The Application of Cyanobacteria as a Biofertilizer for Okra (Abelmoschus esculentus) Production with a Focus on Environmental and Ecological Sustainability
Abstract
:1. Introduction
2. Materials and Methods
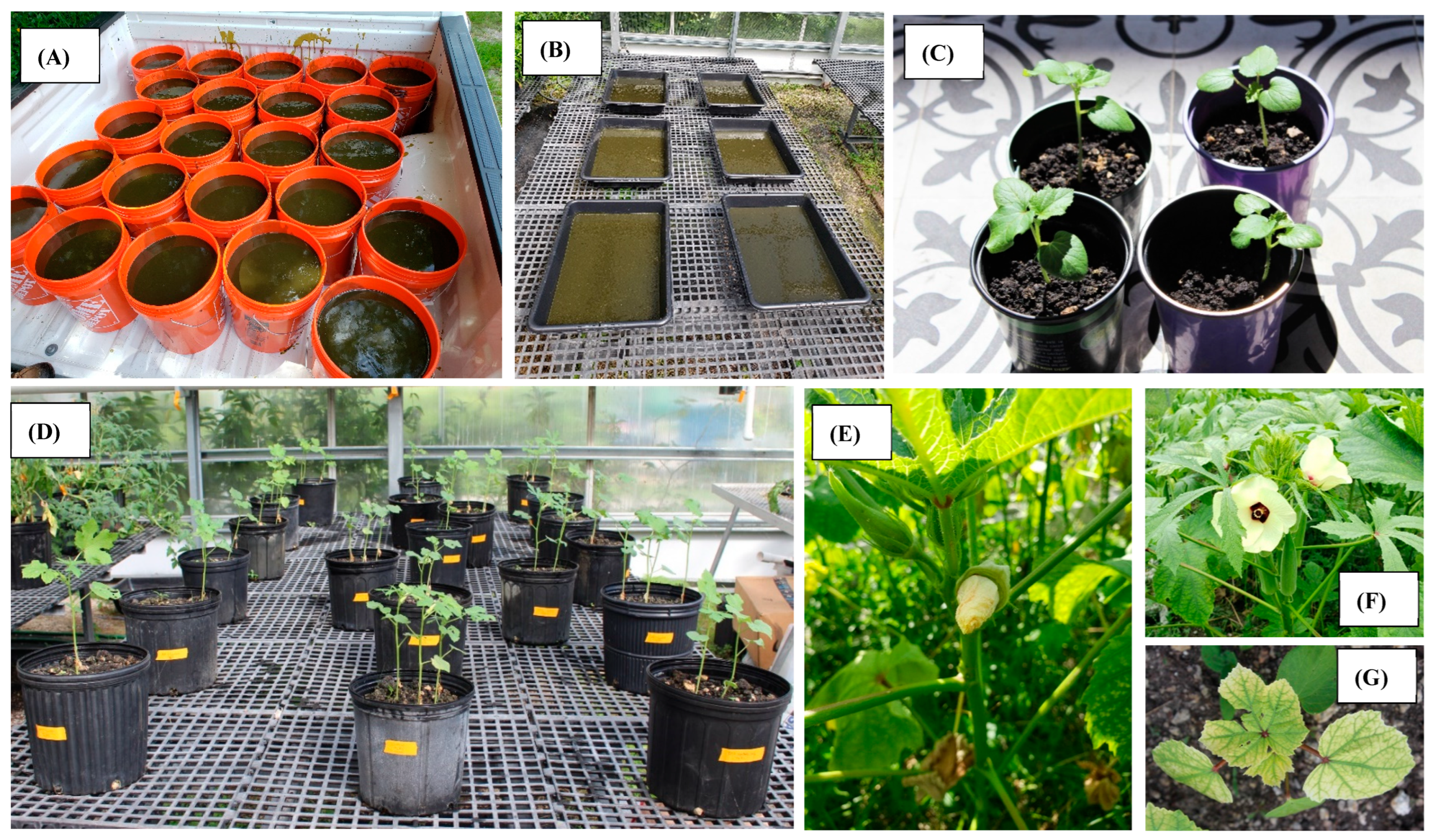
2.1. Cyanobacteria Collection and Biofertilizer Processing
2.2. Experimental Design and Sample Collection
2.3. Plant Height, Stem Diameter, Leaf Chlorophyll Content, Crop Biomass, and Okra Fruit Yield
2.4. Laboratory Analyses
2.5. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Properties of the Biofertilizer
3.2. Plant Physiological Parameters
- (a)
- Leaf chlorophyll content—SPAD values
- (b)
- Plant height, stem diameter, and crop biomass production and okra yield
3.3. Soil Properties at Harvesting
3.4. Environmental Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Simranjit, K.; Kanchan, A.; Prasanna, R.; Ranjan, K.; Ramakrishnan, B.; Singh, A.K.; Shivay, Y.S. Microbial inoculants as plant growth stimulating and soil nutrient availability enhancing options for cucumber under protected cultivation. World J. Microbiol. Biotechnol. 2019, 35, 51. [Google Scholar] [CrossRef]
- Manjunath, M.; Kanchan, A.; Ranjan, K.; Venkatachalam, S.; Prasanna, R.; Ramakrishnan, B.; Hossain, F.; Nain, L.; Shivay, Y.S.; Rai, A.B.; et al. Beneficial cyanobacteria and eubacteria synergistically enhance bioavailability of soil nutrients and yield of okra. Heliyon 2016, 2, e00066. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Olguín, E.J.; Diels, L.; De Philippis, R. Microbial fixation of CO2 in water bodies and in drylands to combat climate change, soil loss and desertification. New Biotechnol. 2015, 32, 109–120. [Google Scholar] [CrossRef]
- Li, Y.C.; Klassen, W.; Lamberts, M.; Olczyk, T.; Liu, G. Okra Production in Miami-Dade County, Florida. HS-87/TR009. 2021. Available online: https://edis.ifas.ufl.edu/publication/TR009 (accessed on 30 August 2022).
- Karthikeyan, N.; Prasanna, R.; Nain, L.; Kaushik, B.D. Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur. J. Soil Biol. 2007, 43, 23–30. [Google Scholar] [CrossRef]
- Prasanna, R.; Babu, S.; Rana, A.; Kabi, S.R.; Chaudhary, V.; Gupta, V.; Kumar, A.; Shivay, Y.S.; Nain, L.; Pal, R.K. Evaluating the establishment and agronomic proficiency of cyanobacterial consortia as organic options in wheat–rice cropping sequence. Exp. Agric. 2013, 49, 416–434. [Google Scholar] [CrossRef]
- Jha, M.N.; Prasad, A.N. Efficacy of new inexpensive cyanobacterial biofertilizer including its shelf-life. World J. Microbiol. Biotechnol. 2006, 22, 73–79. [Google Scholar] [CrossRef]
- Asmamaw, M.; Wolde, G.; Yohannes, M.; Yigrem, S.; Woldemeskel, E.; Chala, A.; Davis, J.G. Comparison of cyanobacterial bio-fertilizer with urea on three crops and two soils of Ethiopia. Afr. J. Agric. Res. 2019, 14, 588–596. [Google Scholar]
- Chittapun, S.; Limbipichai, S.; Amnuaysin, N.; Boonkerd, R.; Charoensook, M. Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: A pot experiment. J. Appl. Phycol. 2018, 30, 79–85. [Google Scholar] [CrossRef]
- Yoder, N.; Davis, J.G. Organic fertilizer comparison on growth and nutrient content of three kale cultivars. HortTechnology 2020, 30, 176–184. [Google Scholar] [CrossRef]
- Faridmarandi, S.; Khare, Y.P.; Naja, G.M. Long-term regional nutrient contributions and in-lake water quality trends for Lake Okeechobee. Lake Reserv. Manag. 2020, 37, 77–94. [Google Scholar] [CrossRef]
- Pereira, I.; Ortega, R.; Barrientos, L.; Moya, M.; Reyes, G.; Kramm, V. Development of a biofertilizer based on filamentous nitrogen-fixing cyanobacteria for rice crops in Chile. J. Appl. Phycol. 2009, 21, 135–144. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Szufa, S.; Grzesik, M.; Piotrowski, K.; Janas, R. The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies 2021, 14, 5262. [Google Scholar]
- Dattamudi, S.; Wang, J.J.; Dodla, S.K.; Arceneaux, A.; Viator, H.P. Effect of nitrogen fertilization and residue management practices on ammonia emissions from subtropical sugarcane production. Atmos. Environ. 2016, 139, 122–130. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef]
- Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of salinity stress and microbial inoculations on glomalin production and plant growth parameters of snap bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. [Google Scholar] [CrossRef]
- Kandel, B.P. Spad value varies with age and leaf of maize plant and its relationship with grain yield. BMC Res. Notes 2020, 13, 475. [Google Scholar] [CrossRef]
- Meng, Z.; Duan, A.; Chen, D.; Dassanayake, K.B.; Wang, X.; Liu, Z.; Gao, S.; Liu, H. Suitable indicators using stem diameter variation-derived indices to monitor the water status of greenhouse tomato plants. PLoS ONE 2017, 12, e0171423. [Google Scholar] [CrossRef]
- Clough, A.; Hunter, M.N. Stem diameter: A rapid accurate parameter for monitoring growth of sorghum. In Proceedings of the 11th Australian Agronomy Conference, Geelong, Victoria, 2–6 February 2003; Volume 22, p. 2005. [Google Scholar]
- Toribio, A.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Moreno, J. Prospection of cyanobacteria producing bioactive substances and their application as potential phytostimulating agents. Biotechnol. Rep. 2020, 26, e00449. [Google Scholar] [CrossRef]
- Essa, A.M.; Ibrahim, W.M.; Mahmud, R.M.; ElKassim, N.A. Potential impact of cyanobacterial exudates on seed germination and antioxidant enzymes of crop plant seedlings. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 1010–1024. [Google Scholar]
- Agwa, O.K.; Ogugbue, C.J.; Williams, E.E. Research Article Field Evidence of Chlorella vulgaris Potentials as a Biofertilizer for Hibiscus esculentus. Int. J. Agric. Res. 2017, 12, 181–189. [Google Scholar] [CrossRef]



| Parameters | Unit | Value |
|---|---|---|
| pH | 8.01 | |
| Total C (TC) | % | 6.07 ± 0.42 |
| Total N (TN) | % | 0.34 ± 0.06 |
| Total P (TP) * | ppm | 103 ± 27 |
| Potassium (K) | ppm | 175 ± 42 |
| Calcium (Ca) | ppm | 13,604 ± 2722 |
| Magnesium (Mg) | ppm | 255 ± 29 |
| Sulphur (S) | ppm | 79.20 ± 8.24 |
| Zinc (Zn) | ppm | 24.48 ± 5.69 |
| Copper (Cu) | ppm | 32.80 ± 4.91 |
| Sodium (Na) | ppm | 51.73 ± 11.73 |
| Parameters | Unit | Value |
|---|---|---|
| Total C (TC) | % | 19.56 ± 0.71 |
| Total N (TN) | % | 1.79 ± 0.07 |
| Total P (TP) | % | 0.02 ± 0.00 |
| Total S (TS) | % | 0.13 ± 0.01 |
| Potassium (K) | % | 0.06 ± 0.01 |
| Calcium (Ca) | % | 6.12 ± 0.70 |
| Magnesium (Mg) | % | 0.12 ± 0.01 |
| Iron (Fe) | ppm | 2005 ± 160 |
| Manganese (Mn) | ppm | 132.56 ± 13.35 |
| Zinc (Zn) | ppm | 53.34 ± 0.69 |
| Copper (Cu) | ppm | 29.51 ± 4.80 |
| Boron (B) | ppm | 95.37 ± 4.21 |
| Molybdenum (Mo) | ppm | 1.82 ± 1.01 |
| Nickel (Ni) | ppm | 4.34 ± 1.78 |
| Treatments | Plant Height * | Stem Diameter * | Shoot Dry Weight¶ | Root Dry Weight * | Shoot/Root | Yield¶ |
|---|---|---|---|---|---|---|
| cm | cm | g | g/pot | |||
| Control | 45.3 ± 3.68 b | 0.78 ± 0.08 a | 26.71 ± 4.61 b | 5.61 ± 0.88 b | 4.69 | 73.38 ± 5.27 b |
| TS | 62.8 ± 4.81 a | 1.08 ± 0.16 a | 47.92 ± 6.99 ab | 8.37 ± 1.95 ab | 6.14 | 120.98 ± 9.42 a |
| TB | 61.3 ± 5.16 a | 1.11 ± 0.13 a | 55.01 ± 7.62 a | 11.58 ± 2.16 a | 3.78 | 130.34 ± 8.78 a |
| HH | 58.3 ± 3.68 ab | 1.05 ± 0.13 a | 37.07 ± 3.92 ab | 7.59 ± 0.78 ab | 4.21 | 110.18 ± 12.36 ab |
| Treatments | C | N | P | K | Ca | Mg | S | Fe | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|
| % | ppm | |||||||||
| Control | 27 ± 6 b | 1.9 ± 0.4 b | 0.18 ± 0.04 b | 1.3 ± 0.41 a | 2.9 ± 0.7 b | 0.62 ± 0.18 a | 0.32 ± 0.14 a | 152 ± 27 c | 17 ± 4 b | 48 ± 11 a |
| TS | 36 ± 11 ab | 2.9 ± 0.6 ab | 0.26 ± 0.07 ab | 2.0 ± 0.62 a | 4.1 ± 1.1 ab | 0.85 ± 0.29 a | 0.33 ± 0.14 a | 173 ± 42 bc | 37 ± 8 a | 40 ± 9 a |
| TB | 44 ± 7 a | 3.7 ± 0.5 a | 0.32 ± 0.07 a | 2.3 ± 0.59 a | 4.8 ± 1.0 a | 0.95 ± 0.21 a | 0.35 ± 0.17 a | 258 ± 31 a | 28 ± 11 ab | 42 ± 13 a |
| HH | 38 ± 10 ab | 3.1 ± 0.6 a | 0.22 ± 0.07 ab | 1.7 ± 0.24 a | 4.5 ± 0.9 ab | 0.88 ± 0.27 a | 0.30 ± 0.16 a | 220 ± 29 ab | 33 ± 7 a | 51 ± 7 a |
| Treatments | pH | C | N | P | K | Ca | Mg | S | Na |
|---|---|---|---|---|---|---|---|---|---|
| % | ppm | ||||||||
| Control | 7.69 | 5.46 ± 0.49 b | 0.296 ± 0.05 c | 86 ± 19 b | 154 ± 28 b | 12,064 ± 1223 b | 242 ± 37 b | 37 ± 6 b | 47 ± 15 a |
| TS | 7.84 | 8.44 ± 1.27 a | 0.438 ± 0.07 ab | 117 ± 39 ab | 388 ± 111 a | 13,289 ± 1361 b | 354 ± 38 a | 102 ± 18 a | 67 ± 14 a |
| TB | 6.63 | 10.77 ± 1.53 a | 0.533 ± 0.06 a | 196 ± 41 a | 301 ± 120 ab | 20,850 ± 2209 a | 384 ± 29 a | 89 ± 11 a | 82 ± 24 a |
| HH | 7.89 | 9.15 ± 1.11 a | 0.407 ± 0.05 b | 141 ± 22 a | 256 ± 62 a | 13,927 ± 1621 b | 325 ± 41 a | 116 ± 22 a | 74 ± 22 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanda, S.; Dattamudi, S.; Jayachandran, K.; Scinto, L.J.; Bhat, M. The Application of Cyanobacteria as a Biofertilizer for Okra (Abelmoschus esculentus) Production with a Focus on Environmental and Ecological Sustainability. Environments 2024, 11, 45. https://doi.org/10.3390/environments11030045
Chanda S, Dattamudi S, Jayachandran K, Scinto LJ, Bhat M. The Application of Cyanobacteria as a Biofertilizer for Okra (Abelmoschus esculentus) Production with a Focus on Environmental and Ecological Sustainability. Environments. 2024; 11(3):45. https://doi.org/10.3390/environments11030045
Chicago/Turabian StyleChanda, Saoli, Sanku Dattamudi, Krishnaswamy Jayachandran, Leonard J. Scinto, and Mahadev Bhat. 2024. "The Application of Cyanobacteria as a Biofertilizer for Okra (Abelmoschus esculentus) Production with a Focus on Environmental and Ecological Sustainability" Environments 11, no. 3: 45. https://doi.org/10.3390/environments11030045






