christodoulou, T. Environmental isotope data in the Western Thessaly valley, Greece: Use of mathematical model for quantitative evaluations with tritium. Bull. Geol. Soc. Greece 1976, 12, 29–94. [Google Scholar]
- Stratikopoulos, K. Hydrogeological and Hydrochemical Study of Thermometallic Springs of Western Peloponnese Using Stable Isotopes. Ph.D. Thesis, University of Patras, Patras, Greece, 2007. [Google Scholar]
- Kolios, N. Low Enthalpy Geochemical Study—The Geothermcal Field of Nea Kessani. Ph.D. Thesis, University of Athens, Athens, Greece, 1993. [Google Scholar]
- Leontiadis, E.D.; Romaidis, I. Isotope hydrology study of the wider area of Orestias. In Proceedings of the 5th Panhellenic Hydrogeological Symposium, Limasol, Cyprus, 1999; pp. 463–479, (In Greek with English Abstract). [Google Scholar]
- Leontiadis, A.S. Isotope hydrology study of the upper flow of Aliakmon river. In Proceedings of the 5th Panhellenic Hydrogeological Symposium, Limasol, Cyprus, 1999; pp. 481–496, (In Greek with English Abstract). [Google Scholar]
- Leontiadis, C.S. Isotope hydrology study of the Louros Riverplain area, Epirus, Greece. In Proceedings of the 5th International Symposium on Underground Water Tracing, Athens, Greece, 22–27 September 1986; pp. 75–90. [Google Scholar]
- Dotsika, E.; Lykoudis, S.; Poutoukis, D. Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Glob. Planet. Chang. 2010, 71, 141–149. [Google Scholar] [CrossRef]
- Epstein, S.; Mayeda, T. Variation of 18O content of water from natural sources. Geochim. Cosmochim. Acta 1953, 4, 213–224. [Google Scholar] [CrossRef]
- Coleman, M.L.; Shepherd, T.J.; Durham, J.J.; Rouse, J.E.; Moore, G.R. Reduction of water with zinc for hydrogen isotope analysis. Anal. Chem. 1982, 54, 993–995. [Google Scholar] [CrossRef]
- Argiriou, A.A.; Lykoudis, S. Isotopic composition of precipitation in Greece. J. Hydrol. 2006, 327, 486–495. [Google Scholar] [CrossRef]
- Bowen, G.J.; Wilkinson, B. Spatial distribution of δ18O in meteoric precipitation. Geology 2002, 30, 315–318. [Google Scholar] [CrossRef]
- Lykoudis, S.P.; Argiriou, A.A. Gridded data set of the stable isotopic composition of precipitation over the eastern and central Mediterranean. J. Geophys. Res. Atmos. 2007. [Google Scholar] [CrossRef]
- GTOPO30 (Global 30 Arc-Second Elevation Data Set). USGS, Ed. Available online: https://lta.cr.usgs.gov/GTOPO30 (accessed on 1 January 2015).
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Craig, H.; Gordon, L.; Horibe, Y. Isotopic exchange effects in the evaporation of water: 1. Low-temperature experimental results. J. Geophys. Res. 1963, 68, 5079–5087. [Google Scholar] [CrossRef]
- Ehhalt, D.; Knott, K.; Nagel, J.; Vogel, J. Deuterium and oxygen 18 in rain water. J. Geophys. Res. 1963, 68, 3775–3780. [Google Scholar] [CrossRef]
- Longinelli, A.; Anglesio, E.; Flora, O.; Iacumin, P.; Selmo, E. Isotopic composition of precipitation in Northern Italy: Reverse effect of anomalous climatic events. J. Hydrol. 2006, 329, 471–476. [Google Scholar] [CrossRef]
- Rozanski, K.; Sonntag, C.; Münnich, K. Factors controlling stable isotope composition of European precipitation. Tellus 1982, 34, 142–150. [Google Scholar] [CrossRef]







| NO | Spring | Location | Region | North | East | Sampling Date | δ18O (‰, VSMOW) | δ2H (‰, VSMOW) |
|---|---|---|---|---|---|---|---|---|
| 1 | Chaladra | Chios | Aegean Isl. | 38.55 | 25.94 | 2018 | −7.2 | −43.5 |
| 2 | Nagos | Chios | Aegean Isl. | 38.56 | 26.07 | 2018 | −7.3 | −45.1 |
| 3 | Ag. Fokas1 | Kos | Aegean Isl. | 36.85 | 27.25 | 2018 | −6.3 | −38.1 |
| 4 | Ag. Fokas2 | Kos | Aegean Isl. | 36.86 | 27.26 | 2018 | −6.0 | −31.1 |
| 5 | Makriammos | Thasos | Aegean Isl. | 40.76 | 24.73 | 2018 | −8.1 | −50.1 |
| 6 | Potamia | Thasos | Aegean Isl. | 40.71 | 24.72 | 2018 | −8.1 | −49.4 |
| 7 | Therisso | Chania | Crete | 35.24 | 23.59 | 2014 | −8.8 | −52.5 |
| 8 | Sterna | Heraklion | Crete | 35.01 | 25.08 | 2014 | −7.7 | −53.1 |
| 9 | Foteino | Arta | Epirus | 39.09 | 21.04 | 2016 | −5.8 | −35.1 |
| 10 | Graikiko | Ioannina | Epirus | 39.42 | 21.04 | 2016 | −7.1 | −41.5 |
| 11 | Kranoula | Ioannina | Epirus | 39.55 | 29.45 | 2015 | −8.3 | −52.5 |
| 12 | Kranoula | Ioannina | Epirus | 39.55 | 29.45 | 2016 | −8.1 | −51.5 |
| 13 | Mili | Ioannina | Epirus | 39.78 | 21.1 | 2016 | −7.3 | −43.9 |
| 14 | Sepeta (s) | Ioannina | Epirus | 38.45 | 20.5 | 2015 | −8.3 | −52.3 |
| 15 | Sepeta (s) | Ioannina | Epirus | 39.45 | 20.5 | 2016 | −7.7 | −50.5 |
| 16 | Vathipedo | Ioannina | Epirus | 39.62 | 21.08 | 2016 | −8.1 | −52.6 |
| 17 | Vikos | Ioannina | Epirus | 39.57 | 20.42 | 2015 | −8.0 | −54.9 |
| 18 | Vikos | Ioannina | Epirus | 39.57 | 20.42 | 2016 | −7.7 | −52.6 |
| 19 | Nea Tenedos | Chalkidiki | Macedonia | 40.32 | 23.25 | 2014 | −6.8 | −46.9 |
| 20 | Petralona | Chalkidiki | Macedonia | 40.37 | 23.17 | 2014 | −8.6 | −62.1 |
| 21 | Polygiros | Chalkidiki | Macedonia | 40.23 | 23.27 | 2018 | −8.2 | −51.5 |
| 22 | Kato Nevrokopi | Drama | Macedonia | 40.88 | 24.09 | 2016 | −8.2 | −54.1 |
| 23 | Anthemia | Imathia | Macedonia | 40.28 | 23.02 | 2014 | −7.6 | −49.3 |
| 24 | Anthemia | Imathia | Macedonia | 40.28 | 23.02 | 2018 | −7.7 | −50.3 |
| 25 | Korifes | Kavala | Macedonia | 41.06 | 24.45 | 2014 | −8.0 | −47.0 |
| 26 | Makrinitsa | Kilkis | Macedonia | 41.3 | 22.99 | 2016 | −7.2 | −45.3 |
| 27 | Notia | Kilkis | Macedonia | 41.06 | 22.07 | 2016 | −9.3 | −64.5 |
| 28 | Kopanou | Kozani | Macedonia | 40.64 | 22.12 | 2014 | −9.3 | −65.2 |
| 29 | Drosia | Pella | Macedonia | 40.47 | 21.52 | 2016 | −9.1 | −62.8 |
| 30 | Polichni | Thessaloniki | Macedonia | 40.67 | 22.96 | 2014 | −7.1 | −44.9 |
| 31 | Vlacherna | Arkadia | Peloponnese | 37.71 | 22.23 | 2015 | −7.9 | −53.1 |
| 32 | Vytina | Arkadia | Peloponnese | 37.67 | 22.19 | 2014 | −7.2 | −43.1 |
| 33 | Vytina | Arkadia | Peloponnese | 37.67 | 22.19 | 2015 | −7.3 | −45.2 |
| 34 | Loutraki | Corinthia | Peloponnese | 37.98 | 22.99 | 2014 | −6.8 | −47.9 |
| 35 | Kaliani | Korinthia | Peloponnese | 37.89 | 22.49 | 2014 | −9.1 | −61.4 |
| 36 | Kaliani | Korinthia | Peloponnese | 37.89 | 22.49 | 2015 | −9.1 | −56.9 |
| 37 | Monastiraki Vonitsas | Aitoloakarnanias | Sterea | 38.84 | 20.96 | 2016 | −7.5 | −56.8 |
| 38 | Helliniko | Attiki | Sterea | 37.9 | 23.73 | 2018 | −6.1 | −34.9 |
| 39 | Kalamos | Attiki | Sterea | 38.14 | 23.74 | 2018 | −7.7 | −43.8 |
| 40 | Kalamos | Attiki | Sterea | 38.14 | 23.74 | 2018 | −7.6 | −42.8 |
| 41 | Velouchi | Evrytania | Sterea | 38.94 | 21.8 | 2015 | −9.7 | −66.5 |
| 42 | Velouchi | Evrytania | Sterea | 38.94 | 21.8 | 2018 | −9.6 | −65.9 |
| 43 | Mornos | Fokida | Sterea | 38.53 | 22.17 | 2015 | −6.2 | −37.1 |
| 44 | Mornos | Fokida | Sterea | 38.53 | 22.17 | 2018 | −6.3 | −38.5 |
| 45 | Kamena Vourla | Fthiotida | Sterea | 38.77 | 22.79 | 2014 | −8.2 | −48.8 |
| 46 | kamena Vourla | Fthiotida | Sterea | 38.77 | 22.79 | 2015 | −5.0 | −33.0 |
| 47 | kamena Vourla | Fthiotida | Sterea | 38.77 | 22.79 | 2018 | −5.1 | −30.8 |
| 48 | Monastery | Fthiotida | Sterea | 38.78 | 22.75 | 2015 | −7.8 | −51.0 |
| 49 | Aliartos | Viotia | Sterea | 38.37 | 23.1 | 2015 | −7.9 | −49.9 |
| 50 | Kanalia | Karditsa | Thessaly | 39.4 | 21.81 | 2018 | −8.3 | −52.4 |
| 51 | Neo Monastiri | Karditsa | Thessaly | 39.26 | 22.26 | 2018 | −8.2 | −54.0 |
| 52 | Zografia | Karditsa | Thessaly | 39.43 | 21.73 | 2018 | −7.7 | −46.8 |
| 53 | Apostoli | Trikala | Thessaly | 39.57 | 21.73 | 2018 | −8.0 | −48.9 |
| 54 | Kotroni | Trikala | Thessaly | 39.46 | 21.56 | 2018 | −8.3 | −51.9 |
| 55 | Pyli | Trikala | Thessaly | 39.47 | 21.62 | 2018 | −7.8 | −50.1 |
| 56 | Agitis | Drama | Thraki | 41.22 | 23.89 | 2014 | −8.6 | −57.0 |
| 57 | Avas | Evros | Thraki | 40.97 | 25.9 | 2014 | −6.9 | 42.1 |
| 58 | Doriskos | Evros | Thraki | 40.86 | 26.13 | 2014 | −6.3 | −40.8 |
| 59 | kounia | Evros | Thraki | 41.28 | 25.9 | 2015 | −7.9 | −50.9 |
| 60 | kounia | Evros | Thraki | 41.28 | 25.9 | 2018 | −7.8 | −49.6 |
| 61 | Pentalofos | Evros | Thraki | 41.64 | 26.18 | 2015 | −9.1 | −61.4 |
| 62 | Pentalofos | Evros | Thraki | 41.64 | 26.18 | 2016 | −8.8 | −61.1 |
| 63 | Plati | Evros | Thraki | 41.58 | 26.32 | 2015 | −7.8 | −53.0 |
| 64 | Plati | Evros | Thraki | 41.58 | 26.32 | 2018 | −7.9 | −53.9 |
| 65 | Polia | Evros | Thraki | 41.43 | 26.22 | 2015 | −6.1 | −40.1 |
| 66 | Polia | Evros | Thraki | 41.43 | 26.22 | 2018 | −6.2 | −41.0 |
| 67 | Rizia | Evros | Thraki | 41.63 | 26.37 | 2016 | −8.1 | −55.0 |
| 68 | Valtos | Evros | Thraki | 41.55 | 26.31 | 2014 | −8.6 | −58.9 |
| 69 | Elatia | Komotini | Thraki | 41.5 | 24.35 | 2015 | −8.1 | −53.0 |
| 70 | Elatia | Komotini | Thraki | 41.5 | 24.35 | 2018 | −8.0 | −51.3 |
| 71 | Nea Kessani | Rodopi | Thraki | 41.13 | 25.08 | 2015 | −7.2 | −42.9 |
| 72 | Nea Kessani | Rodopi | Thraki | 41.13 | 25.08 | 2018 | −6.1 | −38.1 |
| 73 | Agkistro | Serres | Thraki | 41.38 | 23.73 | 2015 | −8.5 | −55.8 |
| 74 | Ochiro | Serres | Thraki | 41.21 | 23.89 | 2016 | −8.7 | −56.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsika, E.; Diamantopoulos, G.; Lykoudis, S.; Poutoukis, D.; Kranioti, E. Isotopic Composition of Spring Water in Greece: Spring Waters Isoscapes. Geosciences 2018, 8, 238. https://doi.org/10.3390/geosciences8070238
Dotsika E, Diamantopoulos G, Lykoudis S, Poutoukis D, Kranioti E. Isotopic Composition of Spring Water in Greece: Spring Waters Isoscapes. Geosciences. 2018; 8(7):238. https://doi.org/10.3390/geosciences8070238
Chicago/Turabian StyleDotsika, Elissavet, George Diamantopoulos, Spyridon Lykoudis, Dimitrios Poutoukis, and Elena Kranioti. 2018. "Isotopic Composition of Spring Water in Greece: Spring Waters Isoscapes" Geosciences 8, no. 7: 238. https://doi.org/10.3390/geosciences8070238