Abstract
Gymnema sylvestre is a plant that is enriched in bioactive compounds. In particular, gymnemic acids (GA) and phenolic compounds (PC) are pharmaceutically important. There is a commercial demand for naturally occurring bioactive compounds, but their availability is limited due to geographical and seasonal variations. The elicitation approach can enhance the biosynthesis of phytochemicals during in vitro culture of G. sylvestre. Here, to further improve gymnemic acid II (GA II) and phenolic compounds (PC) production by G. sylvestre, cell suspension cultures (CSC), which has attracted attention for the production of essential phytochemicals, was explored using copper oxide nanoparticles (CuO NPs). Callus was obtained on MS medium containing 2,4-dichlorophenoxyacetic acid, kinetin, phytoagar, and sucrose. Agar-free MS medium was used to initiate CSC, which was treated with three concentrations of CuO NPs (1, 3 or 5 mg/L). Treatment for 48 h with 3 mg/L CuO NPs resulted in the greatest yields of GA II, total phenolics, and flavonoids. The cultures also displayed pronounced antioxidant, antidiabetic, anti-inflammatory, antibacterial, antifungal, and anticancer activities. The use of CuO NPs (3 mg/L) significantly increased the production of GA II (nine-fold) and PC compared to unamended CSC. We propose that CSC and use of nanoparticles (NPs) as a new generation of elicitors, offer a suitable prospect for the production of bioactive compounds.
1. Introduction
Homeopathic plants are the ideal source of medicinal compounds that enhance the quality and longevity of life. Gymnema sylvestre (Retz.) R. Br. (Family: Asclepiadaceae) is a woody climbing shrub that has medicinal value because of the presence of various bioactive compounds like gymnemic acids (GA) and phenolic compounds (PC), which are used to treat diabetes mellitus [1]. The plant is also used as an ingredient of tea, confections, and many food preparations for the regulation of sugar homeostasis and to control obesity and the level of blood cholesterol [2]. The pharmacologic potential of GA includes antimicrobial, antihypercholesterolemic, hepatoprotective and anti-saccharine activities [3]. GA selectively suppresses the urge for sweet flavors in humans. The leaves of G. sylvestre contain triterpenoid saponins, such as oleananes (e.g., GA) and dammarane (gymnemasides), which are considered the active constituents [4].
The medicinal value of G. sylvestre could lead to its overharvesting. In vitro plant tissue culture or cell suspension culture (CSC) techniques are eco-friendly biotechnological methods for the production of phytochemicals that are an alternative to overexploitation of naturally growing G. sylvestre sources. The phytochemicals derived using these techniques can be used as food additives, nutraceuticals, and pharmaceuticals [5]. Elicitation is one of the most effective biotechnological approaches to increase the production of phytochemicals in tissue culture or CSC [6]. Elicitors are biological and non-biological molecules that recognize receptors on the cytoplasmic membrane of plant cells. The binding of the compounds to the receptors elicits a signal that stimulates the expression of genes involved in the synthetic pathway of plant secondary metabolites. Elicitors can stimulate defensive responses in cells, tissues, organs, and plants because of the alteration of various biosynthetic pathways. Thus, the production of preferred phytochemicals requires elicitor-mediated external stimuli [7]. Enhanced accumulation of secondary metabolites by the addition of elicitors to the CSCs has been reported [8,9]. Application of elicitors to improve the production of GA from G. sylvestre has been accomplished using callus and CSC methods [10,11,12]. Copper (Cu) is a vital micronutrient for plant development and a co-factor in various physiological processes that include cell wall metabolism, photosynthesis and lignification. However, Cu is toxic at higher levels [13]. Cu is also present in catalytic enzymes, assists in protein transport, and functions in the transcription of some genes [14]. Elicitation of CuSO4 increases the content of phytochemicals that include bacoside, betacyanin, and phenylpropanoids during in vitro tissue culture of Bacopa monnieri [15], Alternanthera philoxeroides [16], and Ocimum basilicum [14]. Cu is essential in plant growth and stimulates phytochemical production.
Nanotechnology is an advanced technology that has diverse scientific applications. Recent developments in nanotechnology and extensive uses of engineered nanoparticles (NPs) have advanced biotechnology. Reflecting this importance, the manufacture of engineered NPs is predicted to increase from 0.27 million tons in 2012 to 1.663 million tons by 2020 [17]. Nanotechnology also has huge benefits in agriculture for the control of diseases and in crop production [18]. Nanomaterials with a diameter from 1 to 100 nm are often used commercially. A recent focus has been on the use of nanomaterials as an elicitor to evaluate the stress responses in different economically and medicinally important plants. NPs are extensively used as abiotic elicitors in plant biotechnology to enhance the production of valuable bioactive compounds [19]. In the past several years metallic NPs have become popular due to their enhanced physical and chemical properties [20]. Nanoscale nutrients and insecticides are being increasingly used in agriculture. Cu-containing nano-pesticides have become a popular commercial product for agriculture because of its excellent antimicrobial activities [21]. The application of Cu oxide (CuO) NPs can increase the yield and fruit quality of tomato [22] and pepper [23]. Cu, silver, and gold NPs improved the accumulation of phenolics, flavonoids, and protein in callus cultures of Prunella vulgaris and Momordica charantia [24,25].
Limited evidence is available on the influence of CuO NPs on plants. However, promising results were reported recently for Mentha longifolia [26], Verbena bipinnatifida [27], Ocimum basilicum [28] and Brassica rapa spp. pekinensis [29]. There have been no reports on the impacts of CuO NPs on the CSC of G. sylvestre. Therefore, this study explored the influence of CuO NPs on gymnemic acid II (GA II) and phenolic compounds (PC) production and on the antioxidant, antidiabetic, antibacterial, antifungal, anti-inflammatory and anticancer activities.
2. Materials and Methods
2.1. CSC
G. sylvestre seeds were obtained from Malayandi Kamaraj, Department of Botany, Jamal Mohamed College, Tiruchirappalli, TN, India. The seeds were disinfected with 2% NaOCl solution for 20 min, followed by 0.5% HgCl2 solution for 5 min and five washes with sterile deionized water (H2O). Disinfected seeds were grown in MS medium [30] containing 8.0 g/L phytoagar and 30 g/L sucrose. Cultures were placed in a plant growth chamber at a 24 ± 2 °C with a light/dark cycle of 16 h/8 h for 4 weeks. Leaf segments were incubated on a medium containing 2,4-dichlorophenoxyacetic acid (2,4-D; 0.5–3 mg/L) combined with 0.1 mg/L kinetin (KIN) for callus development. The cultures were kept in a plant growth chamber with the aforementioned temperature and light/dark conditions for 21 days. Friable callus (1 g) was cultured in conical flasks containing MS basal salt solution containing 0.1 mg/L KIN and 2 mg/L 2,4-D for initiation of CSC. The flasks were aerated in a shaking incubator (110 rpm) using the aforementioned light/dark cycle at 25 ± 2 °C. The cultures were re-passaged every 7 days intervals of the new passage of culture. The growth of cell suspensions in terms of fresh cell mass (FCM), dry cell mass (DCM), and phytochemical contents were evaluated after 6, 12, 18 and 24 days of culture.
2.2. Elicitation of CuO NPs in CSC
Pure preparation (99%) of CuO NPs 25 to 55 nm in size was purchased from US Research Nanomaterials, Inc. (Houston, TX, USA). After 18 days, the CSC was treated with 0, 1, 3 or 5 mg/L CuO NPs. After 2 days, a subculture was made in fresh MS liquid medium lacking CuO NPs. The CSC was incubated on a rotatory shaker at 110 rpm using the 16/8 h light and dark cycle at 25 ± 2 °C. The cells were collected from the medium at 23 days for determinations of FCM and DCM. CSC collected from the medium by passage through a filter was washed with sterile deionized H2O and blotted on sterile Whatman #1 filter paper to remove water drops before FCM determination. The filtered cells were frozen, lyophilized overnight, and then used for determination of DCM. Fifty grams DCM of G. sylvestre harvested from each treatment, the control, and CuO NP treatments were digested with 70% nitric acid for 1 h at 115 °C. The samples were individually diluted with high-performance liquid chromatography (HPLC) water, filtered into an Eppendorf tube (1.5 mL) using nylon filters (0.2 μm), and used for inductively coupled plasma mass spectrometry (ICP-MS) using a model 820-MS device (Varian, Palo Alto, CA, USA).
2.3. Extraction and Estimation of Gymnemic Acid II (GA II) in CSC
CSC (500 mg DCM) was extracted using an equal volume of ethanol (EtOH) and H2O, and 10 mL of KOH solution (12%) was added and refluxed. After 1 h, 11 mL of HCl (4 N) was added at room temperature and refluxed. After 1 h, samples were filtered using nylon filters (0.22 µM) and used for HPLC. The HPLC apparatus (Waters, Milford, MA, USA) was equipped with a model 2487 variable dual photo diode array detector (Waters) and examined at a wavelength of 210 nm. Separations were done with a C18 column (5 μm, 2.1 × 100 mm) with acetonitrile: H2O (80:20) at a flow rate of 1 mL/min at 27 °C [4]. The GA standard was acquired from Chromadex (Irvine, CA, USA). The molecular mass conversion of gymnemagenin to GA was calculated as 809.0/506.7 [4].
2.4. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in CSC
Total phenolic content (TPC) was determined by a spectrophotometric method using Folin−Ciocalteu (FC) reagent [25]. Briefly, 200 μL (1 mg/mL) of a CSC sample was mixed with 200 μL FC reagent. After 5 min, 600 μL of sodium carbonate solution (20%) was added and the mixture was left at room temperature for 1 h. The absorbance was read at 765 nm using an ultraviolet–visible spectrophotometer (UV-2120 Optizen, Mecasys, Korea). TPC was calculated as mg gallic acid equivalent (GAE) based on a gallic acid calibration curve.
CuO NP treated and non-treated CSC samples (100 μL, 100 mg/mL) received FC reagent followed by 15% sodium carbonate. The absorbance was read at 755 nm. Total flavonoid content (TFC) of CuO NP treated and non-treated CSC samples (100 μL, 100 mg/mL) were measured using an established calorimetric method [31]. TFC was calculated as mg quercetin equivalent (QE) based on a quercetin calibration curve.
2.5. Biological Activities in CSC
2.5.1. Extract Preparation
Powder of CuO NP treated and non-treated CSC samples (1 g DCM) were extracted with 50 mL of methanol (95%) and kept at room temperature for 24 h with repeated shaking using a rotary shaker at 100 rpm. The solution was passed through Whatman #1 filter paper, and the filtrate was rotary evaporated. The dry methanolic extract was then dissolved with methanol and kept at 4 °C for the following analyses of pharmacological activities.
2.5.2. Antioxidant Activity
Radical Scavenging Activity
The radical scavenging activity of CuO NP treated and non-treated CSC extracts were assessed using an established 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay [25,32]. CSC extracts (100 μL) were added to 1.4 mL DPPH solution and incubated in the dark at room temperature for 45 min. The absorbance (A) was read at 517 nm in UV–visible spectrophotometer (UV-2120 Optizen, Mecasys, Korea). Inhibition (%) was calculated as = [(Ablank − Asample)/Ablank] × 100.
Reductive Potential
The reducing potential of G. sylvestre cell extracts was determined as previously described [25,33]. Each CSC extract (100 μL) was mixed with 2.5 mL sodium phosphate buffer (200 mM), followed by 2.5 mL potassium ferricyanide (1%) and incubation at 50 °C for 20 min. Then, 2.5 mL trichloroacetic acid (10%) was added and the mixture was centrifuged for 10 min at 650 g. The supernatant (2.5 mL) was recovered and 2.5 mL distilled water and 0.5 mL ferric chloride (0.1%) was added. After thorough vortexing, the absorbance was measured at 700 nm.
Phosphomolybdenum Method
The total antioxidant potential of extracts was assessed using the phosphomolybdenum method [25]. Each CSC extract (100 µL, 1 mg/mL) was mixed with 1 mL of phosphomolybdenum reagent (4 mM ammonium molybdate, 0.6 M sulfuric acid, and 28 mM sodium phosphate) and incubated at 95 °C for 90 min in a thermal block. The sample was cooled to 23 °C and the optical density (OD) was determined at 695 nm. Total antioxidant activity was expressed as equivalents of alpha-tocopherol (μg/g of extract).
2.5.3. Antidiabetic Activity
CuO NP treated and non-treated CSC extracts were carried out the antidiabetic activity. Alpha-amylase inhibition was determined using the dinitrosalicylic acid (DNSA) method [25,34]. The test mixture contained 500 μL of 0.02 M Na3PO4 buffer containing α-amylase solution (1 U/mL) and each CSC extract at 20 to 100 μg/mL mixed with 100 mL of starch (1%). The mixture was incubated for 20 min at 37 °C. The reaction was terminated by adding 500 μL of DNSA reagent and placing the tube in a boiling water bath for 10 min. The absorbance was measured at 540 nm. Inhibition (%) was calculated as [(Ablank − Asample)/Ablank] × 100.
Non-enzymatic glycosylation of hemoglobin was determined as previously described [34]. Briefly, 1 mL of hemoglobin (0.06%), 5 µL of gentamycin (0.02%), 1 mL CSC extracts (1 mg/mL), and 1 mL of glucose solution 0.2% were mixed. The mixture was kept at 37 °C in the dark for 3 days and absorbance was determined at 443 nm. Alpha-tocopherol was used as a standard drug having similar concentration as that of extract sample solutions. Inhibition (%) was calculated as [(Ablank − Asample)/Ablank] × 100.
2.5.4. Anti-Inflammatory Activity
Lipoxygenase activities in CuO NP treated and non-treated CSC extracts were determined as previously described [35]. Briefly, 200 µL of a mixture containing 160 µL sodium phosphate buffer (100 mM, pH 8.0), 10 µL CSC extract (25 to 100 µg in 100 mM Tris buffer pH 7.4), and 20 µL 5-lipoxygenase was preincubated for 10 min at 25 °C. The reaction was initiated by the addition of 10 µL linoleic acid solution as a substrate. After 6 min, the absorbance was determined at 234 nm. All reactions were carried out in triplicate in 96-well microplate reader (BioTek Instruments, Winooski, VT, USA). Positive and negative controls were included in the experiment. Inhibition (%) was calculated as [(Ablank − Asample)/Ablank] × 100.
Inhibition of albumin denaturation was determined as previously described [36]. The reaction mixture consisted of CSC extract at different concentrations and 1% bovine serum albumin (aqueous solution). The reaction mixture was incubated for approximately 20 min at 37 °C and then heated at 51 °C for 20 min. The turbidity of the mixture was then estimated at 660 nm by cooling the mixture. Finally, the percentage of inhibition of protein denaturation was calculated as [(Acontrol − Asample)/Acontrol] × 100.
2.5.5. Antibacterial and Antifungal Activities
CuO NP treated and non-treated CSC extracts were examined for their antimicrobial activity as previously described [25,29]. Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis), Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli), and fungi (Aspergillus niger, Fusarium oxysporum, and Candida albicans) were used for the disc diffusion method [25,29].
2.5.6. Anticancer Activity
HT-29 colorectal adenocarcinoma cells and MCF-7 breast cancer cells were treated with 12.5 to 200 μg/L of G. sylvestre cell extracts, and the cytotoxicity of CuO NP treated and non-treated CSC extracts were evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) based assay [25]. The control and extract treated cells were treated with the MTT reagent (20 μL well). The cells were incubated at 37 °C for 4 h, and then dimethyl sulfoxide DMSO, 200 μL was added to all wells to dissolve the formazan crystals that had formed in viable cells. The observation was read using a microplate reader at an absorbance of 492 nm. The inhibition of growth (%) was calculated as = (ODsample/ODcontrol × 100).
2.6. Statistical Analyses
All the tests were performed in triplicate (n = 3). Results are expressed as mean ± SD and followed by Duncan’s multiple range test (DMRT). Significance was defined at a p-value ≤ 0.05. The analyses were done using the SPSS Ver. 20 statistical software package (SPSS, Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Gymnemic Acid II (GA II) Content in CSC
Callus formation was evident in all media conditions. Callus induction was considerably improved with increasing levels of 2,4-D up to 2 mg/L but was detrimentally affected at higher levels. The best callus initiation frequency (94.66%) was attained on MS medium with 2 mg/L 2,4-D and 0.1 mg/L KIN (Figure 1a). These results agree with those of a prior study with G. sylvestre [37]. Presently, the maximum accumulation of biomass was recorded at day 18 (12.26 g DCM) and GA II production (10.20 mg/g DCM) (Figure 1b,c). With time, biomass growth was closely correlated with GA II accumulation. Similar results were attained during the CSC of G. sylvestre and Polygonum multiflorum for the production of gymnemic acid and anthraquinones [11,12,37,38].
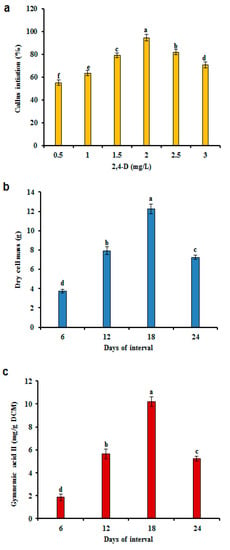
Figure 1.
Callus induction and biomass accumulation in Gymnema sylvestre. (a) Effect of different concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) in combination with 0.1 mg/L kinetin (KIN) for callus induction in G. sylvestre. (b) Biomass accumulation. (c) Gymnemic Acid II (GA II) production on MS liquid medium with 2,4-D (2 mg/L), KIN (0.1 mg/L), and sucrose (30 g/L) with time. Different letters indicate a significant difference at p ≤ 0.05.
3.2. Biomass, Cu, and GA II Content in CuO NP Treated and Non-Treated CSC
Cu accumulation was noted when the cells were exposed to CuO NPs. The Cu level was higher (1.1 mg/L) in 5 mg/L CuO NP treated CSC compared with the value in 1 or 3 mg/L of CuO NP treated CSC (Figure 2a). Similarly, Cu accumulation in B. rapa was reportedly enhanced depending on treatment with CuO NPs [29]. A high concentration of Cu ions may be toxic; for example, binding of Cu to sulfhydryl groups of proteins can inhibit enzyme activities and may induce a deficiency of other elements through competition with ions like iron or by imposing oxidative stress in plant cells [39]. The redox reactions that make Cu a significant essential element is also responsible for its toxicity [13]. Thus, the transition from Cu2+ to Cu+ and the reverse (i.e., the redox cycle) catalyzes the interaction between superoxide ion (O2−) and hydrogen peroxide (H2O2) to produce hydroxyl radicals (OH), which are highly toxic and can cause oxidative stress that involves cell proteins, lipids and DNA [40]. Presently, the maximum level of biomass (14.51 g DCM) accumulation was obtained on medium with 1 mg/L CuO NPs compared to the levels in the control and in samples containing >1 mg/L CuO NPs. Increasing the level of CuO NPs above 1 mg/L decreased the DCM (Figure 2b). A similar observation was also noted in the CSC of bitter gourd after elicitation with silver NPs (AgNPs). The authors corroborated that a higher dose of CuO NPs decreased the biomass accumulation in hairy root culture of Chinese cabbage [25]. Cu has been studied as a stimulatory element for in vitro culture in many plants [41,42]. For example, CuO NPs stimulate phytochemical production in Stevia rebaudiana in vitro [42]. The present study focused on the impact of CuO NPs on GA II content in CSC as determined using HPLC. The content of GA II in elicited CSC was significantly higher (four- to nine-fold) than non-elicited CSC (Figure 3a). The nine-fold increase of GA II (89.25 mg/g DCM) was evident with 3 mg/L CuO NPs (Figure 3a). However, 5 mg/L of CuO NPs inhibited the synthesis of GA II (Figure 3a). The abiotic elicitation of cadmium chloride produced the maximum amount of GA (59.97 mg/g DCM), i.e., a 6.8-fold increase in comparison to the non-treated CSC of G. sylvestre [12]. GA content was improved in the CSC of G. sylvestre using signaling molecules, such as methyl jasmonate and salicylic acid [43]. An increase in the GA content (7.78-fold) was evident in the presence of linolenic acid treatment compared to that of the non-elicited roots of G. sylvestre [4]. The results indicate that CuO NPs are effective in improving the production of GA II when compared to metal salts and signaling molecules in the CSC of G. sylvestre. CuO NPs have a useful role, which is mainly due to the release of Cu ions from the NPs that are taken up by the cells and which play a pivotal role in plant biochemistry [44]. Significant variations in cell biomass, GA II, PC, and pharmacological activities were observed, indicating that CuO NPs are abiotic stress elicitors that enhance the quantity of GA II content in G. sylvestre.
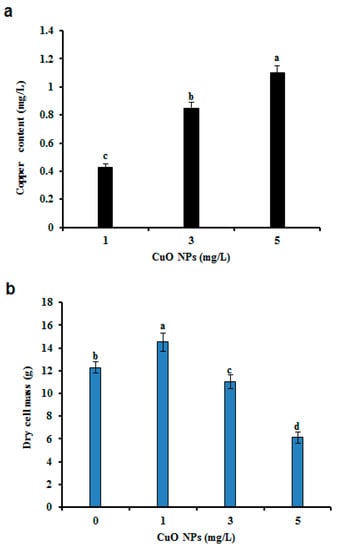
Figure 2.
Effect of copper oxide nanoparticles (CuO NPs) on copper content and biomass accumulation in cell suspension cultures (CSC) of G. sylvestre. (a) Copper content, (b) biomass accumulation. Different letters indicate a significant difference at p ≤ 0.05.
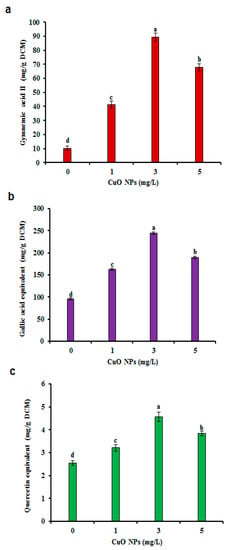
Figure 3.
Effect of CuO NPs on bioactive compounds in CSC of G. sylvestre. (a) Gymnemic acid II (GA II), (b) Total phenolic content (TPC), (c) Total flavonoid content (TFC). Different letters indicate a significant difference at p ≤ 0.05.
3.3. TPC and TFC in CSC
Several studies reported that the inclusion of an abiotic or biotic elicitor to the growth medium notably elevated the synthesis of bioactive compounds by activating a defense system [29]. We investigated the impact of CuO NPs on the accumulation of TPC and TFC in CSC of G. sylvestre. CuO NPs at the concentration of 3 mg/L led to the maximum production of TPC (245.10 mg/g DCM GAE) and TFC (4.57 mg/g DCM QE) compared to control CSC (95.75 GAE and 2.55 QE; Figure 3b,c). However, the use of 5 mg/L of CuO NPs in CSC decreased the amount of TPC (190.15 GAE) and TFC (3.85 QE). TPC and TFC were improved by the elicitation of CuO NPs in hairy roots of Chinese cabbage [29] and AgNPs in CSC of bitter gourd [25]. Elicitation with silicon dioxide NPs elevates the PC accumulation in hairy roots of Dracocephalum kotschyi [45]. The mechanism of an elicitor’s impact on cells, organs and plants is not well-documented. However, biotic or abiotic elicitors easily attack the cell membrane and stimulate defensive signals like electrolyte leakage, oxidative burst, production of reactive oxygen species (ROS) and protein phosphorylation and dephosphorylation. Phytoalexins like PC are increased by secondary messenger molecules including H2O2. The over-expression of PAL (a defense gene) leads to the enhanced production of PC [46]. Several studies have demonstrated that treatments of plants with cadmium oxide NPs or CuO NPs produce an increase in PC, which could act as an antioxidant to scavenge ROS [47,48]. ROS may act as a normal signal for adaptation to Cu stress and could induce the accumulation of TPC in lentil roots [49]. Elicitation of magnetite NPs and static magnetic field elicitation can increase the content of phenolic compounds and polyphenol oxidase in the CSC of Dracocephalum polychaetum [9]. TPC and TFC can also be determined by both the surface area and size of the NPs used. Smaller NPs will induce more stress and, in response, more TPC and TFC will be biosynthesized. Our results suggest that treatment with CuO NPs improved the amount of TPC and TFC in CSC of G. sylvestre.
3.4. Antioxidant Activity
Bioactive compounds with antioxidant activity play a significant role in protection against ROS and reactive nitrogen species. The major anti-oxidant compounds are bioactive compounds, whose production increases when a particular factor in the medium is changed. The factor causes stress, which leads to improved accumulation of bioactive compounds, such as GA, phenols, and flavonoids. Previous results reported changes in radical scavenging activity (57.10%), reducing potential (absorbance at 0.15) and antioxidant capacity (17.54 mg/g) in G. sylvestre leaf extracts [50]. The phenolic and flavonoids were significantly greater in CSC treated with CuO NPs, which correlated with their antioxidant activity (Figure 4). The free radical scavenging activity of the CSC extracts was confirmed through the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay. Of the different concentrations of CuO NPs used to treat CSC, 3 mg/L CuO NPs treated CSC extracts displayed the highest antioxidant activity of 86.15% as compared to the control (72.62%) (Figure 4a). The CuO NPs elicited CSC extracts exhibited the highest reducing potential compared with non-elicited CSC (Figure 4b). The reducing potential is commonly connected with the number of reductones in the test samples [4]. The chelating capacity is essential to determine the antioxidant power of crude extract or single compound because it decreases the number of metal ions by catalyzing malondialdehyde [51]. The antioxidant activity of the CuO NPs (3 mg/L) elicited CSC extracts was higher (80.55 mg/g) than non-elicited CSC extracts (69.50 mg/g) (Figure 4c). The present results are consistent with the previous report that the elicitation of polyunsaturated fatty acids triggered the antioxidant defense system and the marked production of GA and PC in hairy root cultures of G. sylvestre [4]. Correspondingly, the antioxidant potential was improved in CuO NPs elicitation in hairy roots of Brassica rapa spp. pekinensis [29] and regenerated shoots of Stevia rebaudiana [42]. Similar to the present study, CuO NPs were associated with the development of intracellular oxidative stress by the release of metal ions (e.g., Cu+2) or free radicals. The oxidative damage that occurred at higher concentration impaired growth reduced the quantity of bioactive compounds in steviol glycoside and mitigated antioxidant activities [42].
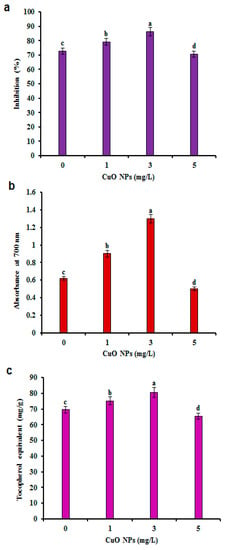
Figure 4.
Effect of CuO NPs on antioxidant activities in CSC of G. sylvestre. (a) Free radical-scavenging activity by the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay, (b) total Fe3+– Fe2+ reductive potential reference antioxidants (butylated hydroxytoluene), (c) total antioxidant capacity (TAC) by the phosphomolybdenum method. TAC is expressed as equivalents of α-tocopherol (μg/g of extract). Different letters indicate a significant difference at p ≤ 0.05.
3.5. Antidiabetic Activity
Oxidative stress in the body is one of the most serious factors in the development of diabetes [52]. Free radical scavengers are used to manage the oxidative damage and to constrain enzymes like α-amylase and α-glucosidase, which are responsible for diabetes [53]. The inhibition of carbohydrate-hydrolyzing enzymes like α-amylase can be an important approach to lower postprandial blood glucose levels. In this study, the activity of α-amylase was potently inhibited in CuO NP-elicited CSC extracts (Figure 5a). Marked suppression of α-amylase in a dose-dependent manner was observed after incubation with different amount of extracts. CuO NP-elicited CSC extracts (100 μg/mL) displayed an 86.75% rate of α-amylase enzyme inhibition compared to the rate of 70.50% for non-elicited CSC extracts. The inhibition rate achieved using acarbose was 92.00% (Figure 5a). Similarly, the GA fraction displayed a significant reduction in amylase activity (14.25%) using the extract of G. sylvestre leaves [54]. Non-enzymatic glycosylation of hemoglobin was increased in CuO NP treated and non-treated CSC extracts. The inhibition of glycosylation was concentration-dependent with CuO NP treated and non-treated CSC extracts and tocopherol, which was used as a standard. The inhibition of glycosylation in CuO NP treated (3 mg/L) and non-treated CSC extracts were 90.50% and 75.25%, respectively (Figure 5b). The results offer clues that effective treatment for diabetes may be possible using the bioactive elements present in plant extracts. GA obtained from G. sylvestre inhibits glucose absorption in the small intestine and suppresses hyperglycemia and hyperinsulinemia in oral glucose tolerance test. The efficiency of GA in inhibiting glucose absorption in the small intestine was increased by a combined effect with acarbose and voglibose [55]. GA and PC have potent inhibitory effects on α-amylase and α-glucosidase [55,56]. We hypothesize that the presence of GA II and PC in CuO NPs elicited by CSC extracts of G. sylvestre could significantly inhibit α-amylase and glycosylation activities.
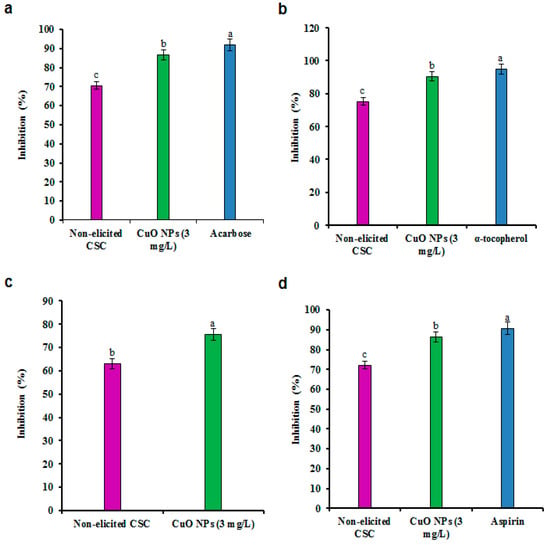
Figure 5.
Effect of CuO NPs on antidiabetic and anti-inflammatory activities in CSC of G. sylvestre. (a) In vitro α-amylase activity, (b) non-enzymatic glycosylation of hemoglobin activity, (c) lipoxygenase inhibition activity, (d) albumin denaturation inhibition assay. Different letters indicate a significant difference at p ≤ 0.05.
3.6. Anti-Inflammatory Activity
Inflammation is a biological reaction triggered by the disturbance of the tissue homeostasis, which occurs in response to the presence of a biological, chemical, or physical agent in the body. PC-mediated interference with these mechanisms that occur by the prevention of prolonged inflammation could be useful for human health [57]. PC frequently reduces the inflammatory process by altering cyclooxygenase and lipoxygenase activities. Lipoxygenase inhibitors are involved in various inflammatory diseases like cancer, asthma, leukemia, lymphoma, and autoimmune disorders and increase the immune response to viral and bacterial infections [35]. Presently, anti-inflammatory capacity was evaluated by lipoxygenase activity (Figure 5c). CuO NP-elicited CSC extracts showed the maximum inhibitory activity of 75.55%, which was higher than that of non-elicited CSC extracts (63.00%). The inhibitory effects PC on 15-lipoxygenase and lipoxygenase have been well documented [57]. Likewise, the denaturation of protein molecules is well recognized in the literature, and it is due to an inflammation process in conditions like arthritis. Inhibition of protein disruption might be responsible for the anti-rheumatic activity of nonsteroidal anti-inflammatory drugs [58]. CuO NP-elicited CSC extracts can inhibit the membrane stabilization by 86.25%, which is near the 90.75% of standard aspirin (Figure 5d). CuO NP-elicited CSC extracts strongly inhibited the denaturation of protein in the membrane stabilization test. The results of the current study corroborate that flavonoids could constrain the enzymes, along with reactive C protein or adhesion molecules [57].
3.7. Antibacterial and Antifungal Activities
The antimicrobial properties of GA and PC against pathogenic bacteria and fungus have been reported [25,59]. CuO NP treated CSC extracts of G. sylvestre displayed strong antibacterial and antifungal activities compared to non-treated CSC extracts (Figure 6). CuO NP treated CSC extracts showed more distinct activities against Gram-positive than Gram-negative bacteria. Consistent with our results, the absence of the lipopolysaccharide containing outer membrane surrounding the cell wall of Gram-positive bacteria allows increased permeability of Hypericum perforatum antimicrobial metabolites into the bacteria [60]. The obtained results support an earlier study of antimicrobial activity of G. sylvestre, which demonstrated a zone of inhibition in B. subtilis and S. aureus agar cultures, while the extracts had no activity against the gram-negative bacterium E. coli [61]. Furthermore, leaf extracts of G. sylvestre displayed good antimicrobial activity [59]. AgNP-treated plants and CuO NP-elicited hairy root extracts were reported to have stronger antimicrobial activities in Achillea millefolium [62] and Brassica rapa spp. pekinensis [29], in which NP elicitation enhanced the amount of bioactive compounds. Similar to the present observations, GA and PC reportedly displayed potent antimicrobial activities [25,59]. Our data confirm that the CuO NP treated CSC extracts have potent antimicrobial activity against clinically important microorganisms due to the higher amounts of GA II and PC.
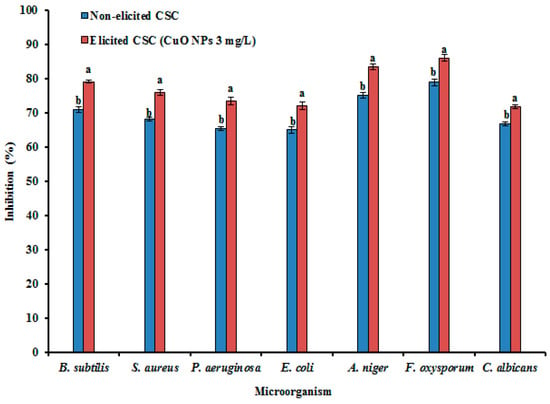
Figure 6.
Effect of CuO NPs on antimicrobial activity in CSC of G. sylvestre using the disc diffusion method. Different letters indicate a significant difference at p ≤ 0.05.
3.8. Anticancer Activity
GA, saponins, and PC display anticancer activities [25,63]. G. sylvestre extracts were active against human lung adenocarcinoma and breast carcinoma cell lines [64]. Presently, the cytotoxic activity of CuO NP treated and non-treated CSC extracts against MCF-7 and HT-29 cancer cells were investigated. The cells were exposed to various levels of CuO NP treated and non-treated CSC extracts. The percentage inhibition of the cells increased with increasing concentrations of CSC extracts (Figure 7). Greater inhibition was noted at 200 μg/mL of the CSC extracts (Figure 7a,b), at which the CuO NP treated CSC extracts exhibited high cancer inhibition, whereas the non-treated CSC extracts displayed less inhibition. Similarly, G. sylvestre and Eclipta prostrata extracts obtained from AgNP treated plants also displayed higher cytotoxicity against HeLa cells [63]. The present findings agree with the earlier observation studies that that the CuO NP-elicited hairy roots displayed more potent cytotoxic activities than non-elicited hairy roots [29]. This avid cytotoxic activity in these extracts may be due to the high amounts of GA II and PC.
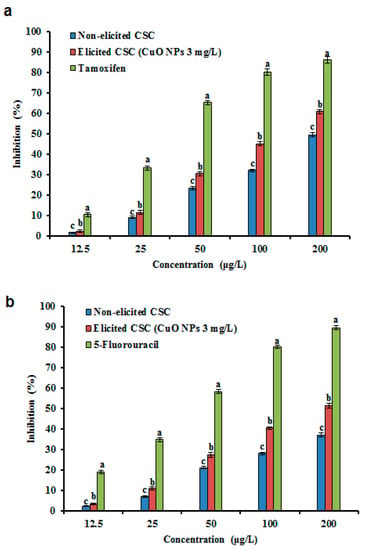
Figure 7.
Effect of CuO NPs on cell viability of MCF-7 cells (a) and HT-29 cells (b) in CSC of G. sylvestre. Different letters indicate a significant difference at p ≤ 0.05.
4. Conclusions
CSC of G. sylvestre has the potential for scale-up on a commercial level by pharmaceutical industries. Higher amounts of GA II and PC synthesis were observed in the CSC when a MS liquid medium with CuO NPs (3 mg/L) was used. Antioxidant, antidiabetic, anti-inflammatory, antibacterial, antifungal and anticancer activities were also increased in the 3 mg/L CuO NP-elicited CSC in comparison to the non-elicited CSC extracts. The results will inform forthcoming studies on the potential relation of these bioactive compounds to the plant abiotic stress response in G. sylvestre. Therefore, our protocol could be useful for the industrial production of GA II and PC and their use for pharmaceutical activities concerned with significant health benefits using a CSC of G. sylvestre.
Author Contributions
I.-M.C., Supervision, Investigation, Resources, G.R., Formal Analysis, Review and Editing, U.S., Methodology, Formal Analysis, B.V., Data Curation, Formal Analysis, M.T., Conceptualization, Methodology, Validation, Project Administration, Writing—Original Draft Preparation.
Funding
This paper was supported by the KU Research Professor Program of Konkuk University, Seoul, South Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pothuraju, R.; Sharma, R.K.; Chagalamarri, J.; Jangra, S.; Kumar Kavadi, P. A systematic review of Gymnema sylvestre in obesity and diabetes management. J. Sci. Food Agric. 2014, 94, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. BioMed Res. Int. 2014, 18, 830285. [Google Scholar]
- Nagella, P.; Thiruvengadam, M.; Jung, S.J.; Murthy, H.N.; Chung, I.M. Establishment of Gymnema sylvestre hairy root cultures for the production of gymnemic acid. Acta Physiol. Plant 2013, 35, 3067. [Google Scholar] [CrossRef]
- Praveen, N.; Thiruvengadam, M.; Yang, Y.S.; Kim, S.H.; Murthy, H.N.; Chung, I.M. Production of gymnemic acid from hairy root cultures of Gymnema sylvestre R. Br. as influenced by polyunsaturated fatty acids (PUFAs) and their antioxidant activity. Ind. Crop. Prod. 2014, 54, 54–61. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Weber, J.; Maciuk, A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Tahsili, J.; Sharifi, M.; Safaie, N.; Esmaeilzadeh-Bahabadi, S.; Behmanesh, M. Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. J. Plant Interact. 2014, 9, 412–417. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Ghanati, F. Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation. Plant Cell Tiss. Org. Cult. 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Rao, A.S.; Rao, M.V. In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate β-cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine 2010, 17, 1033–1039. [Google Scholar] [CrossRef]
- Veerashree, V.; Anuradha, C.M.; Kumar, V. Elicitor-enhanced production of gymnemic acid in cell suspension cultures of Gymnema sylvestre R. Br. Plant Cell Tiss. Org. Cult. 2012, 108, 27–35. [Google Scholar] [CrossRef]
- Bhuvaneswari, C.; Rao, K.; Gandi, S.; Giri, A. Abiotic elicitation of gymnemic acid in the suspension cultures of Gymnema sylvestre. World J. Microbiol. Biotechnol. 2012, 28, 741–747. [Google Scholar]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Trettel, J.R.; Gazim, Z.C.; Gonçalves, J.E.; Stracieri, J.; Magalhães, H.M. Effects of copper sulphate (CuSO4) elicitation on the chemical constitution of volatile compounds and the in vitro development of Basil. Sci. Horticul. 2018, 234, 19–26. [Google Scholar] [CrossRef]
- Sharma, M.; Ahuja, A.; Gupta, R.; Mallubhotla, S. Enhanced bacoside production in shoot cultures of Bacopa monnieri under the influence of abiotic elicitors. Nat. Prod. Res. 2015, 29, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Perotti, J.C.; Rodrigues, I.C.; Kleinowski, A.M.; Ribeiro, M.V.; Einhardt, A.M.; Peters, J.A.; Bacarin, M.A.; Braga, E.J.B. Betacyanin production in alligator weed, grown in vitro, with different concentrations of copper sulfate. Cienc. Rural. 2010, 40, 1874–1880. [Google Scholar] [CrossRef]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ. Pollut. 2018, 240, 802–816. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, G.; García-Castañeda, C.; Vázquez-Núñez, E.; Alonso-Castro, Á.J.; Basurto-Islas, G.; Mendoza, Á.; Cruz-Jiménez, G.; Molina, C. Physiological and biochemical response of plants to engineered NMs: Implications on future design. Plant Physiol. Biochem. 2017, 110, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.D.; Hossain, Z.; Afroz, H. Prospects and applications of nanobiotechnology: A medical perspective. J. Nanobiotechnol. 2012, 10, 1–8. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A Review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Minakova, A.S.; Anumol, T.; Keller, A.A. Quantitative analysis of changes in amino acids levels for cucumber (Cucumis sativus) exposed to nano copper. Nano Impact. 2018, 12, 9–17. [Google Scholar] [CrossRef]
- Juarez-Maldonado, A.; Ortega-Ortíz, H.; Pérez-Labrada, F.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Cu nanoparticles absorbed on chitosan hydrogels positively alter morphological, production, and quality characteristics of tomato. J. Appl. Bot. Food Qual. 2016, 89, 183–189. [Google Scholar]
- Pinedo-Guerrero, Z.H.; Hernández-Fuentes, A.D.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules 2017, 22, 926. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Talankova-Sereda, T.E.; Liapina, K.V.; Shkopinskij, E.A.; Ustinov, A.I.; Kovalyova, A.V.; Dulnev, P.G.; Kucenko, N.I. The influence of Cu and Co nanoparticles on growth characteristics and biochemical structure of Mentha longifolia in vitro. Nanosci. Nanoeng. 2016, 4, 31–39. [Google Scholar]
- Genady, E.A.; Qaid, E.A.; Fahmy, A.H. Copper sulfate nanoparticales in vitro applications on Verbena bipinnatifida Nutt. Stimulating growth and total phenolic content increasments. Int. J. Pharm. Res. Allied Sci. 2016, 5, 196–202. [Google Scholar]
- Genady, E.A.; Ahmed, S.S.; Fahmy, A.H.; Ashour, R.M. Copper sulfate nanoparticles enhance growth parameters, flavonoid content and antimicrobial activity of Ocimum basilicum Linnaeus. J. Am. Sci. 2017, 13, 108–114. [Google Scholar]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of bioactive compounds and gene expression alterations in hairy root cultures of Chinese cabbage elicited by copper oxide nanoparticles. Plant Cell Tiss. Org. Cult. 2018, 134, 95–106. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bao, J.S.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Saleem, B.; Islam, M.; Saeed, H.; Imtiaz, F.; Asghar, M.; Saleem, Z.; Mehmood, A.; Naheed, S. Investigations of Acacia modesta Wall. leaves for in vitro anti-diabetic, proliferative and cytotoxic effects. Braz. J. Pharm. Sci. 2018, 54, e17467. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashraf, M.; Ahmad, I.; Arshad, S.; Yar, M.; Latif, A. Anti-lipoxygenase activity of some indigenous medicinal plants. J. Med. Plants Res. 2013, 7, 219–222. [Google Scholar]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400. [Google Scholar] [CrossRef]
- Nagella, P.; Chung, I.M.; Murthy, H.N. In vitro production of gymnemic acid from cell suspension cultures of Gymnema Sylvestre R. Br. Eng. Life Sci. 2011, 11, 537–540. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rekha, K.; Rajakumar, G.; Lee, T.J.; Kim, S.H.; Chung, I.M. Enhanced production of anthraquinones and phenolic compounds and biological activities in the cell suspension cultures of Polygonum multiflorum. Int. J. Mol. Sci. 2016, 17, 1912. [Google Scholar] [CrossRef]
- Van Assche, F.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Tahiliani, S.; Kothari, S.L. Increased copper content of the medium improves plant regeneration from immature embryos derived callus of wheat (Triticum aestivum). J. Plant Biochem. Biotechnol. 2004, 13, 85–88. [Google Scholar] [CrossRef]
- Javed, R.; Mohamed, A.; Yücesan, B.; Gürel, E.; Kausar, R.; Zia, M. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana. Bertoni. Plant Cell Tiss. Org. Cult. 2017, 131, 611–620. [Google Scholar] [CrossRef]
- Bhuvaneswari, C.; Rao, K.; Gandi, S.; Giri, A. Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules-methyl jasmonate and salicylic acid. In Vitro Cell. Dev. Biol. Plant 2015, 51, 88–92. [Google Scholar]
- Zafar, H.; Ali, A.; Zia, M. CuO nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. Appl. Biochem. Biotechnol. 2017, 181, 365–378. [Google Scholar] [CrossRef]
- Nourozia, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Pharmaceutical important phenolic compounds overproduction and gene expression analysis in Dracocephalum kotschyi hairy roots elicited by SiO2 nanoparticles. Ind. Crop. Prod. 2019, 133, 435–446. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl. Microbiol. Biotechnol. 2010, 88, 437–449. [Google Scholar] [CrossRef]
- Večeřová, K.; Večeřa, Z.; Dočekal, B.; Oravec, M.; Pompeiano, A.; Tříska, J.; Urban, O. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. Pollut. 2016, 218, 207–218. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Venkidasamy, B.; Thiruvengadam, M. Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. rapa seedlings. Water Air Soil Pollut. 2019, 230, 48. [Google Scholar] [CrossRef]
- Janas, K.M.; Amarowicz, R.; Zielińska-Tomaszewska, J.; Kosińska, A.; Posmyk, M.M. Induction of phenolic compounds in two darkgrown lentil cultivars with different tolerance to copper ions. Acta Physiol. Plant. 2009, 31, 587–595. [Google Scholar] [CrossRef]
- Rachh, P.R.; Patel, S.R.; Hirpara, H.V.; Rupareliya, M.T.; Rachh, M.R.; Bhargava, A.S.; Patel, N.M.; Modi, D.C. In vitro evaluation of antioxidant activity of Gymnema sylvestre R. br. leaf extract. Rom. J. Biol. Plant Biol. 2009, 54, 141–148. [Google Scholar]
- Ruiz-Ruiz, J.C.; Matus-Basto, A.J.; Acereto-Escoffié, P.; Segura-Campos, M.R. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric. Immunol. 2018, 28, 61424–61437. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress - A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Singh, V.P. Role of Tamra bhasma, an Ayurvedic preparation, in the management of lipid peroxidation in liver of albino rats. Indian J. Exp. Biol. 1996, 34, 66–70. [Google Scholar] [PubMed]
- Nirmala, S.; Ravichandiran, V.; Vijayalakshmi, A. In vitro assay of alpha amylase inhibitory activity of gymnemic acid isolated from Gymnema Sylvestre leaves. Der Pharm. Lett. 2016, 8, 29–32. [Google Scholar]
- Shenoy, R.S.; Prashanth, K.V.H.; Manonmani, H.K. In vitro antidiabetic effects of isolated triterpene glycoside fraction from Gymnema sylvestre. Evid.-Based Complement. Alternat. Med. 2018, 7154702. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef] [PubMed]
- Ratnasari, N.; Walters, M.; Tsopmo, A. Antioxidant and lipoxygenase activities of polyphenol extracts from oat brans treated with polysaccharide degrading enzymes. Heliyon 2017, 3, e00351. [Google Scholar] [CrossRef]
- Umapathy, E.; Ndebia, E.J.; Meeme, A.; Adam, B.; Menziwa, P.; Nkeh-Chungag, B.N.; Iputo, J.E. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res. 2010, 4, 789–795. [Google Scholar]
- David, B.C.; Sudarsanam, G. Antimicrobial activity of Gymnema sylvestre (Asclepiadaceae). J. Acute Dis. 2013, 222–225. [Google Scholar] [CrossRef]
- Tusevski, B.; Vinterhalter, D.; Krstic Milosevic, M.; Sokovic, A.; Ciric, D.; Vinterhalter, S.; Zdravkovic Korac, J.; Petreska Stanoeva, M.; Stefova, S.; Simic, G. Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tiss. Org. Cult. 2017, 128, 589–605. [Google Scholar] [CrossRef]
- Saumendu, D.R.; Sarkar, K.; Dipankar, S.; Singh, T.; Prabha, B. In vitro antibiotic activity of various extracts of Gymnema sylvestre. Int. J. Pharm. Res. Dev. 2010, 2, 1–3. [Google Scholar]
- Ghanati, F.; Bakhtiarian, S.; Parast, B.M.; Behrooz, M.K. Production of new active phytocompounds by Achillea millefolium L. after elicitation with silver nanoparticles and methyl jasmonate. Biosci. Biotechnol. Res. Asia 2014, 11, 391–399. [Google Scholar] [CrossRef]
- Khanna, V.G.; Kannabiran, K. Anticancer-cytotoxic activity of saponins isolated from the leaves of Gymnema sylvestre and Eclipta prostrata on HeLa cells. Int. J. Green Pharm. 2009, 3, 227–229. [Google Scholar]
- Srikanth, A.V.; Maricar, S.; Lakshmi, M.N.; Ravi Kumar, P.; Madhava Reddy, B. Anticancer activity of Gymnema Sylv. R. Br. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 2–4. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).