Effect of Silica Nanoparticles on Fluid/Rock Interactions during Low Salinity Water Flooding of Chalk Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brines
2.2. Isothermal Static Adsorption
2.3. Dynamic Adsorption of NPs in Chalk Core
2.4. Effect of Oil on the Interaction of the NPs and Mineral
3. Results and Discussion
3.1. NF Characterization
3.2. Isothermal Static Adsorption
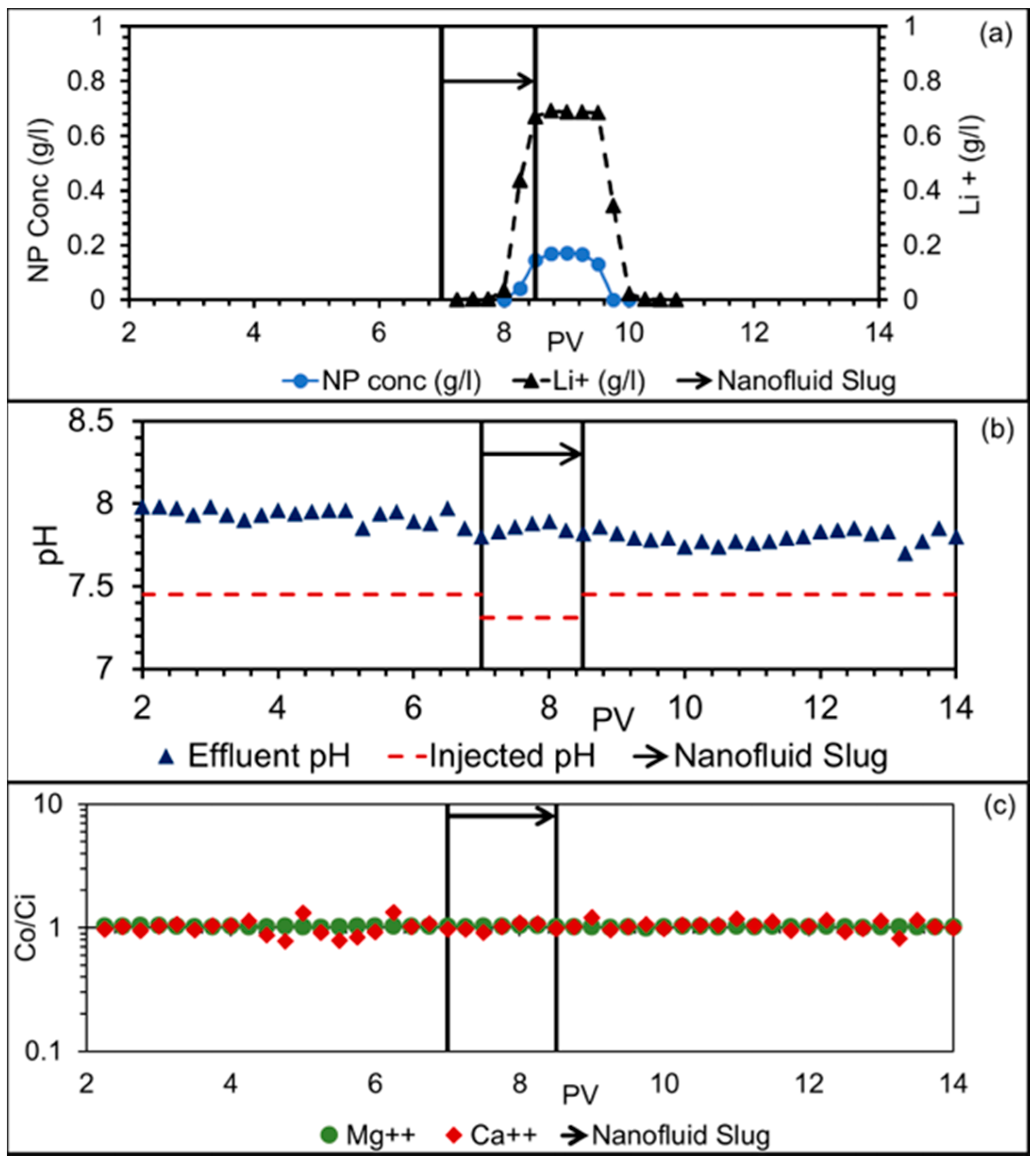
3.3. Dynamic Adsorption
3.4. NPs Interaction with Chalk Mineral in Presence of Hydrocarbon
4. Conclusions
- Silica NPs showed an adsorption affinity to calcite surface. Salinity was shown to enhance the adsorption by about 40%.
- Dynamic adsorption of the NPs in the chalk core showed high irreversible adsorption at elevated salinity (SSW) and desorption of NPs was observed in low salinity and ion free condition.
- Adsorption/desorption mechanisms for the NPs have been proposed. Further, it can be concluded that NPs adsorption during these experiments led to significant reduction of calcite dissolution both in DIW and LSW.
- In spite of the NPs affinity to adsorb on the chalk surface, no pore throat blockage was observed from SEM imaging. The SEM images were done on spots along horizontally cut core. However, they are small fractions of the whole core. Further the differential pressure drop across the chalk core during nanofluid injection also indicated reduced resistance to flow wherein lower pressure drop was recorded, compared to with injection of LSW alone.
- The nanofluid at 1 g/L prepared in LSW reduced the produced calcium ion concentration by about 30% as compared to the case of LSW alone. This indicates that silica NF hinders calcite dissolution i.e., has less effect on chalk matrix integrity which is one of the major concern in chalk reservoir, if low salinity for EOR is to be employed.
- The combination of silica NPs with low salinity EOR technique reduces the risk of matrix integrity loss and the subsidence degree of the water flooded chalk.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayatollahi, S.; Zerafat, M.M. Nanotechnology-Assisted Eor Techniques: New Solutions to Old Challenges. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Zhang, H.; Nikolov, A.; Wasan, D. Enhanced oil recovery (eor) using nanoparticle dispersions: Underlying mechanism and imbibition experiments. Energy Fuels 2014, 28, 3002–3009. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Pet. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Chávez-Miyauchi, T.S.E.; Firoozabadi, A.; Fuller, G.G. Nonmonotonic elasticity of the crude oil–brine interface in relation to improved oil recovery. Langmuir 2016, 32, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Suleimanov, B.; Ismailov, F.; Veliyev, E. Nanofluid for enhanced oil recovery. J. Pet. Sci. Eng. 2011, 78, 431–437. [Google Scholar] [CrossRef]
- Fletcher, A.; Davis, J. How eor can be transformed by nanotechnology. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Hendraningrat, L.; Li, S.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Pet. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohammadi, S.; Ghazanfari, M.H.; Kharrat, R.; Masihi, M. Monitoring wettability alteration by silica nanoparticles during water flooding to heavy oils in five-spot systems: A pore-level investigation. Exp. Therm. Fluid Sci. 2012, 40, 168–176. [Google Scholar] [CrossRef]
- Li, S.; Torsæter, O. Experimental investigation of the influence of nanoparticles adsorption and transport on wettability alteration for oil wet berea sandstone. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8–11 March 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- Behzadi, A.; Mohammadi, A. Environmentally responsive surface-modified silica nanoparticles for enhanced oil recovery. J. Nanopart. Res. 2016, 18, 1–19. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. A stabilizer that enhances the oil recovery process using silica-based nanofluids. Transp. Porous Media 2015, 108, 679–696. [Google Scholar] [CrossRef]
- Ogolo, N.; Olafuyi, O.; Onyekonwu, M. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Shahrabadi, A.; Bagherzadeh, H.; Roostaie, A.; Golghanddashti, H. Experimental investigation of hlp nanofluid potential to enhance oil recovery: A mechanistic approach. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Ortega, D.J.S.; Kim, H.B.; James, L.A.; Johansen, T.E.; Zhang, Y. The effectiveness of silicon dioxide sio 2 nanoparticle as an enhanced oil recovery agent in ben nevis formation, hebron field, offshore eastern canada. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 7–10 November 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Haroun, M.R.; Alhassan, S.; Ansari, A.A.; Al Kindy, N.A.M.; Abou Sayed, N.; Kareem, A.; Ali, B.; Sarma, H.K. Smart nano-eor process for abu dhabi carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Agista, M.; Guo, K.; Yu, Z. A state-of-the-art review of nanoparticles application in petroleum with a focus on enhanced oil recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Nielson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of nanoparticle adsorption during transport in porous media. SPE J. 2015, 20, 667–677. [Google Scholar] [CrossRef]
- Zhang, T.; Murphy, M.; Yu, H.; Huh, C.; Bryant, S.L. Mechanistic model for nanoparticle retention in porous media. Transp. Porous Media 2016, 115, 387–406. [Google Scholar] [CrossRef]
- Li, S.; Torsæter, O. The impact of nanoparticles adsorption and transport on wettability alteration of water wet berea sandstone. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Nusa Dua, Bali, Indonesia, 20–22 October 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- Al-Anssari, S.; Wang, S.; Barifcani, A.; Lebedev, M.; Iglauer, S. Effect of temperature and sio 2 nanoparticle size on wettability alteration of oil-wet calcite. Fuel 2017, 206, 34–42. [Google Scholar] [CrossRef]
- Roustaei, A.; Bagherzadeh, H. Experimental investigation of sio2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2015, 5, 27–33. [Google Scholar] [CrossRef]
- Abhishek, R.; Kumar, G.S.; Sapru, R. Wettability alteration in carbonate reservoirs using nanofluids. Pet. Sci. Technol. 2015, 33, 794–801. [Google Scholar] [CrossRef]
- Abhishek, R.; Bagalkot, N.; Kumar, G.S. Effect of transverse forces on velocity of nanoparticles through a single fracture in a fractured petroleum reservoir. Int. J. Oil Gas Coal Technol. 2016, 12, 379–395. [Google Scholar] [CrossRef]
- Nwidee, L.N.; Al-Anssari, S.; Barifcani, A.; Sarmadivaleh, M.; Lebedev, M.; Iglauer, S. Nanoparticles influence on wetting behaviour of fractured limestone formation. J. Pet. Sci. Eng. 2017, 149, 782–788. [Google Scholar] [CrossRef]
- Nazari Moghaddam, R.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative study of using nanoparticles for enhanced oil recovery: Wettability alteration of carbonate rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Barifcani, A.; Wang, S.; Iglauer, S. Wettability alteration of oil-wet carbonate by silica nanofluid. J. Colloid Interface Sci. 2016, 461, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hendraningrat, L.; Torsaeter, O. Improved oil recovery by hydrophilic silica nanoparticles suspension: 2 phase flow experimental studies. In Proceedings of the IPTC 2013: International Petroleum Technology Conference, Beijing, China, 26 March 2013. [Google Scholar]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica nanofluids in an oilfield polymer polyacrylamide: Interfacial properties, wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Wang, S.; Barifcani, A.; Iglauer, S. Oil-water interfacial tensions of silica nanoparticle-surfactant formulations. Tenside Surfactants Deterg. 2017, 54, 334–341. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, P.; Colon, T.; Huh, C.; Balhoff, M. A microfluidic investigation of the synergistic effect of nanoparticles and surfactants in macro-emulsion-based enhanced oil recovery. SPE J. 2017, 22, 459–469. [Google Scholar] [CrossRef]
- Binks, B.P.; Whitby, C.P. Nanoparticle silica-stabilised oil-in-water emulsions: Improving emulsion stability. Coll. Surf. A Physicochem. Eng. Asp. 2005, 253, 105–115. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Chon, B.H.; Sangwai, J.S. Thermal stability of oil-in-water pickering emulsion in the presence of nanoparticle, surfactant, and polymer. J. Ind. Eng. Chem. 2015, 22, 324–334. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Sangwai, J.S. Comparative effectiveness of production performance of pickering emulsion stabilized by nanoparticle–surfactant–polymerover surfactant–polymer (sp) flooding for enhanced oil recoveryfor brownfield reservoir. J. Pet. Sci. Eng. 2015, 129, 221–232. [Google Scholar] [CrossRef]
- Monfared, A.D.; Ghazanfari, M.H.; Jamialahmadi, M.; Helalizadeh, A. Adsorption of silica nanoparticles onto calcite: Equilibrium, kinetic, thermodynamic and dlvo analysis. Chem. Eng. J. 2015, 281, 334–344. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Rezaei Gomari, K.A. Influence of temperature on wettability alteration of carbonate reservoirs. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006; Society of Petroleum Engineers: Richardson, TX, USA, 2006. [Google Scholar]
- Hamouda, A.; Valderhaug, O.; Munaev, R.; Stangeland, H. Possible mechanisms for oil recovery from chalk and sandstone rocks by low salinity water (lsw). In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Zahid, A.; Shapiro, A.A.; Skauge, A. Experimental studies of low salinity water flooding carbonate: A new promising approach. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Mahani, H.; Keya, A.L.; Berg, S.; Bartels, W.-B.; Nasralla, R.; Rossen, W.R. Insights into the mechanism of wettability alteration by low-salinity flooding (lsf) in carbonates. Energy Fuels 2015, 29, 1352–1367. [Google Scholar] [CrossRef]
- Al-Nofli, K.; Pourafshary, P.; Mosavat, N.; Shafiei, A. Effect of initial wettability on performance of smart water flooding in carbonate reservoirs—An experimental investigation with ior implications. Energies 2018, 11, 1394. [Google Scholar] [CrossRef]
- Wang, X.; Alvarado, V. Kaolinite and silica dispersions in low-salinity environments: Impact on a water-in-crude oil emulsion stability. Energies 2011, 4, 1763. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Gupta, S. Enhancing oil recovery from chalk reservoirs by a low-salinity water flooding mechanism and fluid/rock interactions. Energies 2017, 10, 576. [Google Scholar] [CrossRef]
- Rezaei Gomari, S.; Joseph, N. Study of the effect of clay particles on low salinity water injection in sandstone reservoirs. Energies 2017, 10, 322. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Maevskiy, E. Oil recovery mechanism (s) by low salinity brines and their interaction with chalk. Energy Fuels 2014, 28, 6860–6868. [Google Scholar] [CrossRef]
- Abhishek, R.; Hamouda, A.A. Effect of various silica nanofluids: Reduction of fines migrations and surface modification of berea sandstone. Appl. Sci. 2017, 7, 1216. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergy between nanoparticles and surfactants in stabilizing foams for oil recovery. Energy Fuels 2015, 29, 467–479. [Google Scholar] [CrossRef]
- Frykman, P. Spatial variability in petrophysical properties in upper maastrichtian chalk outcrops at stevns klint, denmark. Mar. Pet. Geol. 2001, 18, 1041–1062. [Google Scholar] [CrossRef]
- Tabrizy, V.A.; Denoyel, R.; Hamouda, A. Characterization of wettability alteration of calcite, quartz and kaolinite: Surface energy analysis. Coll. Surf. A Physicochem. Eng. Asp. 2011, 384, 98–108. [Google Scholar] [CrossRef]
- Griffith, N.; Ahmad, Y.; Daigle, H.; Huh, C. Nanoparticle-stabilized natural gas liquid-in-water emulsions for residual oil recovery. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Murphy, M.J. Experimental Analysis of Electrostatic and Hydrodynamic Forces Affecting Nanoparticle Retention in Porous Media. Ph.D. Thesis, The University of Texas at Austin Department of Petroleum and Geosystems Engineering, Austin, TX, USA, 2012. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic chemistry. In An Introduction Emphasizing Chemical Equilibria in Natural Waters; Wiley-Interscience: New York, NY, USA, 1970. [Google Scholar]
- Hendraningrat, L.; Torsæter, O. A study of water chemistry extends the benefits of using silica-based nanoparticles on enhanced oil recovery. Appl. Nanosci. 2016, 6, 83–95. [Google Scholar] [CrossRef]
- Dehghan Monfared, A.; Ghazanfari, M.H.; Jamialahmadi, M.; Helalizadeh, A. Potential application of silica nanoparticles for wettability alteration of oil–wet calcite: A mechanistic study. Energy Fuels 2016, 30, 3947–3961. [Google Scholar] [CrossRef]
- Austad, T.; Shariatpanahi, S.; Strand, S.; Black, C.; Webb, K. Conditions for a low-salinity enhanced oil recovery (eor) effect in carbonate oil reservoirs. Energy Fuels 2011, 26, 569–575. [Google Scholar] [CrossRef]
- Chukwudeme, E.; Hamouda, A. Oil recovery from polar components (asphaltene and sa) treated chalk rocks by low salinity water and water containing so42− and mg2+ at different temperatures. Coll. Surf. A Physicochem. Eng. Asp. 2009, 336, 174–182. [Google Scholar] [CrossRef]
- Yi, Z.; Sarma, H.K. Improving waterflood recovery efficiency in carbonate reservoirs through salinity variations and ionic exchanges: A promising low-cost “smart-waterflood” approach. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012; Society of Petroleum Engineers: Abu Dhabi, UAE, 2012. [Google Scholar]
- Tang, G.; Morrow, N.R. Salinity, temperature, oil composition, and oil recovery by waterflooding. SPE Reserv. Eng. 1997, 12, 269–276. [Google Scholar] [CrossRef]
- Petrovich, R.; Hamouda, A. Dolomitization of ekofisk oil field reservoir chalk by injected seawater. In Proceedings of the Ninth International Symposium on Water–Rock Interactions, Taupo, New Zealand, 30 March–3 April 1998. [Google Scholar]
- Van Oort, E.; Van Velzen, J.; Leerlooijer, K. Impairment by suspended solids invasion: Testing and prediction. SPE Prod. Facil. 1993, 8, 178–184. [Google Scholar] [CrossRef]
- Jolma, I.; Strand, D.; Stavland, A.; Fjelde, I.; Hatzignatiou, D. When size matters-polymer injectivity in chalk matrix. In Proceedings of the IOR 2017-19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar]









| Temperature °C | Viscosity (cP) | Density (g/mL) |
|---|---|---|
| 20 | 0.92 | 0.73 |
| 50 | 0.5802 | 0.7683 |
| 70 | 0.4812 | 0.7525 |
| Ion | SSW (mol/L) | LSW (mol/L) |
|---|---|---|
| HCO3− | 0.002 | 0.0002 |
| Cl– | 0.525 | 0.0525 |
| SO42– | 0.0240 | 0.0024 |
| Mg2+ | 0.045 | 0.0045 |
| Ca2+ | 0.013 | 0.0013 |
| Na+ | 0.450 | 0.045 |
| K+ | 0.010 | 0.0010 |
| Core Id | Porosity (%) | Permeability (mD) | Length (cm) | Dia (cm) | Pre/Post Flush Fluid | Slug Composition |
|---|---|---|---|---|---|---|
| SK1 | 48.10 | 3.9 | 5.31 | 3.78 | DIW | 1 (g/L) DP in DIW with 0.1M LiCl tracer |
| SK2 | 49.00 | 3.9 | 7.80 | 3.78 | DIW | 90 μL 0.1M HCl + 0.1M LiCl in DIW |
| SK3 | 51.71 | 3.9 | 3.9 | 3.78 | SSW | 1 g/L DP in SSW + 0.1M LiCl |
| SK4 | 47.38 | 3.9 | 3.35 | 3.78 | LSW | 1 g/L DP in LSW + 0.1M LiCl |
| Core Id | Porosity (%) | Permeability (mD) | Length (cm) | Dia (cm) | Swi | Primary Fluid | Secondary Fluid (Nanofluid) |
|---|---|---|---|---|---|---|---|
| SK5 | 50.7 | 3.9 | 8.83 | 3.785 | 0.13 | SSW | DP (1 g/L) in SSW |
| SK6 | 50 | 3.9 | 5.96 | 3.785 | 0.28 | LSW | DP (1 g/L) in LSW |
| SK7 | 50.24 | 3.9 | 4.658 | 3.785 | 0.275 | SSW | DP (1 g/L) in LSW |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhishek, R.; Hamouda, A.A.; Ayoub, A. Effect of Silica Nanoparticles on Fluid/Rock Interactions during Low Salinity Water Flooding of Chalk Reservoirs. Appl. Sci. 2018, 8, 1093. https://doi.org/10.3390/app8071093
Abhishek R, Hamouda AA, Ayoub A. Effect of Silica Nanoparticles on Fluid/Rock Interactions during Low Salinity Water Flooding of Chalk Reservoirs. Applied Sciences. 2018; 8(7):1093. https://doi.org/10.3390/app8071093
Chicago/Turabian StyleAbhishek, Rockey, Aly A. Hamouda, and Amr Ayoub. 2018. "Effect of Silica Nanoparticles on Fluid/Rock Interactions during Low Salinity Water Flooding of Chalk Reservoirs" Applied Sciences 8, no. 7: 1093. https://doi.org/10.3390/app8071093





