The Effect of Computerized Testing on Sun Bear Behavior and Enrichment Preferences
Abstract
:1. Introduction
2. Method
2.1. Subjects
2.2. Procedure
2.2.1. Computer Training
2.2.2. Behavioral Observations
2.2.3. Preference Assessment
3. Results
3.1. Computer Training
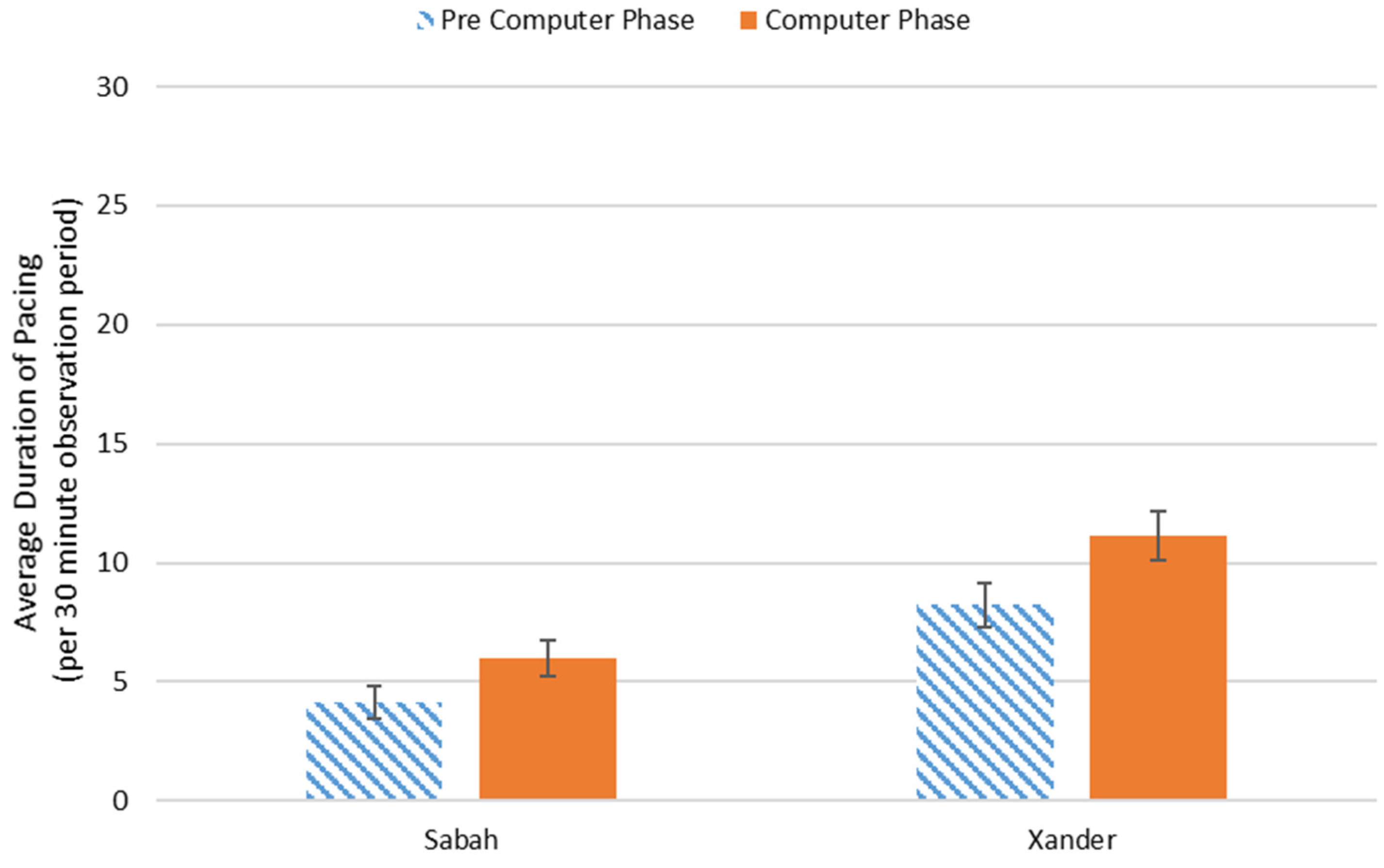
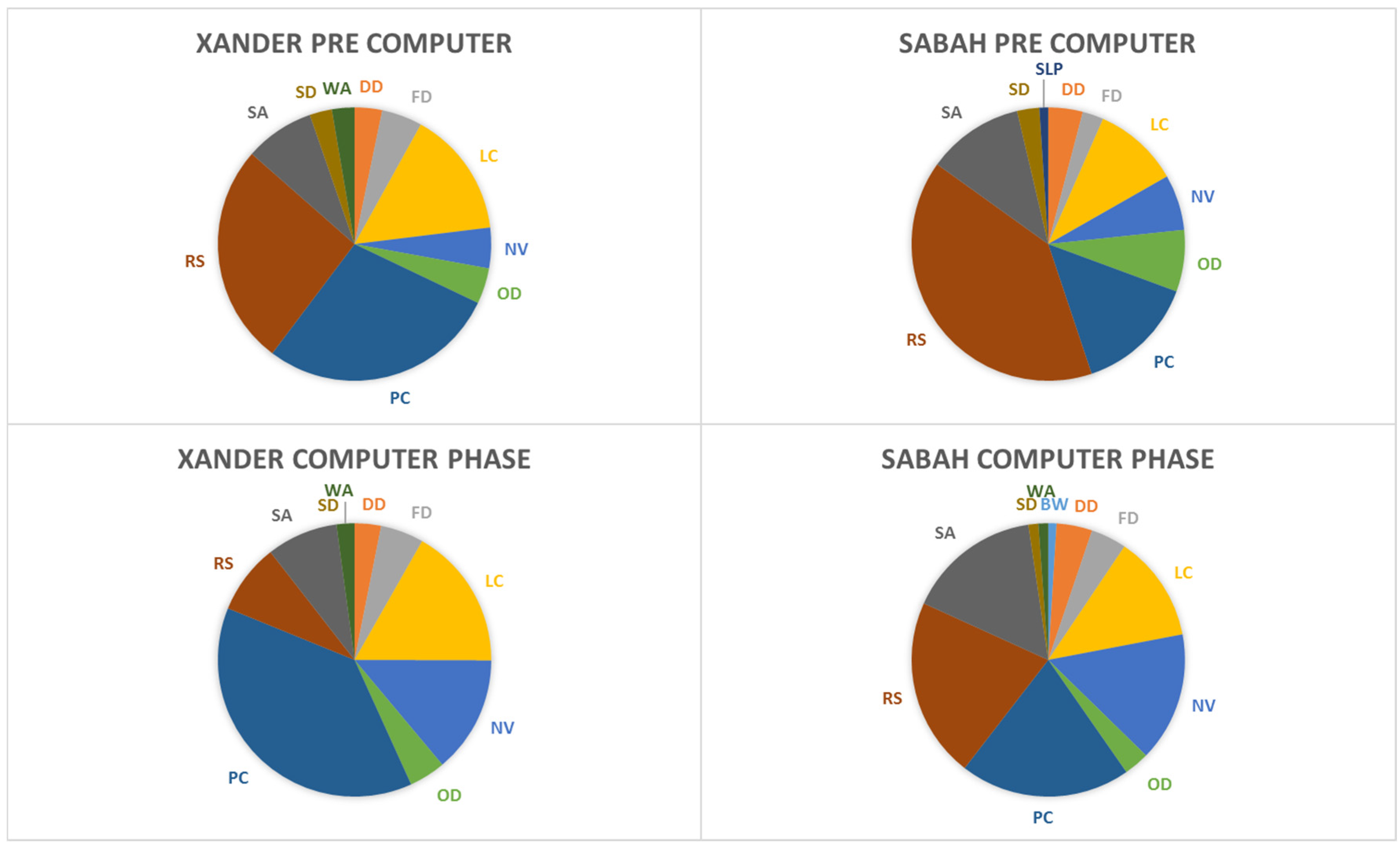
3.2. Behavioral Observations
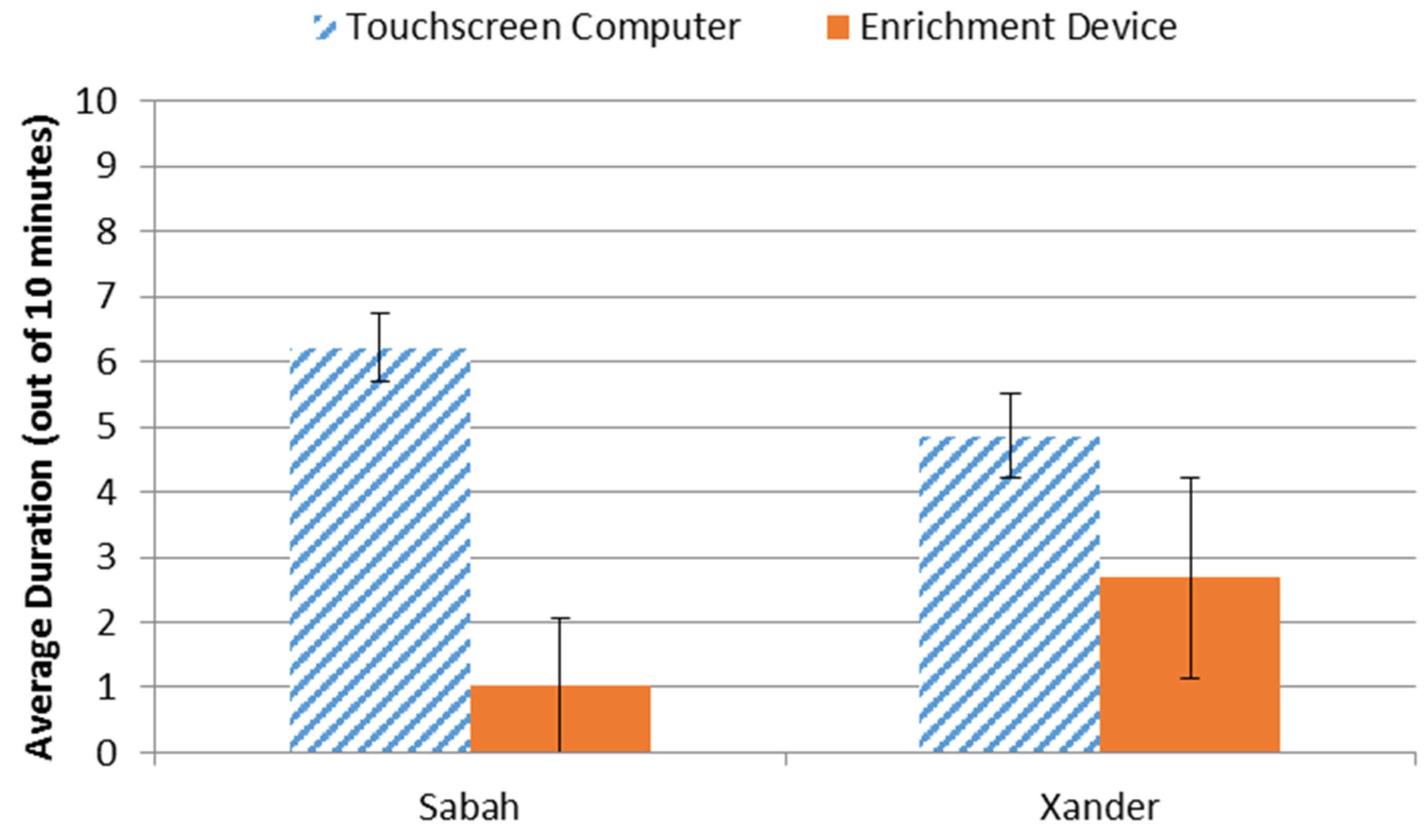
3.3. Preference Assessment
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
Appendix A. Sun Bear Ethogram
A.1. Distance
A.2. Solitary Behaviors
A.3. Instantaneous AND All-occurrence
A.4. Social Behaviors
References
- Beran, M.J.; Parrish, A.E.; Perdue, B.M.; Washburn, D.A. Comparative cognition: Past, present, and future. Int. J. Comp. Psychnol. 2014, 27, 3–30. [Google Scholar]
- Dungl, E.; Schratter, D.; Huber, L. Discrimination of face-like patterns in the giant panda (Ailuropoda melanoleuca). J. Comp. Psychnol. 2008, 122, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kelling, A.S.; Snyder, R.J.; Marr, M.J.; Bloomsmith, M.A.; Gardner, W.; Maple, T.L. Color vision in the giant panda (Ailuropoda melanoleuca). Learn. Behav. 2006, 34, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Perdue, B.M.; Snyder, R.J.; Pratte, J.; Marr, M.J.; Maple, T.L. Spatial memory recall in the giant panda (Ailuropoda melanoleuca). J. Comp. Psychnol. 2009, 123, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Perdue, B.M.; Snyder, R.J.; Zhang, Z.; Marr, M.J.; Maple, T.L. Sex differences in spatial ability: A test of the range size hypothesis in the order Carnivora. Biol. Lett. 2011, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Swaisgood, R.R.; Lindburg, D.G.; Zhou, X. Giant pandas discriminate individual differences in conspecific scent. Anim. Behav. 1999, 57, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Ulrich, Z.; Vonk, J.; Humbyrd, M.; Crowley, M.; Wojtkowski, E.; Yates, F.; Allard, S. Picture object recognition in an American black bear (Ursus americanus). Anim. Cogn. 2016. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Jett, S.E.; Mosteller, K.W. Concept formation in American black bears, Ursus americanus. Anim. Behav. 2012, 84, 953–964. [Google Scholar] [CrossRef]
- Vonk, J.; Beran, M.J. Bears ‘count’ too: Quantity estimation and comparison in black bears, Ursus americanus. Anim. Behav. 2012, 84, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Johnson-Ulrich, Z. Social and nonsocial category discriminations in a chimpanzee (Pan troglodytes) and American black bears (Ursus americanus). Learn. Behav. 2014, 42, 231. [Google Scholar] [CrossRef] [PubMed]
- Zamisch, V.; Vonk, J. Spatial memory in captive American black bears (Ursus americanus). J. Comp. Psychnol. 2012, 126, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Bacon, E.S. Curiosity in the American black bear. Bears: Their Biol. Manag. 1980, 4, 153–157. [Google Scholar] [CrossRef]
- Bacon, E.S.; Burghardt, G.M. Learning and color discrimination in the American black bear. Bears: Their Biol. Manag. 1976, 3, 27–36. [Google Scholar] [CrossRef]
- Bacon, E.S.; Burghardt, G.M. Food preference testing of captive black bears. Bears: Their Biol. Manag. 1983, 5, 102–105. [Google Scholar] [CrossRef]
- Cunningham, E.; Janson, C. A socioecological perspective on primate cognition, past and present. Anim. Cogn. 2007, 10, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Gaulin, S.J.C.; Fitzgerald, R.W. Sex differences in spatial ability: An evolutionary hypothesis and test. Am. Nat. 1986, 127, 74–88. [Google Scholar]
- Gaulin, S.J.C.; Fitzgerald, R.W. Sexual selection for spatial-learning ability. Anim. Behav. 1989, 37, 322–331. [Google Scholar] [CrossRef]
- Gray, J.A.; Buffery, A.W. Sex differences in emotional and cognitive behaviour in mammals including man: Adaptive and neural bases. Acta Psychol. 1971, 35, 89–111. [Google Scholar] [CrossRef]
- Diamond, R.F.; Stoinski, T.S.; Mickelberg, J.L.; Basile, B.M.; Gazes, R.P.; Templer, V.L.; Hampton, R.R. Similar stimulus features control visual classification in orangutans and rhesus monkeys. J. Exp. Anal. Behav. 2016, 105, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Servheen, C. Sun bear conservation action plan. In Bears: Status Survey and Conservation Action Plan; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland; Cambridge, UK, 1999; pp. 219–224. [Google Scholar]
- Yamanashi, Y.; Hayashi, M. Assessing the effects of cognitive experiments on the welfare of captive chimpanzees (Pan troglodytes) by direct comparison of activity budget between wild and captive chimpanzees. Am. J. Primatol. 2011, 73, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Maple, T.L.; Perdue, B.M. Zoo Animal Welfare; Animal Welfare Series; Springer: Berlin, Germany, 2013. [Google Scholar]
- Clay, A.W.; Perdue, B.M.; Gaalema, D.E.; Dolins, F.; Bloomsmith, M.A. The use of technology to enhance zoological parks. Zoo Biol. 2011, 30, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Perdue, B.M.; Clay, A.W.; Stoinski, T.S.; Gaalema, D.E.; Maple, T.L. Technology at the zoo: The influence of a touchscreen computer on orangutans and zoo visitors. Zoo Biol. 2012, 31, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Tarou, L.R.; Kuhar, C.W.; Adcock, D.; Bloomsmith, M.A.; Maple, T.L. Computer-assisted enrichment for zoo-housed orangutans (Pongo pygmaeus). Anim. Welf. 2004, 13, 445–453. [Google Scholar]
- Burghardt, G.M. Behavioral research on common animals in small zoos. In Research in Zoos and Aquariums; Institute of Laboratory Animal Resources, Ed.; National Academy of Sciences: Washington, DC, USA, 1975; pp. 103–133. [Google Scholar]
- Schwarzenberger, F.; Fredriksson, G.; Schaller, K.; Kolter, L. Fecal steroid analysis for monitoring reproduction in the sun bear (Helarctos malayanus). Theriogenology 2004, 62, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Frederick, C.; Hunt, K.E.; Kyes, R.; Collins, D.; Wasser, S.K. Reproductive timing and aseasonality in the sun bear (Helarctos malayanus). J. Mammal. 2012, 93, 522–531. [Google Scholar] [CrossRef]

© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdue, B.M. The Effect of Computerized Testing on Sun Bear Behavior and Enrichment Preferences. Behav. Sci. 2016, 6, 19. https://doi.org/10.3390/bs6040019
Perdue BM. The Effect of Computerized Testing on Sun Bear Behavior and Enrichment Preferences. Behavioral Sciences. 2016; 6(4):19. https://doi.org/10.3390/bs6040019
Chicago/Turabian StylePerdue, Bonnie M. 2016. "The Effect of Computerized Testing on Sun Bear Behavior and Enrichment Preferences" Behavioral Sciences 6, no. 4: 19. https://doi.org/10.3390/bs6040019
APA StylePerdue, B. M. (2016). The Effect of Computerized Testing on Sun Bear Behavior and Enrichment Preferences. Behavioral Sciences, 6(4), 19. https://doi.org/10.3390/bs6040019




