Interface Growth and Void Formation in Sn/Cu and Sn0.7Cu/Cu Systems
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
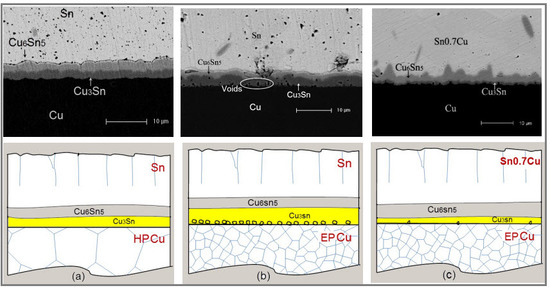
3.1. Sn/HP Cu and Sn/EP Cu Joints after Aging at 150 °C
3.2. Sn0.7Cu/EP Cu Joints after Aging at 150 °C
3.3. Sn/EP Cu Joints after Aging at 150 °C and 180 °C
3.4. Growth Behaviors of IMC Layer
3.5. Discussion
4. Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Tu, K.N.; Hsiao, H.Y.; Chen, C. Transition from flip chip solder joint to 3D IC micro-bump: Its effect on microstructure anisotropy. Microelectron. Reliab. 2013, 53, 2–6. [Google Scholar] [CrossRef]
- Che, F.X.; Pang, H.L.J. Characterization of IMC layer and its effect on thermomechanical fatigue life of Sn–3.8 Ag–0.7 Cu solder joints. J. Alloys Compd. 2012, 541, 6–13. [Google Scholar] [CrossRef]
- Chen, K.; Kunz, M.; Tamura, N.; Wenk, H.R. Residual stress preserved in quartz from the San Andreas Fault Observatory at Depth. Geology 2015, 43, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.; Tu, K.N. Six cases of reliability study of Pb-free solder joints in electronic packaging technology. Mater. Sci. Eng. R Rep. 2002, 38, 55–105. [Google Scholar] [CrossRef]
- Tu, K.N.; Zeng, K. Tin–lead (SnPb) solder reaction in flip chip technology. Mat. Sci. Eng. R Rep. 2001, 34, 1–58. [Google Scholar] [CrossRef]
- Tsao, L.C. Suppressing effect of 0.5 wt.% nano-TiO2 addition into Sn–3.5Ag–0.5Cu solder alloy on the intermetallic growth with Cu substrate during isothermal aging. J. Alloys Compd. 2011, 509, 8441–8448. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C. Growth mechanism of intermetallic compounds and damping properties of Sn–Ag–Cu-1wt% nano-ZrO2 composite solders. Microelectron. Reliab. 2014, 54, 945–955. [Google Scholar] [CrossRef]
- Song, J.Y.; Yu, J.; Lee, T.Y. Effects of reactive diffusion on stress evolution in Cu–Sn films. Scr. Mater. 2004, 51, 167–170. [Google Scholar] [CrossRef]
- Buchovecky, E.; Jadhav, N.; Bower, A.F.; Chason, E. Finite element modeling of stress evolution in Sn films due to growth of the Cu6Sn5 intermetallic compound. J. Electron. Mater. 2009, 38, 2676–2684. [Google Scholar] [CrossRef]
- Yen, Y.W.; Lin, C.Y.; Hermana, G.N.; Chen, P.Y.; Wu, Y.P. Interfacial reactions in the Au/Sn-xZn/Cu sandwich couples. J. Alloys Compd. 2017, 710, 479–490. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Gu, Q.; Terada, Y.; Uesugi, K.; Yasuda, H.; Nogita, K. The influence of Ni and Zn additions on microstructure and phase transformations in Sn–0.7 Cu/Cu solder joints. Acta Mater. 2015, 83, 357–371. [Google Scholar] [CrossRef]
- Wang, Y.W.; Lin, Y.W.; Tu, C.T.; Kao, C.R. Effects of minor Fe, Co, and Ni additions on the reaction between SnAgCu solder and Cu. J. Alloys Compd. 2009, 478, 121–127. [Google Scholar] [CrossRef]
- Yoon, J.W.; Noh, B.I.; Jung, S.B. Effects of third element and surface finish on interfacial reactions of Sn–Ag–xCu (or Ni)/(Cu or ENIG) solder joints. J. Alloys Compd. 2010, 506, 331–337. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, K.N. Structure and properties of lead-free solders bearing micro and nano particles. Mater. Sci. Eng. R Rep. 2014, 82, 1–32. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.D.; Mannan, S.H. A review: On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Yu, C.; Chen, J.S.; Wang, K.Y.; Chen, J.Q.; Lu, H. Suppression effect of Cu and Ag on Cu3Sn layer in solder joints. J. Mater. Sci. Mater. Electron. 2013, 24, 4630–4635. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yu, J.; Kim, S.H. Effects of sulfide-forming element additions on the Kirkendall void formation and drop impact reliability of Cu/Sn–3.5 Ag solder joints. Acta Mater. 2009, 57, 5001–5012. [Google Scholar] [CrossRef]
- Yu, J.; Kim, J.Y. Effects of residual S on Kirkendall void formation at Cu/Sn–3.5 Ag solder joints. Acta Mater. 2008, 56, 5514–5523. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Yin, L.; Kondos, P.; Parks, C.; Borgesen, P.; Dimitrov, N. Influence of plating parameters and solution chemistry on the voiding propensity at electroplated copper–solder interface. J. Appl. Electrochem. 2008, 38, 1695–1705. [Google Scholar] [CrossRef]
- Zou, J.; Mo, L.; Wu, F.; Wang, B.; Liu, H.; Zhang, J.; Wu, Y. Effect of Cu substrate and solder alloy on the formation of kirkendall voids in the solder joints during thermal aging. In Proceedings of the 2010 11th International Conference on Electronic Packaging Technology & High Density Packaging (ICEPT-HDP), Xi’an, China, 16–19 August 2010; pp. 944–948. [Google Scholar]
- Zou, H.F.; Yang, H.J.; Zhang, Z.F. Morphologies, orientation relationships and evolution of Cu6Sn5 grains formed between molten Sn and Cu single crystals. Acta Mater. 2008, 56, 2649–2662. [Google Scholar] [CrossRef]
- Choi, S.; Bieler, T.R.; Lucas, J.P.; Subramanian, K.N. Characterization of the growth of intermetallic interfacial layers of Sn-Ag and Sn-Pb eutectic solders and their composite solders on Cu substrate during isothermal long-term aging. J. Electron. Mater. 1999, 28, 1209–1215. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, C.O. Residual stress effect on self-annealing of electroplated copper. Jpn. J. Appl. Phys. 2003, 42, 4484–4488. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.; Yu, C. Void formation at the interface in Sn/Cu solder joints. Microelectron. Reliab. 2011, 51, 2314–2318. [Google Scholar] [CrossRef]
- Kim, S.; Yu, J. Recrystallization-induced void migration in electroplated Cu films. Scr. Mater. 2012, 67, 312–315. [Google Scholar] [CrossRef]
- Jung, K.; Conrad, H. Retardation of grain growth in electrodeposited Cu by an electric field. J. Mater. Sci. 2007, 42, 3994–4003. [Google Scholar] [CrossRef]
- Wang, Y.W.; Lin, Y.W.; Kao, C.R. Inhibiting the formation of microvoids in Cu3Sn by additions of Cu to solders. J. Alloys Compd. 2010, 493, 233–239. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, Y.; Yu, Z.; Zhang, P.; Zhao, W.; Yang, J.; Wu, D. Interface Growth and Void Formation in Sn/Cu and Sn0.7Cu/Cu Systems. Appl. Sci. 2018, 8, 2703. https://doi.org/10.3390/app8122703
Chen J, Zhang Y, Yu Z, Zhang P, Zhao W, Yang J, Wu D. Interface Growth and Void Formation in Sn/Cu and Sn0.7Cu/Cu Systems. Applied Sciences. 2018; 8(12):2703. https://doi.org/10.3390/app8122703
Chicago/Turabian StyleChen, Jieshi, Yongzhi Zhang, Zhishui Yu, Peilei Zhang, Wanqin Zhao, Jin Yang, and Di Wu. 2018. "Interface Growth and Void Formation in Sn/Cu and Sn0.7Cu/Cu Systems" Applied Sciences 8, no. 12: 2703. https://doi.org/10.3390/app8122703