Combined Sous-Vide and High Hydrostatic Pressure Treatment of Pork: Is the Order of Application Decisive When Using Minimal Processing Technologies?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Treatments
2.2.1. Single Sous-Vide (SV) and High Hydrostatic Pressure (HHP) Processing
2.2.2. Combined Heat and High Hydrostatic Pressure Processing
2.2.3. Treatment Parameters
2.3. Storage Conditions
2.4. Analysis of the Quality Parameters
2.4.1. Weight Loss
2.4.2. pH Value
2.4.3. Moisture Content
2.4.4. Water Holding Capacity (WHC)
2.4.5. Color
2.4.6. Texture
2.4.7. Lipid Oxidation
2.4.8. Differential Scanning Calorimetry (DSC)
2.4.9. Microbial Analysis
2.5. Statistical Analysis
3. Results and Discussion
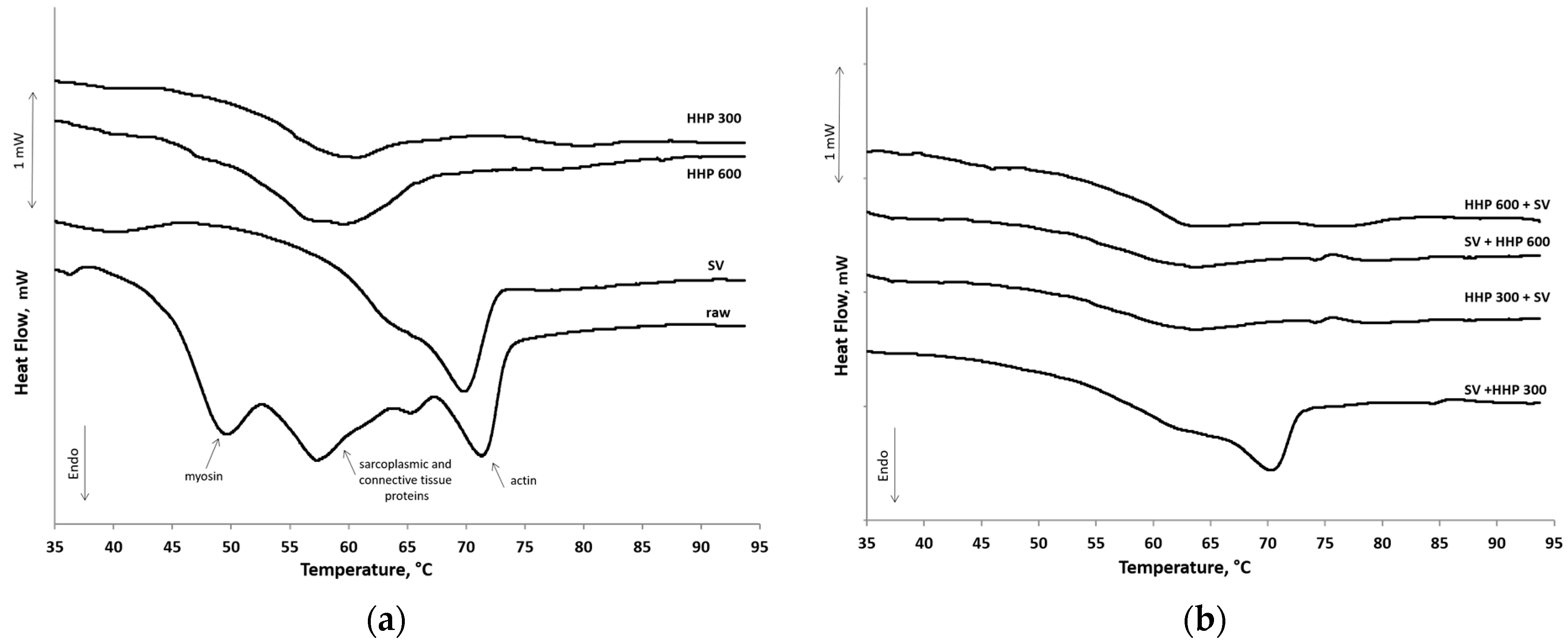
3.1. Comparison of the Effects of the Single Heat and Pressure Treatments Focusing on the Main Differences
3.2. Combined-Treated Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creed, P.G. Sensory and nutritional quality of sous-vide foods. Food Control 1995, 6, 45–52. [Google Scholar] [CrossRef]
- Bejerholm, C.; Aaslyng, M.D. The influence of cooking technique and core temperature on results of a sensory analysis of pork–depending on the raw meat quality. Food Qual. Prefer. 2003, 15, 19–30. [Google Scholar] [CrossRef]
- Mortensen, L.M.; Frost, M.B.; Skibsted, L.H.; Risbo, J. Long-time low temperature cooking of beef: Three dominant time-temperature behaviours of sensory properties. Flavour 2015, 3, 2. [Google Scholar] [CrossRef]
- Becker, A.; Boulaaba, A.; Pingen, S.; Krischek, C.; Klein, G. Low temperature cooking of pork meat-Physicochemical and sensory aspects. Meat Sci. 2016, 118, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sous Vide Advisory Committee (SVAC). Code of Practice for Sous Vide Catering Systems; Sous Vide Advisory Committee (SVAC): Tetbury, Gloucestershire, UK.
- Betts, D.G. Critical factors affecting the safety of minimally processed chilled foods. In Sous-Vide and Cook-Chill Processing for Food Industry, 1st ed.; Aspen Inc.: Gaithersburg, MD, USA, 1998; pp. 135–145. [Google Scholar]
- Food Safety Authority of Ireland Guidance Note No. 20. Industrial Processing of Heat-Chill Foods. 2007. Available online: https://www.fsai.ie/getmedia/68870b71-bd82-4303-90f2-c62b0e9b5afe/10144_fsai-guidance-note-20-accessible.pdf?ext=.pdf (accessed on 21 January 2024).
- Food and Drug Administration (FDA). Fish-and-Fishery-Products-Hazards-and-Controls-Guidance. In Chapter 13: Clostridium Botulinum Toxin Formation; FDA: Silver Spring, MD, USA, 2011; p. 246. [Google Scholar]
- Latoch, A.; Głuchowski, A.; Czarniecka-Skubina, E. Sous-Vide as an Alternative Method of Cooking to Improve the Quality of Meat: A Review. Foods 2023, 12, 3110. [Google Scholar] [CrossRef]
- Hasani, E.; Csehi, B.; Darnay, L.; Ladányi, M.; Dalmadi, I.; Kenesei, G. Effect of Combination of Time and Temperature on Quality Characteristics of Sous Vide Chicken Breast. Foods 2022, 11, 521. [Google Scholar] [CrossRef]
- Hasani, E.; Kiskó, G.; Dalmadi, I.; Hitka, G.; Friedrich, L.F.; Kenesei, G. Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast. Foods 2023, 12, 1213. [Google Scholar] [CrossRef] [PubMed]
- Hasani, E.; Kenesei, G.; Dalmadi, I. Comparison of the single-step and double-step sous-vide treatment effect on the quality attributes of chicken breast: A novel approach to sous-vide. Prog. Agric. Eng. Sci. 2021, 17, 61–68. [Google Scholar] [CrossRef]
- Patterson, M.F. A review: Microbiology of pressure-treated foods. J. Appl. Microbiol. 2005, 98, 1400–1409. [Google Scholar] [CrossRef]
- Buckow, R.; Sikes, A.; Tume, R. Effect of High Pressure on Physicochemical Properties of Meat. Crit. Rev. Food Sci. Nutr. 2013, 53, 770–786. [Google Scholar] [CrossRef]
- Murchie, L.W.; Cruz-Romero, M.; Kerry, J.P.; Linton, M.; Patterson, M.F.; Smiddy, M. High pressure processing of shellfish: A review of microbiological and other quality aspects. Innov. Food Sci. Emerg. Technol. 2005, 6, 257–270. [Google Scholar] [CrossRef]
- Omer, M.K.; Alvseike, O.; Holck, A.; Axelsson, L.; Prieto, M.; Skjerve, E. Application of high pressure processing to reduce verotoxigenic E. coli in two types of dry-fermented sausage. Meat Sci. 2010, 86, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Matejková, K.; Krízek, M.; Vácha, F.; Dadáková, E. Effect of high-pressure treatment on biogenic amines formation in vacuum-packed trout flesh (Oncorhynchus mykiss). Food Chem. 2013, 137, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, Q.; Wang, J.; Wang, Y.; Zhao, Q.; Xue, C. Effects of high-pressure processing on murine norovirus-1 in oysters (Crassostrea gigas) in situ. Food Control 2009, 20, 992–996. [Google Scholar] [CrossRef]
- Novella-Rodriguez, S.; Veciana-Nogues, M.T.; Saldo, J.; Vidal-Carou, M.C. Effects of high hydrostatic pressure treatments on biogenic amine contents in goat cheeses during ripening. J. Agric. Food Chem. 2002, 50, 7288–7292. [Google Scholar] [CrossRef] [PubMed]
- Penas, E.; Frias, J.; Gomez, R.; Vidal-Valverde, C. High hydrostatic pressure can improve the microbial quality of sauerkraut during storage. Food Control 2010, 21, 524–528. [Google Scholar] [CrossRef]
- Boylu, M.; Hitka, G.; Kenesei, G. Effect of alternative pre-treatments and fermentation on quality characteristics of oyster mushrooms. Prog. Agric. Eng. Sci. 2023, 19, 35–45. [Google Scholar] [CrossRef]
- Huang, Y.; Gan, Y.; Li, F.; Yan, C.; Li, H.; Feng, Q. Effects of high pressure in combination with thermal treatment on lipid hydrolysis and oxidation in pork. LWT-Food Sci. Technol. 2015, 63, 136–143. [Google Scholar] [CrossRef]
- Ma, H.J.; Ledward, D.A.; Zamri, A.I.; Frazier, R.A.; Zhou, G.H. Effects of high pressure/thermal treatment on lipid oxidation in beef and chicken muscle. Food Chem. 2007, 104, 1575–1579. [Google Scholar] [CrossRef]
- Hassoun, A.; Ojha, S.; Tiwari, B.; Rustad, T.; Nilsen, H.; Heia, K.; Cozzolino, D.; Bekhit, A.E.-D.; Biancolillo, A.; Wold, J.P. Monitoring Thermal and Non-Thermal Treatments during Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Appl. Sci. 2020, 10, 6802. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Rev. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Raso, J.; Barbosa-Canovas, G.V. Nonthermal preservation of foods using combined processing techniques. Crit. Rev. Food Sci. Nutr. 2003, 43, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Balasubramaniam, V.M. Fundamentals of food processing using high pressure. In Nonthermal Processing Technologies for Food; Wiley: Hoboken, NJ, USA, 2011; pp. 1–19. [Google Scholar]
- Krebbers, B.; Matser, A.M.; Hoogerwerf, S.W.; Moezelaar, R.; Tomassen, M.M.M.; van der Berg, R.W. Combined high-pressure and thermal treatments for processing of tomato puree: Evaluation of microbial inactivation and quality parameters. Innov. Food Sci. Emerg. Technol. 2003, 4, 377–385. [Google Scholar] [CrossRef]
- Matser, A.M.; Krebbers, B.; van der Berg, R.W.; Bartels, P.V. Advantages of high pressure sterilisation on quality of food products. Trends Food Sci. Technol. 2004, 15, 79–85. [Google Scholar] [CrossRef]
- Mathys, A.; Toepfl, S.; Heinz, V.; Knorr, D. Food sterilisation under high pressure–Fundamentals, new insights and challenges. In Proceedings of the European Congress on Chemical Engineering (ECCE-6), Copenhagen, Denmark, 6–21 September 2007; Book of Abstracts. Ghani, R., Dam-Johansen, K., Eds.; Berlin University of Technology: Berlin, Germany, 2007; p. 2768. [Google Scholar]
- Ma, H.J.; Ledward, D.A. High pressure processing of fresh meat-Is it worth it? Meat Sci. 2013, 95, 897–903. [Google Scholar] [CrossRef]
- Hygreeva, D.; Pandey, M.C. Novel approaches in improving the quality and safety aspects of processed meat products through high pressure processing technology—A review. Trends Food Sci. Technol. 2016, 54, 175–185. [Google Scholar] [CrossRef]
- Zamri, A.I.; Ledward, A.; Frazier, R.A. Effect of combined heat and high-pressure treatments on the texture of chicken breast muscle (Pectoralis Fundus). J. Agric. Food Chem. 2006, 54, 2992–2996. [Google Scholar] [CrossRef]
- Picouet, P.A.; Cofan-Carbo, S.; Vilaseca, H.; Carboné Ballbè, L.; Castells, P. Stability of sous-vide cooked salmon loins processed by high pressure. Innov. Food Sci. Emerg. Technol. 2011, 12, 26–31. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, L.; Ding, G.; Hu, X.; Liao, X.; Zhang, Y. High hydrostatic pressure and thermal treatments for ready-to-eat wine-marinated shrimp: An evaluation of microbiological and physicochemical qualities. Innov. Food Sci. Emerg. Technol. 2013, 20, 16–23. [Google Scholar] [CrossRef]
- Chakraborty, S.; Srinivasa, P.; Mishra, H.N. Effect of combined high pressure–temperature treatments on color and nutritional quality attributes of pineapple (Ananas com. L.) puree. Innov. Food Sci. Emerg. Technol. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Espinosa, M.C.; Díaz, P.; Linares, M.B.; Teruel, M.R.; Garrido, M.D. Quality characteristics of sous vide ready to eat seabream processed by high pressure. LWT-Food Sci. Technol. 2015, 64, 657–662. [Google Scholar] [CrossRef]
- Hong, G.P.; Shim, K.B.; Choi, M.J.; Min, S.M. Effects of Thermal Processing Combined with High Hydrostatic Pressure on the Characteristics of Cooked Pork. Korean J. Food Sci. Anim. Resour. 2008, 28, 415–421. [Google Scholar] [CrossRef]
- Sun, S.; Sullivan, G.; Stratton, J.; Bower, C.; Cavender, G. Effect of HPP treatment on the safety and quality of beef steak intended for sous vide cooking. LWT-Food Sci. Technol. 2017, 86, 185–192. [Google Scholar] [CrossRef]
- Zheng, H.; Xiong, G.; Han, M.; Deng, S.; Xu, X.; Zhou, G. High Pressure/Thermal Combinations on Texture and Water Holding Capacity of Chicken Batters. Innov. Food Sci. Emerg. Technol. 2015, 30, 8–14. [Google Scholar] [CrossRef]
- Janardhanan, R.; Virseda, P.; Huerta-Leidenz, N.; Beriain, M.H. Effect of high–hydrostatic pressure processing and sous-vide cooking on physicochemical traits of Biceps femoris veal patties. Meat Sci. 2022, 188, 108772. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Cheftel, J.C.; Culioli, J. Effects of High Pressure on Meat: A Review. Meat Sci. 1997, 46, 212. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Bajovic, B.; Bolumar, T.; Heinz, V. Quality considerations with high pressure processing of fresh and value added meat products. Meat Sci. 2012, 92, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.; Hamm, G. Eine Einfache Methode zur Bestimmung der Wasserbindung in Muskel. Die Naturwissenschaften 1953, 40, 259–277. [Google Scholar] [CrossRef]
- CIE International Commission on Illumination. Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms, Supplement No. 2 to CIE Publication No. 15, Colorimetry; CIE: Vienna, Austria, 1978. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Biliaderis, C.G. Differential Scanning Calorimetry in Food Research-A Review. Food Chem. 1983, 10, 239–265. [Google Scholar] [CrossRef]
- Farkas, J. A DSC-termoanalitikai módszer néhány élelmiszertudományi alkalmazása, (DSC–a thermoanalytical method applied in the food industry). Élelmiszervizsgálati Közlemények 1994, 40, 180–189. [Google Scholar]
- Kenesei, G.; Jónás, G.; Salamon, B.; Dalmadi, I. Thermograms of the combined High Hydrostatic Pressure and Sous-vide treated Longissimus dorsi of pork. J. Phys. Conf. Ser. 2017, 950, 042007. [Google Scholar] [CrossRef]
- Ma, H.J.; Ledward, D.A. High pressure/thermal treatment effects on the texture of beef muscle. Meat Sci. 2004, 68, 347–355. [Google Scholar] [CrossRef]
- Tornberg, E. Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Roldan, M.; Antequera, T.; Armenteros, M.; Ruiz, J. Effect of different temperature-time combunations on lipid and protein oxidation of sous-vide cooked lamb loins. Food Chem. 2014, 149, 129–136. [Google Scholar] [CrossRef]
- Christensen, L.; Bertram, H.C.; Aaslyng, M.D.; Christensen, M. Protein denaturation and water–protein interactions as affected by low temperature long time treatment of porcine longissimus dorsi. Meat Sci. 2011, 88, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Gunvig, A.; Tørngren, M.A.; Aaslyng, M.D.; Knøchel, S.; Christensen, M. Sensory characteristics of meat cooked for prolonged times at low temperature. Meat Sci. 2012, 90, 485–489. [Google Scholar] [CrossRef]
- Kurp, L.; Danowska-Oziewicz, M.; Kłębukowska, L. Sous Vide Cooking Effects on Physicochemical, Microbiological and Sensory Characteristics of Pork Loin. Appl. Sci. 2022, 12, 2365. [Google Scholar] [CrossRef]
- Tokifuji, A.; Matsushima, Y.; Hatchisuka, K.; Yoshioka, K. Texture, sensory and swallowing characteristics of high-pressure-heat treated pork meat gel as a dysphagia diet. Meat Sci. 2013, 93, 843–848. [Google Scholar] [CrossRef]
- Pérez Alcalá, D.; Grande Burgos, M.J.; Rodríguez López, J.; Lucas, R.; Gálvez, A.; Pérez Pulido, R. Effect of High Hydrostatic Pressure Processing on the Microbiological Quality and Bacterial Diversity of Sous-Vide-Cooked Cod. Foods 2023, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ling, Y.; Chen, F.; Wang, C.; Qiao, Y.; Xiong, G.; Wang, L.; Wu, W.; Shi, L.; Ding, A. Effect of High Hydrostatic Pressure Combined with Sous-Vide Treatment on the Quality of Largemouth Bass during Storage. Foods 2022, 11, 1931. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS. FSIS Directive on HPP 6120.2 4. 2012. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-07/6120.2.pdf (accessed on 26 February 2024).
- Fehér, Á. Health Issues of Novel Food Technologies (Új Élelmiszer-Előállítási Technológiák Egészségügyi Megítélése), Guidelines; Semmelweis University OKK-OÉTI: Budapest, Hungary, 2003. [Google Scholar]
| Moisture (%) | 73.29 | ± | 0.19 | |
| Protein (%) | 21.74 | ± | 0.25 | |
| Fat (%) | 1.43 | ± | 0.09 | |
| pH | 5.97 | ± | 0.09 | |
| Color | L* | 48.55 | ± | 0.72 |
| a* | 6.85 | ± | 0.54 | |
| b* | 1.76 | ± | 0.65 | |
| DSC | denaturation heat (J/g) total | 3.09 | ± | 0.18 |
| denaturation heat (J/g)—myosin peak * | 0.76 | ± | 0.08 | |
| denaturation heat (J/g)—sarcoplasmic and connective tissue proteins peak * | 1.31 | ± | 0.14 | |
| denaturation heat (J/g)—actin peak * | 1.26 | ± | 0.12 | |
| Texture | hardness (N) | 2.28 | ± | 0.15 |
| TBARS | mg MDA/kg | 1.78 | ± | 0.16 |
| Microbiology | anaerobic TVC (log10, cfu/g) | 3.02 | ± | 0.09 |
| aerobic TVC (log10, cfu/g) | 3.33 | ± | 0.07 | |
| L. monocytogenes TVC (log10, cfu/g) | 5.33 | ± | 0.36 ** |
| GROUP | VACUUM | HEAT | PRESSURE | ORDER | |||
|---|---|---|---|---|---|---|---|
| Temp., °C | Time, min | MPa | Time, min. | Temperature, °C | |||
| SV | + | 60 | 60 | - | - | - | - |
| HHP 300 | + | - | - | 300 | 5 | RT | - |
| HHP 600 | + | - | - | 600 | 5 | RT | - |
| SV + HHP 300 | + | 60 | 60 | 300 | 5 | RT | Heat + Pressure |
| SV + HHP 600 | + | 60 | 60 | 600 | 5 | RT | Heat + Pressure |
| HHP 300 + SV | + | 60 | 60 | 300 | 5 | RT | Pressure + Heat |
| HHP 600 + SV | + | 60 | 60 | 600 | 5 | RT | Pressure + Heat |
| Day 0 | Day 21/2 °C | Day 21/8 °C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture (%) | SV | 65.91 | ± | 1.59 | a,X | 68.20 | ± | 0.76 | a,X | 67.52 | ± | 1.54 | a,X | |
| HHP 300 | 71.30 | ± | 0.82 | a,X | 69.50 | ± | 0.59 | a,X | 72.20 | ± | 1.16 | a,X | ||
| HHP 600 | 71.63 | ± | 1.16 | a,X | 69.96 | ± | 0.93 | a,X | 71.92 | ± | 0.16 | a,X | ||
| Weight loss (%) | SV | 18.96 | ± | 0.66 | a,Y | 24.28 | ± | 0.52 | a,Y | 24.65 | ± | 1.18 | a,Y | |
| HHP 300 | 4.12 | ± | 0.63 | a,X | 7.73 | ± | 1.09 | b,X | 11.00 | ± | 0.84 | b,X | ||
| HHP 600 | 5.08 | ± | 0.52 | a,X | 9.67 | ± | 0.77 | b,X | 12.61 | ± | 0.77 | c,XY | ||
| pH | SV | 6.08 | ± | 0.09 | a,X | 6.04 | ± | 0.05 | a,X | 5.95 | ± | 0.08 | a,X | |
| HHP 300 | 5.90 | ± | 0.07 | a,X | 6.00 | ± | 0.05 | a,X | 5.82 | ± | 0.03 | a,X | ||
| HHP 600 | 5.99 | ± | 0.07 | a,X | 6.05 | ± | 0.06 | a,X | 6.01 | ± | 0.08 | a,X | ||
| Color | L* | SV | 75.75 | ± | 0.20 | a,X | 76.36 | ± | 0.33 | a,X | 75.42 | ± | 0.30 | a,XY |
| HHP 300 | 74.55 | ± | 0.29 | a,X | 74.04 | ± | 0.88 | a,Y | 73.15 | ± | 0.91 | a,X | ||
| HHP 600 | 77.58 | ± | 0.60 | a,Y | 77.02 | ± | 0.21 | a,X | 77.49 | ± | 0.79 | a,Y | ||
| a* | SV | 8.09 | ± | 0.14 | a,Y | 8.01 | ± | 0.24 | a,Y | 8.04 | ± | 0.13 | a,Y | |
| HHP 300 | 7.71 | ± | 0.23 | a,Y | 8.76 | ± | 0.31 | ab,XY | 9.65 | ± | 0.38 | b,Z | ||
| HHP 600 | 6.36 | ± | 0.41 | a,X | 7.27 | ± | 0.17 | a,X | 7.11 | ± | 0.14 | a,X | ||
| b* | SV | 3.97 | ± | 0.21 | a,X | 5.35 | ± | 0.06 | c,Y | 4.65 | ± | 0.14 | b,Y | |
| HHP 300 | 3.53 | ± | 0.24 | b,X | 2.68 | ± | 0.18 | a,X | 3.22 | ± | 0.25 | ab,X | ||
| HHP 600 | 5.88 | ± | 0.09 | a,Y | 6.11 | ± | 0.16 | a,Z | 6.15 | ± | 0.11 | a,Z | ||
| DSC | denaturation heat (J/g) | SV | 1.05 | ± | 0.03 | a,X | 1.14 | ± | 0.03 | a,X | 1.63 | ± | 0.23 | a,X |
| HHP 300 | 1.07 | ± | 0.05 | a,X | 0.90 | ± | 0.02 | a,X | 0.87 | ± | 0.19 | a,X | ||
| HHP 600 | 0.57 | ± | 0.05 | a,X | 0.63 | ± | 0.11 | a,X | 0.72 | ± | 0.16 | a,X | ||
| Texture | hardness (N) | SV | 5.44 | ± | 0.43 | a,X | 4.78 | ± | 0.07 | a,X | 5.30 | ± | 0.28 | a,X |
| HHP 300 | 2.55 | ± | 0.32 | a,Y | 3.75 | ± | 0.33 | a,Y | 3.03 | ± | 0.81 | a,X | ||
| HHP 600 | 5.93 | ± | 0.14 | a,X | 4.83 | ± | 0.94 | a,X | 5.60 | ± | 1.23 | a,X | ||
| TBARS | mg MDA/kg | SV | 1.84 | ± | 0.23 | a,X | 2.45 | ± | 0.03 | a,Y | 3.20 | ± | 0.11 | b,Y |
| HHP 300 | 1.67 | ± | 0.28 | a,X | 1.67 | ± | 0.05 | a,X | 1.79 | ± | 0.06 | a,X | ||
| HHP 600 | 2.98 | ± | 0.29 | a,Y | 3.44 | ± | 0.05 | a,Z | 4.97 | ± | 0.05 | b,Y | ||
| Microbiology | anaerobic TVC (log10 CFU/g) | SV | 1.05 | ± | 0.20 | a,X | 1.27 | ± | 0.35 | a,X | 4.30 | ± | 0.12 | b,X |
| HHP 300 | 1.53 | ± | 0.48 | a,X | 5.75 | ± | 1.11 | a,X | 7.79 | ± | 0.38 | a,Y | ||
| HHP 600 | <0.70 | ± | 0.00 | a,X | 2.61 | ± | 0.62 | a,X | 7.70 | ± | 0.27 | b,Y | ||
| aerobic TVC (log10 CFU/g) | SV | 2.21 | ± | 0.23 | a,X | 3.80 | ± | 0.00 | a,X | 7.80 | ± | 0.23 | b,X | |
| HHP 300 | 4.13 | ± | 0.77 | a,X | 6.10 | ± | 1.16 | a,X | 8.10 | ± | 0.23 | a,X | ||
| HHP 600 | 2.68 | ± | 1.18 | a,X | 3.22 | ± | 0.59 | b,X | 8.55 | ± | 0.09 | b,X | ||
| L. monocytogenes TVC (log10 CFU/g) | SV | <1.70 | ± | 0.00 | n/a,X | <1.70 | ± | 0.00 | n/a,X | <1.70 | ± | 0.00 | n/a,X | |
| HHP 300 | 2.59 | ± | 0.65 | a,X | 4.00 | ± | 0.19 | a,Y | 7.64 | ± | 0.23 | a,Z | ||
| HHP 600 | <1.70 | ± | 0.00 | a,X | <1.70 | ± | 0.00 | a,X | 4.94 | ± | 0.29 | b,Y | ||
| Treatment Order | Pressure | Day 0 | Day 21/2 °C | Day 21/8 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture (%) | SV + HHP | 300 Mpa | 66.09 | ± | 2.06 | 69.15 | ± | 0.23 | 70.67 | ± | 1.09 | |
| 600 Mpa | 68.91 | ± | 1.57 | 68.01 | ± | 1.66 | 68.07 | ± | 1.83 | |||
| HHP + SV | 300 Mpa | 66.97 | ± | 1.31 | 68.77 | ± | 0.64 | 68.48 | ± | 0.79 | ||
| 600 Mpa | 65.26 | ± | 1.32 | 65.04 | ± | 0.47 | 66.24 | ± | 0.87 | |||
| Weight loss (%) | SV + HHP | 300 Mpa | 19.34 | ± | 0.21 | 20.48 | ± | 0.22 | 22.82 | ± | 0.74 | |
| 600 Mpa | 21.24 | ± | 1.12 | 23.26 | ± | 1.72 | 23.55 | ± | 0.27 | |||
| HHP + SV | 300 Mpa | 20.31 | ± | 0.78 | 19.18 | ± | 1.17 | 21.41 | ± | 0.55 | ||
| 600 Mpa | 31.09 | ± | 0.04 | 28.01 | ± | 0.67 | 31.59 | ± | 0.29 | |||
| pH | SV + HHP | 300 Mpa | 6.02 | ± | 0.07 | 6.03 | ± | 0.09 | 5.94 | ± | 0.05 | |
| 600 Mpa | 6.06 | ± | 0.07 | 6.05 | ± | 0.07 | 5.98 | ± | 0.06 | |||
| HHP + SV | 300 Mpa | 6.11 | ± | 0.07 | 6.03 | ± | 0.06 | 6.01 | ± | 0.07 | ||
| 600 Mpa | 6.10 | ± | 0.08 | 6.07 | ± | 0.05 | 5.99 | ± | 0.07 | |||
| Color | L* | SV + HHP | 300 Mpa | 74.64 | ± | 0.28 | 74.61 | ± | 0.49 | 75.11 | ± | 0.43 |
| 600 Mpa | 74.91 | ± | 0.43 | 73.49 | ± | 0.94 | 74.15 | ± | 0.69 | |||
| HHP + SV | 300 Mpa | 80.82 | ± | 0.50 | 80.21 | ± | 0.75 | 80.07 | ± | 1.05 | ||
| 600 Mpa | 79.78 | ± | 0.36 | 79.99 | ± | 0.70 | 78.79 | ± | 1.17 | |||
| a* | SV + HHP | 300 Mpa | 7.63 | ± | 0.39 | 7.77 | ± | 0.38 | 7.69 | ± | 0.30 | |
| 600 Mpa | 6.48 | ± | 0.30 | 6.44 | ± | 0.33 | 6.40 | ± | 0.30 | |||
| HHP + SV | 300 Mpa | 6.56 | ± | 0.25 | 6.82 | ± | 0.30 | 7.32 | ± | 0.31 | ||
| 600 Mpa | 4.75 | ± | 0.13 | 5.66 | ± | 0.37 | 5.88 | ± | 0.40 | |||
| b* | SV + HHP | 300 Mpa | 4.37 | ± | 0.21 | 4.92 | ± | 0.19 | 4.91 | ± | 0.31 | |
| 600 Mpa | 5.86 | ± | 0.22 | 6.25 | ± | 0.14 | 6.42 | ± | 0.18 | |||
| HHP + SV | 300 Mpa | 4.41 | ± | 0.18 | 5.09 | ± | 0.21 | 5.38 | ± | 0.43 | ||
| 600 Mpa | 6.24 | ± | 0.11 | 6.66 | ± | 0.19 | 6.73 | ± | 0.27 | |||
| DSC | denaturation heat | SV + HHP | 300 Mpa | 1.30 | ± | 0.02 | 1.01 | ± | 0.35 | 0.80 | ± | 0.11 |
| (J/g) | 600 Mpa | 0.46 | ± | 0.04 | 0.49 | ± | 0.04 | 0.54 | ± | 0.11 | ||
| HHP + SV | 300 Mpa | 0.65 | ± | 0.28 | 0.66 | ± | 0.18 | 0.52 | ± | 0.07 | ||
| 600 Mpa | 0.64 | ± | 0.23 | 0.46 | ± | 0.16 | 0.54 | ± | 0.13 | |||
| Texture | hardness (N) | SV + HHP | 300 Mpa | 6.03 | ± | 0.30 | 4.69 | ± | 0.31 | 4.87 | ± | 0.63 |
| 600 Mpa | 7.79 | ± | 0.26 | 6.04 | ± | 0.23 | 8.75 | ± | 1.13 | |||
| HHP + SV | 300 Mpa | 5.37 | ± | 0.01 | 4.42 | ± | 0.21 | 5.35 | ± | 0.15 | ||
| 600 Mpa | 7.69 | ± | 0.67 | 5.75 | ± | 0.41 | 8.00 | ± | 0.81 | |||
| TBARS | mg MDA/kg | SV + HHP | 300 Mpa | 1.93 | ± | 0.41 | 1.94 | ± | 0.19 | 2.79 | ± | 0.02 |
| 600 Mpa | 6.07 | ± | 0.26 | 5.15 | ± | 0.49 | 6.24 | ± | 0.07 | |||
| HHP + SV | 300 Mpa | 2.80 | ± | 0.62 | 1.86 | ± | 0.04 | 2.82 | ± | 0.06 | ||
| 600 Mpa | 5.69 | ± | 0.49 | 5.11 | ± | 0.05 | 5.54 | ± | 0.13 | |||
| Microbiology | anaerobic TVC | SV + HHP | 300 Mpa | <DL | 1.72 | ± | 0.02 | 3.20 | ± | 0.10 | ||
| (log10 CFU/g) | 600 MPa | <DL | 1.24 | ± | 0.24 | 2.70 | ± | 0.20 | ||||
| HHP + SV | 300 MPa | <DL | 1.62 | ± | 0.08 | 2.30 | ± | 0.10 | ||||
| 600 MPa | <DL | <DL | 1.35 | ± | 0.45 | |||||||
| aerobic TVC | SV + HHP | 300 MPa | 1.85 | ± | 0.37 | 3.50 | ± | 0.12 | 5.78 | ± | 0.45 | |
| (log10 CFU/g) | 600 MPa | 1.60 | ± | 0.10 | 3.32 | ± | 0.10 | 4.67 | ± | 1.10 | ||
| HHP + SV | 300 MPa | 1.97 | ± | 0.32 | 3.39 | ± | 0.05 | 3.55 | ± | 0.11 | ||
| 600 MPa | <DL | 2.72 | ± | 0.27 | 2.25 | ± | 0.15 | |||||
| L. monocytogenes | SV + HHP | 300 MPa | <DL | <DL | <DL | |||||||
| TVC (log10 CFU/g) | 600 MPa | <DL | <DL | <DL | ||||||||
| HHP + SV | 300 MPa | <DL | <DL | <DL | ||||||||
| 600 MPa | <DL | <DL | <DL | |||||||||
| p (Order of Treatments) | p (Pressure Level) | p (Order of Treatments × Pressure Level) | ||
|---|---|---|---|---|
| Moisture | (%) | 0.085 | 0.101 | 0.361 |
| Weight loss | (%) | <0.001 *** | <0.001 *** | <0.001 |
| pH | 0.197 | 0.859 | 0.557 | |
| Color | L* | 0.105 | 0.154 | 0.245 |
| a* | <0.001 *** | <0.001 *** | 0.565 | |
| b* | 0.030 * | <0.001 *** | 0.619 | |
| DSC | denaturation heat (J/g) | 0.067 * | 0.003 ** | 0.020 * |
| Texture | hardness (N) | 0.508 | <0.001 *** | 0.817 |
| TBARS | mg MDA/kg | 0.989 | 0.043 * | 0.985 |
| Microbiology | anaerobic TVC (log10, CFU/g) | 0.373 | 0.898 | 0.407 |
| aerob TVC (log10, CFU/g) | 0.268 | 0.523 | 0.957 | |
| L. monocytogenes TVC (log10 CFU/g) | n/a | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenesei, G.; Kiskó, G.; Dalmadi, I. Combined Sous-Vide and High Hydrostatic Pressure Treatment of Pork: Is the Order of Application Decisive When Using Minimal Processing Technologies? Appl. Sci. 2024, 14, 3583. https://doi.org/10.3390/app14093583
Kenesei G, Kiskó G, Dalmadi I. Combined Sous-Vide and High Hydrostatic Pressure Treatment of Pork: Is the Order of Application Decisive When Using Minimal Processing Technologies? Applied Sciences. 2024; 14(9):3583. https://doi.org/10.3390/app14093583
Chicago/Turabian StyleKenesei, György, Gabriella Kiskó, and István Dalmadi. 2024. "Combined Sous-Vide and High Hydrostatic Pressure Treatment of Pork: Is the Order of Application Decisive When Using Minimal Processing Technologies?" Applied Sciences 14, no. 9: 3583. https://doi.org/10.3390/app14093583





