Reuse of Soils Fertilized with Ash as Recycling Derived Fertilizer Revealed Strong Stimulation of Microbial Communities Involved in P Mobilization in Lolium perenne Rhizospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microcosm Trial Set-Up
2.2. Microcosm Harvest, Plant and Soil Analyses
2.3. Cultivation Dependent Microbial Analyses
2.4. Cultivation Independent Microbial Analyses
2.5. Data Analysis
3. Results
3.1. Biomass Yields, Agronomic Efficiency and Elemental Analysis
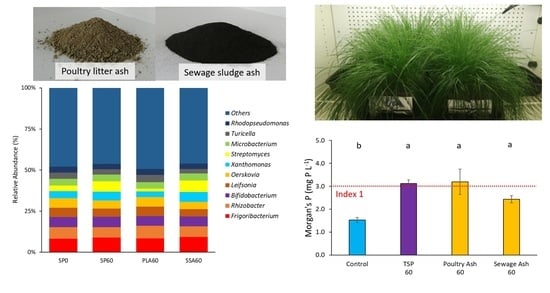
3.2. Microbial P-Mobilization and Solubilization, Soil pH and Morgan’s P
3.3. Phosphatase Activity and Gene Copy Numbers (phoC, phoD) in Soil
3.4. Arbuscular Mycorrhizal Fungi Root Colonization
3.5. Bacterial Community Analysis
3.6. Bacterial Community Analysis Based on phoD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Growing at a Slower Pace, World Population is Expected to Reach 9.7 Billion in 2050 and Could Peak at Nearly 11 Billion around 2100. Available online: https://www.un.org/sustainabledevelopment/blog/2019/06/growing-at-a-slower-pace-world-population-is-expected-to-reach-9-7-billion-in-2050-and-could-peak-at-nearly-11-billion-around-2100-un-report/ (accessed on 24 January 2022).
- Liu, Y.; Villalba, G.; Ayres, R.U.; Schroder, H. Global Phosphorus Flows and Environmental Impacts from a Consumption Perspective. J. Ind. Ecol. 2008, 12, 229–247. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus Resources, Their Depletion and Conservation, A Review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The Global Phosphorus Cycle. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2003; pp. 585–643. [Google Scholar]
- Smit, A.L.; Bindraban, P.S.; Schröder, J.J.; Conijn, J.G.; van der Meer, H.G. Phosphorus in Agriculture: Global Resources, Trends and Developments; Plant Research International: Wageningen, The Netherlands, 2009. [Google Scholar]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A New Sustainability Paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Powers, S.M.; Chowdhury, R.B.; MacDonald, G.K.; Metson, G.S.; Beusen, A.H.W.; Bouwman, A.F.; Hampton, S.E.; Mayer, B.K.; McCrackin, M.L.; Vaccari, D.A. Global Opportunities to Increase Agricultural Independence Through Phosphorus Recycling. Earth’s Future 2019, 7, 370–383. [Google Scholar] [CrossRef]
- Mackey, K.; Paytan, A. Phosphorus Cycle. Encycl. Microbiol. 2009, 3, 322–334. [Google Scholar]
- Barrow, N.J. Comparing two theories about the nature of soil phosphate. Eur. J. Soil Sci. 2021, 72, 679–685. [Google Scholar] [CrossRef]
- Gerke, J. Phytate (Inositol Hexakisphosphate) in Soil and Phosphate Acquisition from Inositol Phosphates by Higher Plants. A Review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.-M.; Richardson, A.E. Phosphate Mobilisation by Soil Microorganisms. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 225–234. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring Phosphorus Fertilizers and Fertilization Strategies for Improved Human and Environmental Health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting Phosphorus Bioavailability from High-Ash Biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Seeling, B.; Zasoski, R.J. Microbial Effects in Maintaining Organic and Inorganic Solution Phosphorus Concentrations in a Grassland Topsoil. Plant Soil 1993, 148, 277–284. [Google Scholar] [CrossRef]
- Mozumder, P.; Berrens, R.P. Inorganic Fertilizer Use and Biodiversity Risk: An Empirical Investigation. Ecol. Econ. 2007, 62, 538–543. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Li, D.; Xu, C.; Xiang, X. Differential Responses of Arbuscular Mycorrhizal Fungal Communities to Mineral and Organic Fertilization. MicrobiologyOpen 2020, 9, e00920. [Google Scholar] [CrossRef] [PubMed]
- Ikoyi, I.; Fowler, A.; Schmalenberger, A. One-Time Phosphate Fertilizer Application to Grassland Columns Modifies the Soil Microbiota and Limits Its Role in Ecosystem Services. Sci. Total Environ. 2018, 630, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Huotari, N.; Tillman-Sutela, E.; Moilanen, M.; Laiho, R. Recycling of Ash—For the Good of the Environment? For. Ecol. Manag. 2015, 348, 226–240. [Google Scholar] [CrossRef]
- Rosenberg, O.; Persson, T.; Högbom, L.; Jacobson, S. Effects of Wood-Ash Application on Potential Carbon and Nitrogen Mineralisation at Two Forest Sites with Different Tree Species, Climate and N Status. For. Ecol. Manag. 2010, 260, 511–518. [Google Scholar] [CrossRef]
- Jacobson, S.; Lundström, H.; Nordlund, S.; Sikström, U.; Pettersson, F. Is Tree Growth in Boreal Coniferous Stands on Mineral Soils Affected by the Addition of Wood Ash? Scand. J. For. Res. 2014, 29, 675–685. [Google Scholar] [CrossRef]
- Perkiömäki, J.; Fritze, H. Does Simulated Acid Rain Increase the Leaching of Cadmium from Wood Ash to Toxic Levels to Coniferous Forest Humus Microbes? FEMS Microbiol. Ecol. 2003, 44, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Huygens, D.; Saveyn, H.G.M. Agronomic Efficiency of Selected Phosphorus Fertilisers Derived from Secondary Raw Materials for European Agriculture. A Meta-Analysis. Agron. Sustain. Dev. 2018, 38, 52. [Google Scholar] [CrossRef]
- Hermann, L.; Schaaf, T. Outotec (AshDec®) Process for P Fertilizers from Sludge Ash. In Phosphorus Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2019; pp. 221–233. [Google Scholar]
- Deinert, L.; Ikoyi, I.; Egeter, B.; Forrestal, P.; Schmalenberger, A. Short-Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne). Plants 2023, 12, 2762. [Google Scholar] [CrossRef]
- Gahan, J.; O’Sullivan, O.; Cotter, P.D.; Schmalenberger, A. Arbuscular Mycorrhiza Support Plant Sulfur Supply through Organosulfur Mobilizing Bacteria in the Hypho- and Rhizosphere. Plants 2022, 11, 3050. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Fox, A.; Kwapinski, W.; Griffiths, B.S.; Schmalenberger, A. The Role of Sulfur- and Phosphorus-Mobilizing Bacteria in Biochar-Induced Growth Promotion of Lolium perenne. FEMS Microbiol. Ecol. 2014, 90, 78–91. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. phoD Alkaline Phosphatase Gene Diversity in Soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of Bacterial Non-Specific Acid (phoC) and Alkaline (phoD) Phosphatase Genes in Bulk and Rhizosphere Soil from Organically Managed Soybean Fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Sakurai, M.; Wasaki, J.; Tomizawa, Y.; Shinano, T.; Osaki, M. Analysis of Bacterial Communities on Alkaline Phosphatase Genes in Soil Supplied with Organic Matter. Soil Sci. Plant Nutr. 2008, 54, 62–71. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s q2-feature-classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.D.; Lynch, D.H.; Bent, E.; Entz, M.H.; Dunfield, K.E. Soil Bacterial phoD Gene Abundance and Expression in Response to Applied Phosphorus and Long-Term Management. Soil Biol. Biochem. 2015, 88, 137–147. [Google Scholar] [CrossRef]
- Fish, J.A.; Chai, B.; Wang, Q.; Sun, Y.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The Functional Gene Pipeline and Repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef] [PubMed]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage Sludge Ash—A Promising Secondary Phosphorus Source for Fertilizer Production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A Review on Wastewater Sludge Valorisation and Its Challenges in the Context of Circular Economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Choudhury, A.; Felton, G.; Moyle, J.; Lansing, S. Fluidized bed combustion of poultry litter at farm-scale: Environmental impacts using a life cycle approach. J. Clean. Prod. 2020, 276, 124231. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Deinert, L.; Ashekuzzaman, S.; Forrestal, P.; Schmalenberger, A. The impact of struvite and ash recycling-derived fertilizers on microbial phosphorus mobilization capabilities and community structure in a Lolium perenne field trial. bioRxiv 2024, arXiv:bioRxiv:2024.2001.2016.575387. [Google Scholar]
- Barrow, N.J. How understanding soil chemistry can lead to better phosphate fertilizer practice: A 68 year journey (so far). Plant Soil 2022, 476, 117–131. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Corneo, P.E.; Mariotte, P.; Kertesz, M.A.; Dijkstra, F.A. Effect of Crop Rotation on Mycorrhizal Colonization and Wheat Yield Under Different Fertilizer Treatments. Agric. Ecosyst. Environ. 2017, 247, 130–136. [Google Scholar] [CrossRef]
- Gryndler, M.; Larsen, J.; Hršelová, H.; Řezáčová, V.; Gryndlerová, H.; Kubát, J. Organic and Mineral Fertilization, Respectively, Increase and Decrease the Development of External Mycelium of Arbuscular Mycorrhizal Fungi in a Long-Term Field Experiment. Mycorrhiza 2006, 16, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.C. Mycelium of Arbuscular Mycorrhizal Fungi (AMF) From Different Genera: Form, Function and Detection. Plant Soil 2000, 226, 131–151. [Google Scholar] [CrossRef]
- Thompson, J.P. Soilless Culture of Vesicular–Arbuscular Mycorrhizae of Cereals: Effects of Nutrient Concentration and Nitrogen Source. Can. J. Bot. 1986, 64, 2282–2294. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH Mediates the Balance Between Stochastic and Deterministic Assembly of Bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Barrow, N.J.; Lambers, H. Phosphate-solubilising microorganisms mainly increase plant phosphate uptake by effects of pH on root physiology. Plant Soil 2022, 476, 397–402. [Google Scholar] [CrossRef]
- Mijangos, I.; Albizu, I.; Epelde, L.; Amezaga, I.; Mendarte, S.; Garbisu, C. Effects of Liming on Soil Properties and Plant Performance of Temperate Mountainous Grasslands. J. Environ. Manag. 2010, 91, 2066–2074. [Google Scholar] [CrossRef]
- Fraser, T.; Lynch, D.H.; Entz, M.H.; Dunfield, K.E. Linking Alkaline Phosphatase Activity with Bacterial phoD Gene Abundance in Soil from a Long-Term Management Trial. Geoderma 2015, 257–258, 115–122. [Google Scholar] [CrossRef]
- Spohn, M.; Treichel, N.S.; Cormann, M.; Schloter, M.; Fischer, D. Distribution of Phosphatase Activity and Various Bacterial Phyla in the Rhizosphere of Hordeum vulgare L. Depending on P Availability. Soil Biol. Biochem. 2015, 89, 44–51. [Google Scholar] [CrossRef]
- Gómez-Muñoz, B.; Efthymiou, A.; Dubey, M.; Sølve, J.; Nicolaisen, M.; Jensen, D.F.; Nybroe, O.; Larsen, J. Cellulose Amendment Promotes P Solubilization by Penicillium aculeatum in Non-Sterilized Soil. Fungal Biol. 2022, 126, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Ragot, S.A.; Kertesz, M.A.; Mészáros, É.; Frossard, E.; Bünemann, E.K. Soil phoD and phoX Alkaline Phosphatase Gene Diversity Responds to Multiple Environmental Factors. FEMS Microbiol. Ecol. 2017, 93, fiw212. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Messuti, M.I.; Reiner, G.; Boenel, M.; Vobis, G.; Wall, L.G.; Scervino, J.M. Exploring the Response of Actinobacteria to the Presence of Phosphorus Salts Sources: Metabolic and Co-Metabolic Processes. J. Basic Microbiol. 2019, 59, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Wang, Y.; Tan, J.; Qin, Y.; Zuo, W.; Wu, H.; He, H.; He, D. Alkaline Phosphatase-Harboring Bacterial Community and Multiple Enzyme Activity Contribute to Phosphorus Transformation During Vegetable Waste and Chicken Manure Composting. Bioresour. Technol. 2020, 297, 122406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Ye, L.; Huang, Y.; Kang, Z.; Zhang, B.; Zhang, X. Colonization by Tuber melanosporum and Tuber indicum affects the growth of Pinus armandii and phoD alkaline phosphatase encoding bacterial community in the rhizosphere. Microbiol. Res. 2020, 239, 126520. [Google Scholar] [CrossRef]
- Liu, W.; Ling, N.; Luo, G.; Guo, J.; Zhu, C.; Xu, Q.; Liu, M.; Shen, Q.; Guo, S. Active phoD-Harboring Bacteria are Enriched by Long-Term Organic Fertilization. Soil Biol. Biochem. 2021, 152, 108071. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Li, M.; Yang, S.; Tao, X.; Zheng, Y.; Wang, X.; Chen, R. Biochar reduces bioavailability of phosphorus during swine manure composting: Roles of phoD-harboring bacterial community. Sci. Total Environ. 2023, 858, 159926. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Saju, A.; Schmalenberger, A.; Lagrange, H.; Forrestal, P.; Postma, R.; van Scholl, L.; Verleden, I.; Ryan, D.; Krapinska, A.; et al. Product Information Sheets (WPT1_D3.4). Interreg North-West Europe; ReNu2Farm. Available online: https://www.nmi-agro.nl/wp-content/uploads/2019/09/21022022_WPT1_D3.4-Product-factsheets.pdf (accessed on 26 January 2024).





| Treatment | Biomass FW (g) | Biomass DW (g) | Shoot Count | DW:Shoot Ratio (g Shoot−1) | AE | ||||
|---|---|---|---|---|---|---|---|---|---|
| SP0 | 11.87 b | ±0.33 | 1.49 b | ±0.16 | 87.3 a | ±2.73 | 0.017 a | ±1.7 × 10−03 | |
| SP60 | 14.63 a | ±0.31 | 1.71 ab | ±0.05 | 87.2 a | ±3.72 | 0.020 a | ±7.8 × 10−04 | 13.5 |
| PLA60 | 13.13 ab | ±0.24 | 1.84 a | ±0.02 | 86.3 a | ±2.89 | 0.021 a | ±5.5 × 10−04 | 20.8 |
| SSA60 | 12.76 b | ±0.71 | 1.77 ab | ±0.04 | 84.5 a | ±3.12 | 0.021 a | ±1.1 × 10−03 | 17.0 |
| Treatment | Macronutrients [µg] | |||||
|---|---|---|---|---|---|---|
| Ca | K | Mg | N | P | S | |
| SP0 | 1379 | 5135 | 325 | 4070 | 157 | 291 |
| SP60 | 1381 | 4290 | 360 | 3911 | 214 | 292 |
| PLA60 | 1025 | 4238 | 263 | 3094 | 136 | 245 |
| SSA60 | 1157 | 4036 | 282 | 3565 | 151 | 263 |
| Treatment | Micronutrients [µg] | |||||
| B | Cu | Fe | Mb | Mn | Zn | |
| SP0 | 0.75 | 1.32 | 35.20 | 0.01 | 22.19 | 4.63 |
| SP60 | 0.67 | 1.28 | 21.21 | 0.02 | 16.86 | 4.58 |
| PLA60 | 0.61 | 0.93 | 17.79 | 0.02 | 16.81 | 3.87 |
| SSA60 | 0.58 | 1.02 | 24.74 | 0.01 | 16.03 | 3.95 |
| Treatment | PAA (MPN g−1 Soil) | Phy (MPN g−1 Soil) | R2A (MPN g−1 Soil) | |||
|---|---|---|---|---|---|---|
| SP0 | 6.2 × 1005 b | ±2.0 × 1005 | 3.5 × 1006 b | ±7.2 × 1005 | 2.0 × 1008 a | ±1.4 × 1008 |
| SP60 | 1.6 × 1006 b | ±1.1 × 1006 | 6.7 × 1006 b | ±1.3 × 1006 | 8.7 × 1007 a | ±2.1 × 1007 |
| PLA60 | 3.2 × 1006 a | ±4.2 × 1005 | 1.8 × 1007 a | ±5.1 × 1006 | 1.1 × 1008 a | ±4.7 × 1007 |
| SSA60 | 3.7 × 1006 ab | ±1.1 × 1006 | 1.0 × 1007 ab | ±3.0 × 1006 | 7.8 × 1007 a | ±1.1 × 1007 |
| Treatment | Soil pH | Morgan’s P (mg P L−1) | ||
|---|---|---|---|---|
| SP0 | 4.75 c | ±0.01 | 1.53 b | ±0.11 |
| SP60 | 5.06 b | ±0.03 | 3.12 a | ±0.15 |
| PLA60 | 5.34 a | ±0.03 | 3.20 a | ±0.55 |
| SSA60 | 5.30 a | ±0.04 | 2.43 a | ±0.16 |
| Treatment | ACP (µg pNP g−1 Soil h−1) | ALP (µg pNP g−1 Soil h−1) | phoC (phoC Copies g−1 Soil) | phoD (phoD Copies g−1 Soil) | ||||
|---|---|---|---|---|---|---|---|---|
| SP0 | 1504.9 a | ±79.0 | 121.9 a | ±11.1 | 1.1 × 1007 b | ±1.7 × 1006 | 1.5 × 1009 b | ±1.8 × 1008 |
| SP60 | 1556.1 a | ±65.2 | 109.7 a | ±8.3 | 1.1 × 1007 b | ±9.7 × 1005 | 1.5 × 1009 b | ±5.8 × 1007 |
| PLA60 | 1608.1 a | ±91.4 | 117.4 a | ±6.5 | 2.2 × 1007 ab | ±3.3 × 1006 | 2.8 × 1009 a | ±2.7 × 1008 |
| SSA60 | 1322.1 a | ±94.0 | 115.9 a | ±6.7 | 2.2 × 1007 a | ±8.0 × 1005 | 2.5 × 1009 a | ±1.1 × 1008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deinert, L.; Schmalenberger, A. Reuse of Soils Fertilized with Ash as Recycling Derived Fertilizer Revealed Strong Stimulation of Microbial Communities Involved in P Mobilization in Lolium perenne Rhizospheres. Environments 2024, 11, 49. https://doi.org/10.3390/environments11030049
Deinert L, Schmalenberger A. Reuse of Soils Fertilized with Ash as Recycling Derived Fertilizer Revealed Strong Stimulation of Microbial Communities Involved in P Mobilization in Lolium perenne Rhizospheres. Environments. 2024; 11(3):49. https://doi.org/10.3390/environments11030049
Chicago/Turabian StyleDeinert, Lea, and Achim Schmalenberger. 2024. "Reuse of Soils Fertilized with Ash as Recycling Derived Fertilizer Revealed Strong Stimulation of Microbial Communities Involved in P Mobilization in Lolium perenne Rhizospheres" Environments 11, no. 3: 49. https://doi.org/10.3390/environments11030049