A Simplified Method to Predict the Heat Release Rate of Industrial Nitrocellulose Materials
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussion
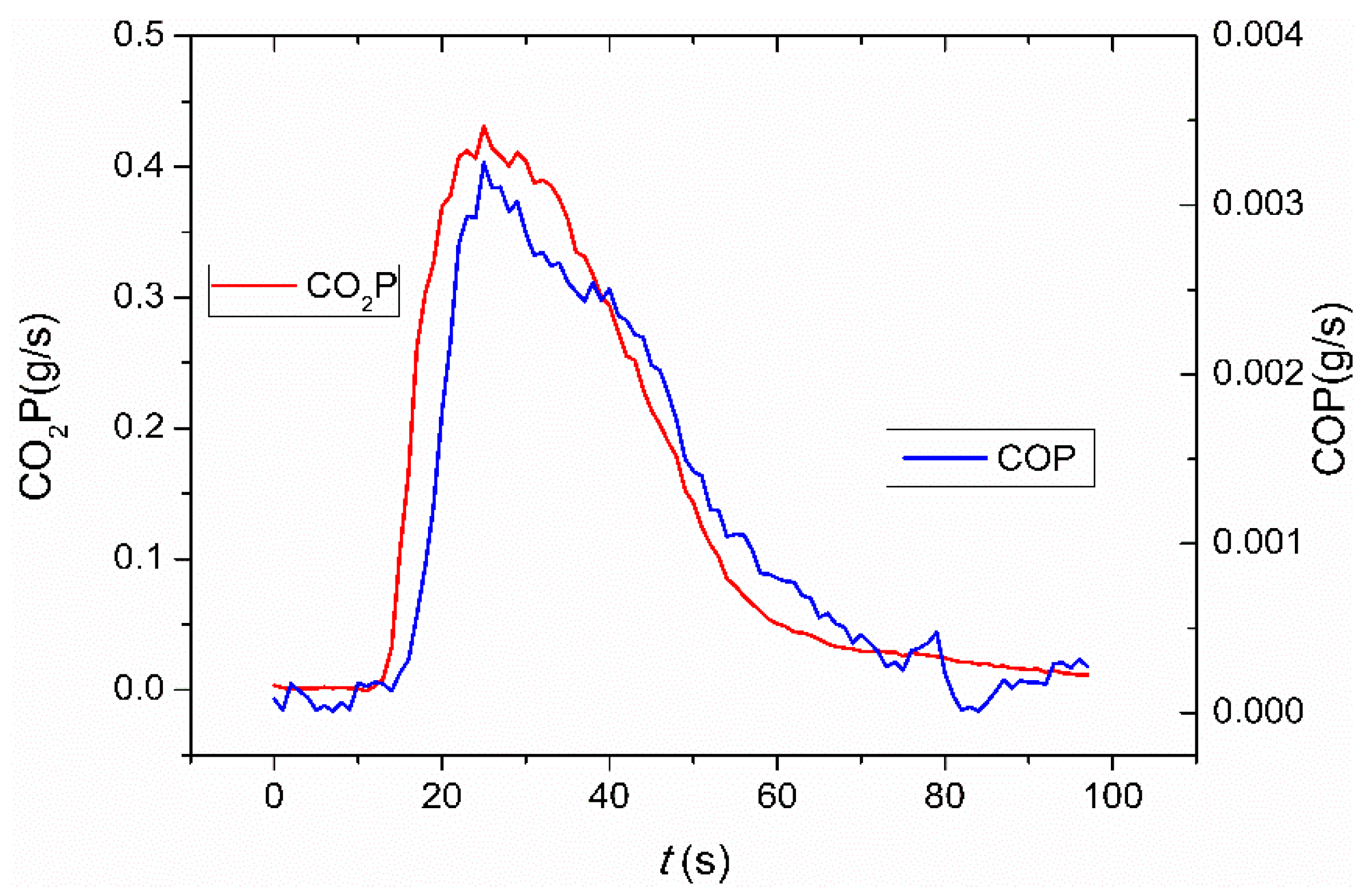
3.1. General Observations
3.2. Flame Height
3.3. Prediction of HRR
3.3.1. Determination of
3.3.2. Determination of
4. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De la Ossa, M.Á.F.; Torre, M.; García-Ruiz, C. Determination of nitrocellulose by capillary electrophoresis with laser-induced fluorescence detection. Anal. Chim. Acta 2012, 745, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hikal, W.M.; Zhang, Y.; Bhattacharia, S.K.; Li, L.; Panditrao, S.; Wang, S.; Weeks, B.L. Direct laser initiation and improved thermal stability of nitrocellulose/graphene oxide nanocomposites. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Zhang, X.; Weeks, B.L. Preparation of sub-micron nitrocellulose particles for improved combustion behavior. J. Hazard. Mater. 2014, 268, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, J. The Chemistry of Explosives; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Pourmortazavi, S.M.; Hosseini, S.G.; Rahimi-Nasrabadi, M.; Hajimirsadeghi, S.S.; Momenian, H. Effect of nitrate content on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 162, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Q.S.; Sun, J.H.; Liao, X.; Wang, Z.S. Study on the influence of moisture content on thermal stability of propellant. J. Hazard. Mater. 2009, 168, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.M.; Liang, W. Modeling the moisture effects of solid ingredients on composite propellant properties. Aerosp. Sci. Technol. 2006, 10, 695–699. [Google Scholar] [CrossRef]
- Książczak, A.; Książczak, T.; Ostrowski, M. Intermolecular interactions and phase equilibria in nitrocellulose-s-diethyldiphenylurea system. J. Therm. Anal. Calorim. 2003, 74, 575–581. [Google Scholar] [CrossRef]
- Makashir, P.; Mahajan, R.; Agrawal, J. Studies on kinetics and mechanism of initial thermal decomposition of nitrocellulose: Isothermal and non-isothermal techniques. J. Therm. Anal. Calorim. 1995, 45, 501–509. [Google Scholar] [CrossRef]
- Sovizi, M.; Hajimirsadeghi, S.; Naderizadeh, B. Effect of particle size on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 168, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Higashi, E.; Nakano, K.; Ito, S.; Wada, Y.; Kasamatsu, J.; Miya, H.; Yamamoto, M.; Wada, Y. Thermal behavior of nitrocellulose with inorganic salts and their mechanistic action. Propell. Explos. Pyrot. 2010, 35, 461–467. [Google Scholar] [CrossRef]
- Fensom, D.; Fordham, S. A microscopical study of the solution of nitrocotton by nitroglycerine. Trans. Faraday Soc. 1947, 43, 538–542. [Google Scholar] [CrossRef]
- Mahajan, R.; Makashir, P.; Agrawal, J. Combustion behaviour of nitrocellulose and its complexes with copper oxide. Hot stage microscopic studies. J. Therm. Anal. Calorim. 2001, 65, 935–942. [Google Scholar] [CrossRef]
- Katoh, K.; Le, L.; Arai, M.; Tamura, M. Study on the spontaneous ignition of cellulose nitrate effect of the type of storage atmosphere (I). Sci. Technol. Energy Mater. 2003, 64, 236–240. [Google Scholar]
- Katoh, K.; Le, L.; Arai, M.; Tamura, M. Study on the spontaneous ignition of cellulose nitrate effect of the type of storage atmosphere (II). Sci. Technol. Energy Mater. 2004, 65, 77–81. [Google Scholar]
- Binke, N.; Rong, L.; Zhengquan, Y.; Yuan, W.; Pu, Y.; Hu, R.; Yang, Q. Studies on the kinetics of the first order autocatalytic decomposition reaction of highly nitrated nitrocellulose. J. Therm. Anal. Calorim. 1999, 58, 403–411. [Google Scholar] [CrossRef]
- Kapoor, I.P.S.; Srivastava, P.; Singh, G. Preparation, characterization and thermolysis of phenylenediammonium dinitrate salts. J. Hazard. Mater. 2008, 150, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Pourmortazavi, S.M.; Fathollahi, M.; Hajimirsadeghi, S.S.; Hosseini, S.G. Thermal behavior of aluminum powder and potassium perchlorate mixtures by DTA and TG. Thermochim. Acta 2006, 443, 129–131. [Google Scholar] [CrossRef]
- Fu, G.; Wang, J.; Yan, M. Anatomy of Tianjin Port fire and explosion: Process and causes. Process Saf. Prog. 2016, 35, 216–220. [Google Scholar] [CrossRef]
- Willey, R.J.; Murphy, J.; Baulch, A. The explosion in Tianjin, China, August 12, 2015. Process Saf. Prog. 2015, 34, 313–314. [Google Scholar] [CrossRef]
- Zhao, B. Facts and lessons related to the explosion accident in Tianjin Port, China. Nat. Hazards 2016, 84, 707–713. [Google Scholar] [CrossRef]
- Jessup, R.S.; Prosen, E. Heats of combustion and formation of cellulose and nitrocellulose (cellulose nitrate). J. Res. Natl. Bureau Stand. 1950, 44, 387–393. [Google Scholar] [CrossRef]
- Yaman, H.; Çelik, V.; Değirmenci, E. Experimental investigation of the factors affecting the burning rate of solid rocket propellants. Fuel 2014, 115, 794–803. [Google Scholar] [CrossRef]
- Degirmenci, E. Effects of grain size and temperature of double base solid propellants on internal ballistics performance. Fuel 2015, 146, 95–102. [Google Scholar] [CrossRef]
- He, Y.; He, Y.P.; Liu, J.H.; Li, P.; Chen, M.Y.; Wei, R.C.; Wang, J. Experimental study on the thermal decomposition and combustion characteristics of nitrocellulose with different alcohol humectants. J. Hazard. Mater. 2017, 340, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.C.; He, Y.P.; Liu, J.H.; He, Y.; Mi, W.Z.; Wang, J. Experimental study on the fire properties of nitrocellulose with different structures. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Babrauskas, V. Heat release rates. In SFPE Handbook of Fire Protection Engineering; Spinger: New York, NY, USA, 2016. [Google Scholar]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- National Defense Science and Technology Industry Committee. Specification for Nitrocellulose of Lacquers; WJ9028–2005; National Defense Science and Technology Industry Committee: Beijing, China, 2005. [Google Scholar]
- Drysdale, D. An Introduction to Fire Dynamics; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Babrauskas, V. Development of the cone calorimeter—A bench-scale heat release rate apparatus based on oxygen consumption. Fire Mater. 1984, 8, 81–95. [Google Scholar] [CrossRef]
- Babrauskas, V. The cone calorimeter. In SFPE Handbook of Fire Protection Engineering; Spinger: New York, NY, USA, 2016. [Google Scholar]
- Street, P.J. The combustion of single droplets of some fuel oils and alternative liquid fuel combinations. J. Inst. Energy 1980, 53, 154. [Google Scholar]
- Jacques, M.T.; Jordan, J.B.; Williams, A.; Hadley-Coates, L. The combustion of water-in-oil emulsions and the influence of asphaltene content. Symp. Int. Combust. 1977, 16, 307–319. [Google Scholar] [CrossRef]
- Hu, X.K.; He, Y.P.; Li, Z.H.; Wang, J. Combustion characteristics of n-heptane at high altitudes. Proc. Combust. Inst. 2011, 33, 2607–2615. [Google Scholar] [CrossRef]
- Heskestad, G. Fire plumes, flame height, and air entrainment. In SFPE Handbook of Fire Protection Engineering; Springer: New York, NY, USA, 2016. [Google Scholar]
- Zhou, Z.H.; Yao, W.; Li, H.H.; Yuen, R.; Wang, J. Experimental analysis of low air pressure influences on fire plumes. Int. J. Heat Mass Transf. 2014, 70, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Cetegen, B.M.; Ahmed, T.A. Experiments on the periodic instability of buoyant plumes and pool fires. Combust. Flame 1993, 93, 157–184. [Google Scholar] [CrossRef]
- Wang, J.W.; Fang, J.; Guan, J.F.; Zeng, Y.; Zhang, Y.M. Flame volume and radiant fraction of jet diffusion methane flame at sub-atmospheric pressures. Fuel 2016, 167, 82–88. [Google Scholar] [CrossRef]
- Heskestad, G. Luminous heights of turbulent diffusion flames. Fire Saf. J. 1983, 5, 103–108. [Google Scholar] [CrossRef]
- Yao, W.; Hu, X.K.; Rong, J.Z.; Wang, J.; Zhang, H. Experimental study of large-scale fire behavior under low pressure at high altitude. J. Fire Sci. 2013, 31, 481–494. [Google Scholar] [CrossRef]











| Additives | Chemical Formula | Molecular Weight (g/mol) | Density (kg/m3) | Heat of Combustion (kJ/g) | |
|---|---|---|---|---|---|
| Humectant | Isopropanol | C3H8O | 60.06 | 786 | 33.08 |
| Ethanol | C2H6O | 46.07 | 789 | 29.68 | |
| Plasticizer | Dibutyl phthalate | C16H22O | 278.34 | 1047 | 30.99 |
| Samples | L (cm) | Bulk Density (kg/m3) | Atmospheric Pressure (kPa) | Ambient Temperature (°C) | Relative Humidity (%) | Measurements |
|---|---|---|---|---|---|---|
| NC-I | 8, 10 | 156.3 | 100.3 ± 0.3 | 28 ± 2 | 40 ± 3 |
|
| NC-E | 8, 10 | 156.3 | ||||
| NC-D | 8, 10 | — |
| Sample | L (cm) | Ave./Max. MLR (g/s) | f (Hz) | zf-m (m) | If |
|---|---|---|---|---|---|
| NC-I | 8 | 0.52 | 5.13 | 0.35 | 0.77 |
| 10 | 1.03 | 4.55 | 0.50 | 0.64 | |
| NC-E | 8 | 0.51 | 5.02 | 0.33 | 0.72 |
| 10 | 0.95 | 4.52 | 0.42 | 0.71 | |
| NC-D | 8 | 1.14 | 4.42 | 0.53 | 0.44 |
| 10 | 1.79 | 4.93 | 0.70 | 0.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, M. A Simplified Method to Predict the Heat Release Rate of Industrial Nitrocellulose Materials. Appl. Sci. 2018, 8, 910. https://doi.org/10.3390/app8060910
Liu J, Chen M. A Simplified Method to Predict the Heat Release Rate of Industrial Nitrocellulose Materials. Applied Sciences. 2018; 8(6):910. https://doi.org/10.3390/app8060910
Chicago/Turabian StyleLiu, Jiahao, and Mingyi Chen. 2018. "A Simplified Method to Predict the Heat Release Rate of Industrial Nitrocellulose Materials" Applied Sciences 8, no. 6: 910. https://doi.org/10.3390/app8060910




