Early Life Adversity Alters the Developmental Profiles of Addiction-Related Prefrontal Cortex Circuitry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experiment 1: Effects of Early Life Stress on Overall DA Receptors and Glutamatergic Projection Neurons in the plPFC across Development
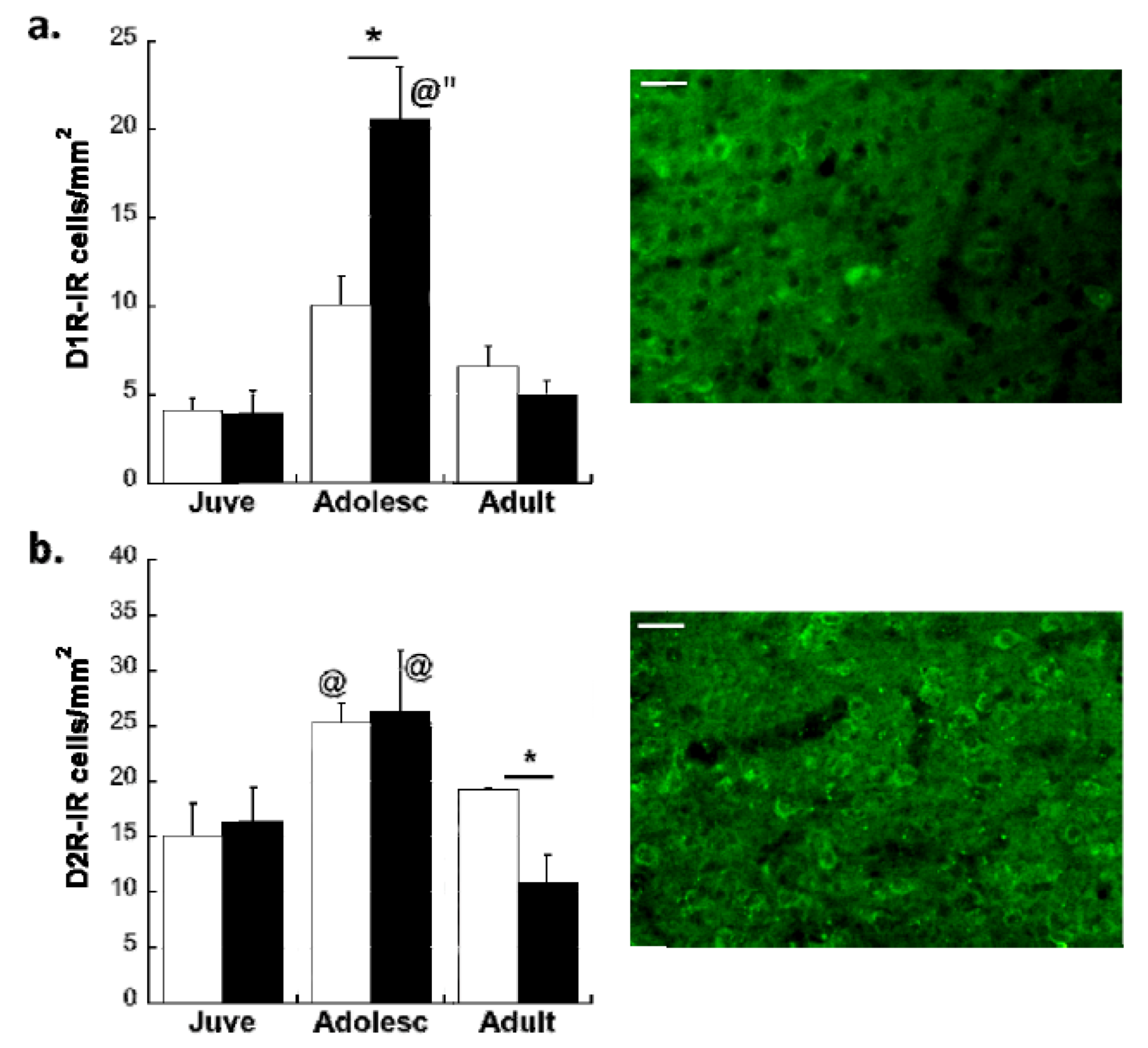
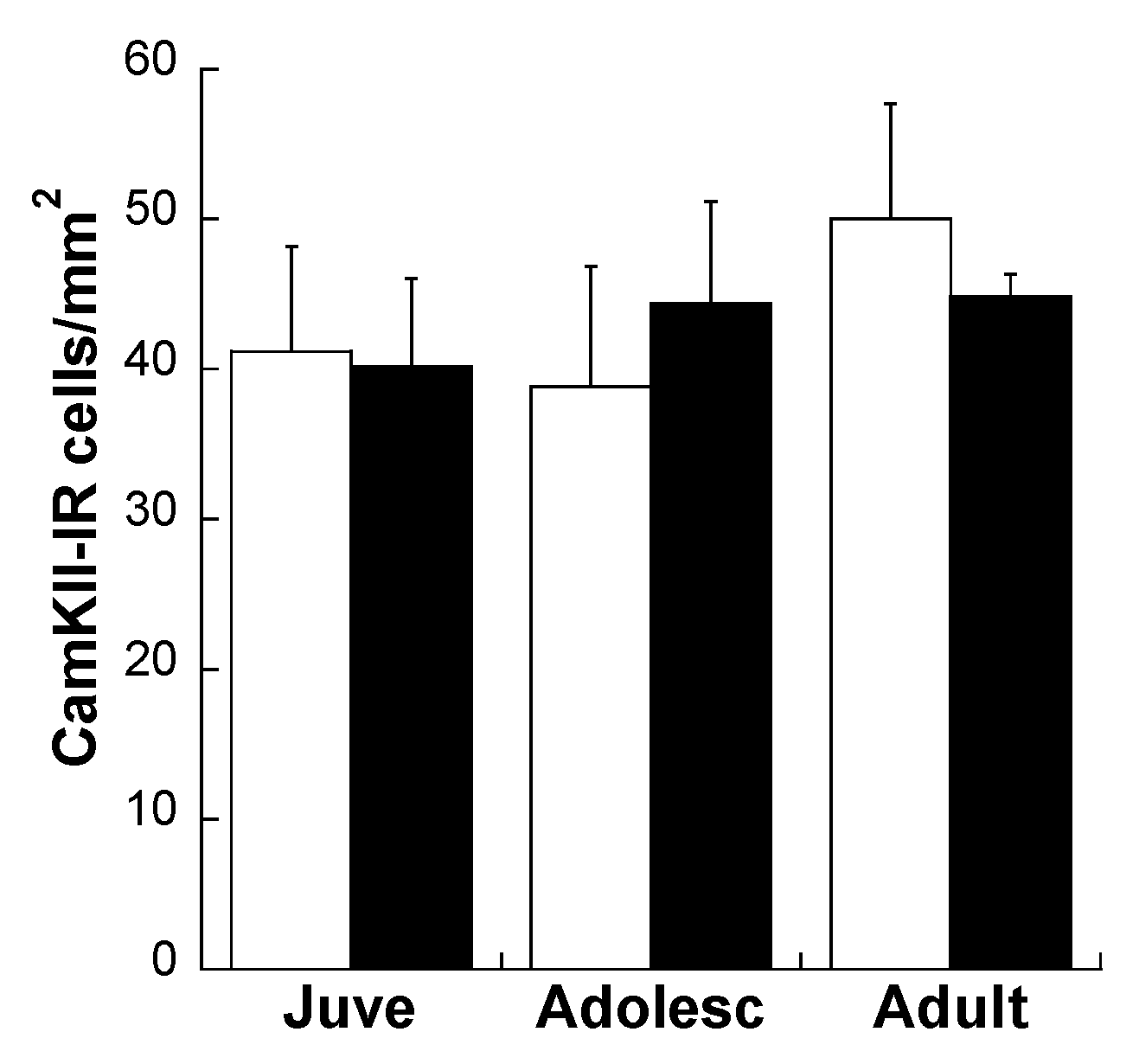
Effects of Age and MS on D1R and D2R Dopamine Expression Distribution on All Glutamatergic Projection Neurons in the plPFC

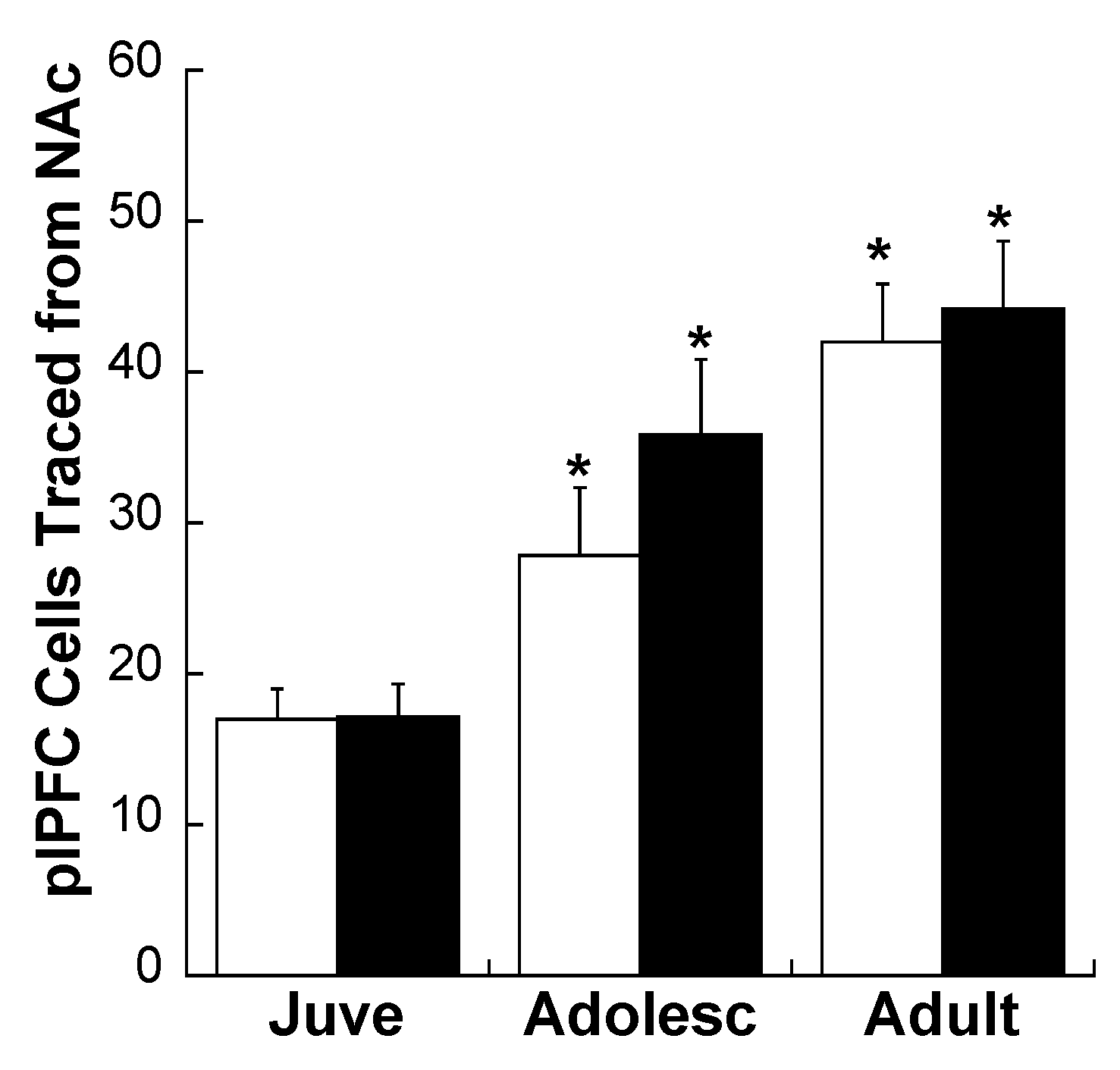
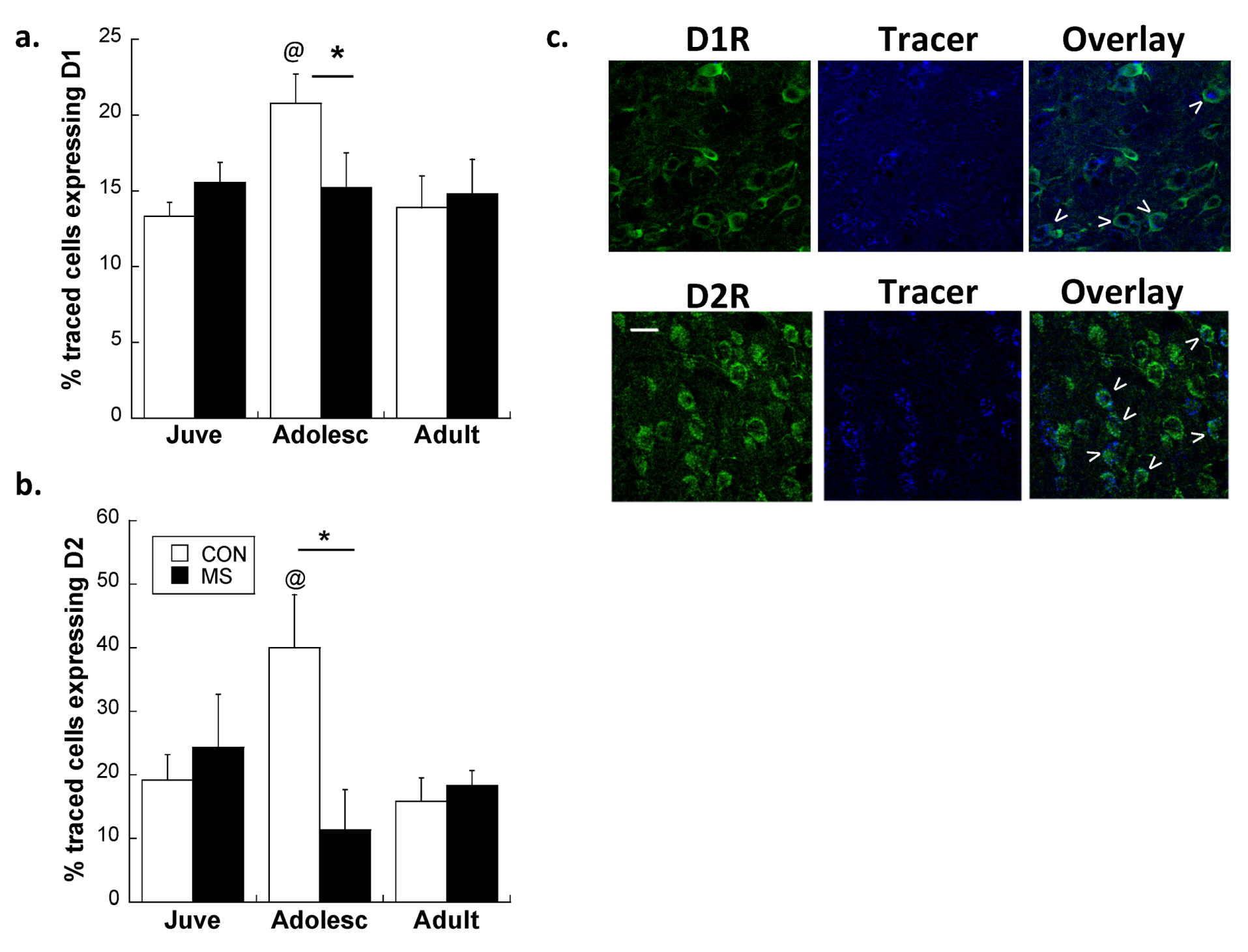
2.2. Experiment 2: Effects of Early Life Stress on D1R and D2R Distribution Specifically on plPFC→NAc Neurons across Development
3. Experimental Section
3.1. Subjects
3.2. Experiment 1: Effects of Early Life Stress on DA Receptors and Glutamatergic Projection Neurons in the plPFC across Development
Double-Label Immunofluorescence
3.3. Experiment 2: Effects of Early Life Stress on D1R and D2R Distribution Specifically on plPFC→NAc Neurons across Development
3.3.1. Retrograde Tracer Microinjections
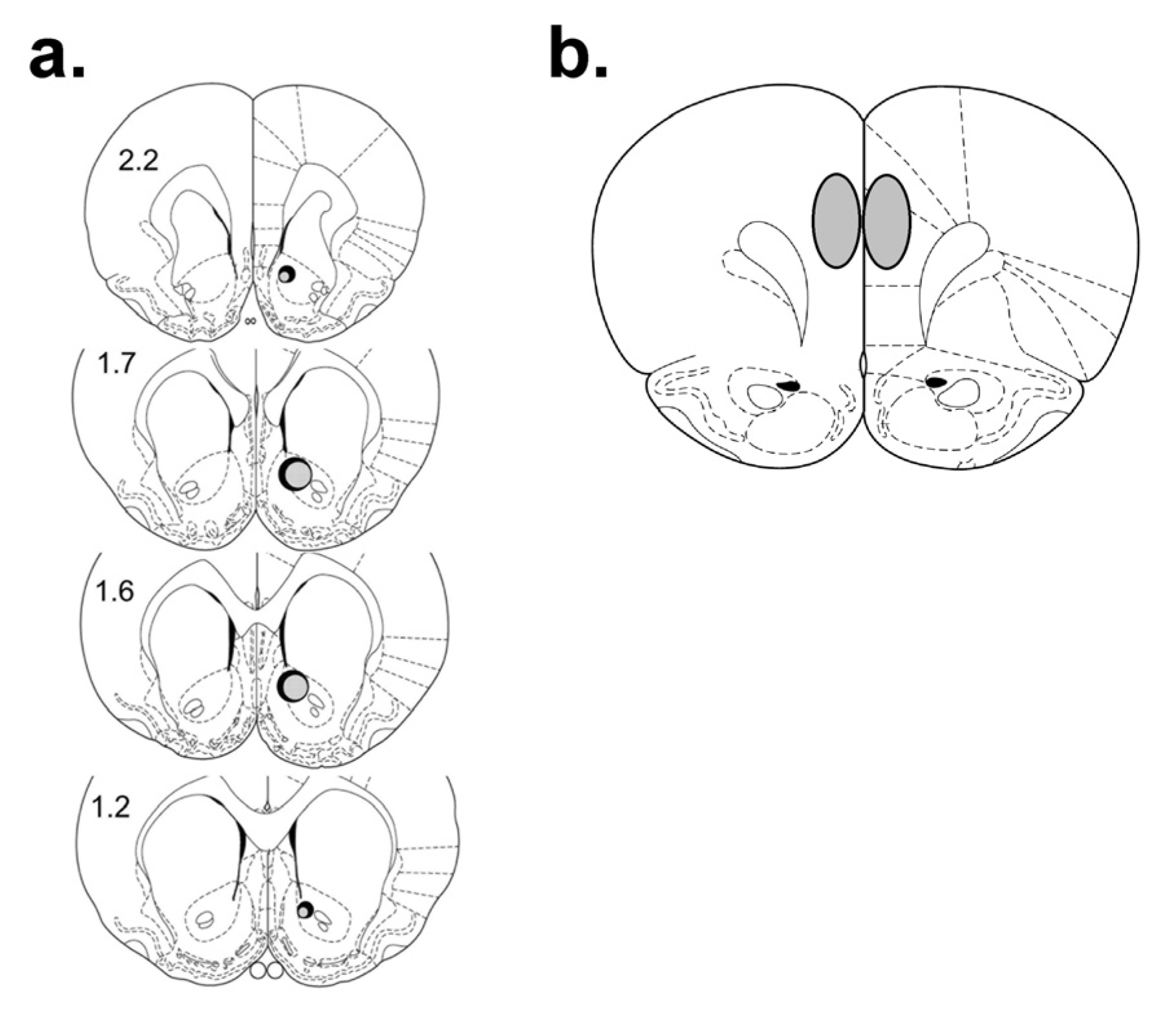
3.3.2. D1 and D2 Fluorescence Immunohistochemistry
3.3.3. Confocal Microscopy and Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Agid, O.; Shapira, B.; Zislin, J.; Ritsner, M.; Hanin, B.; Murad, H.; Troudart, T.; Bloch, M.; Heresco-Levy, U.; Lerer, B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol. Psychiatry 1999, 4, 163–172. [Google Scholar]
- Kessler, R.; Davis, C.; Kendler, K. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol. Med. 1997, 27, 1101–1119. [Google Scholar] [CrossRef]
- Kessler, R.; Avenevoli, S.; Ries Merikangas, K. Mood disorders in children and adolescents: An epidemiologic perspective. Biol. Psychiatry 2001, 49, 1002–1014. [Google Scholar] [CrossRef]
- Davey, C.; Yucel, M.; Allen, N. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci. Biobehav. Rev. 2008, 32, 1–19. [Google Scholar]
- Andersen, S.L.; Teicher, M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008, 31, 183–191. [Google Scholar] [CrossRef]
- Lehmann, J.; Feldon, J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Rev. Neurosci. 2000, 11, 383–408. [Google Scholar] [CrossRef]
- Brenhouse, H.C.; Andersen, S.L. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol. Psychiatry 2011, 70, 434–440. [Google Scholar] [CrossRef]
- Chocyk, A.; Dudys, D.; Przyborowska, A.; Mackowiak, M.; Wedzony, K. Impact of maternal separation on neural cell adhesion molecules expression in dopaminergic brain regions of juvenile, adolescent and adult rats. Pharmacol. Rep. 2010, 62, 1218–1224. [Google Scholar]
- Jahng, J.W.; Ryu, V.; Yoo, S.B.; Noh, S.J.; Kim, J.Y.; Lee, J.H. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience 2010, 171, 144–152. [Google Scholar] [CrossRef]
- Macri, S.; Laviola, G.; Leussis, M.P.; Andersen, S.L. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology 2010, 35, 392–402. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A.; Polcari, A.; Andersen, S.L. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: A retrospective clinical report. J. Clin. Psychiatry 2009, 70, 684–691. [Google Scholar]
- Cruz, F.C.; Quadros, I.M.; Planeta Cda, S.; Miczek, K.A. Maternal separation stress in male mice: Long-term increases in alcohol intake. Psychopharmacology 2008, 201, 459–468. [Google Scholar] [CrossRef]
- Moffett, M.C.; Vicentic, A.; Kozel, M.; Plotsky, P.; Francis, D.D.; Kuhar, M.J. Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol. 2007, 73, 321–330. [Google Scholar]
- Teicher, M.H.; Tomoda, A.; Andersen, S.L. Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Ann. N. Y. Acad. Sci. 2006, 1071, 313–323. [Google Scholar] [CrossRef]
- Kohut, S.; Roma, P.; Davis, C.; Zernig, G.; Saria, A.; Dominguez, J.; Rice, K.; Riley, A. The impact of early environmental rearing condition on the discriminative stimulus effects and fos expression induced by cocaine in adult male and female rats. Psychopharmacology 2009, 203, 383–397. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction 2000, 95 (Suppl. 2), S91–S117. [Google Scholar]
- Robinson, T.E.; Berridge, K.C. Review. The incentive sensitization theory of addiction: Some current issues. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3137–3146. [Google Scholar] [CrossRef]
- Brenhouse, H.C.; Sonntag, K.C.; Andersen, S.L. Transient d1 dopamine receptor expression on prefrontal cortex projection neurons: Relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008, 28, 2375–2382. [Google Scholar]
- Andersen, S.L.; Teicher, M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 2004, 29, 1988–1993. [Google Scholar] [CrossRef]
- Ladd, C.O.; Huot, R.L.; Thrivikraman, K.V.; Nemeroff, C.B.; Meaney, M.J.; Plotsky, P.M. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000, 122, 81–103. [Google Scholar]
- Meaney, M.J.; Diorio, J.; Francis, D.; Widdowson, J.; LaPlante, P.; Caldji, C.; Sharma, S.; Seckl, J.R.; Plotsky, P.M. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev. Neurosci. 1996, 18, 49–72. [Google Scholar] [CrossRef]
- Kosten, T.A.; Lee, H.J.; Kim, J.J. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006, 1087, 142–150. [Google Scholar]
- Heim, C.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 2008, 33, 693–710. [Google Scholar] [CrossRef]
- Heidbreder, C.; Weiss, I.; Domeny, A.; Pryce, C.; Homberg, J.; Feldon, J.; Moran, M.; Nelson, P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrom. Neuroscience 2000, 100, 749–768. [Google Scholar] [CrossRef]
- Stevenson, C.W.; Marsden, C.A.; Mason, R. Early life stress causes FG-7142-induced corticolimbic dysfunction in adulthood. Brain Res. 2008, 1193, 43–50. [Google Scholar] [CrossRef]
- Helmeke, C.; Ovtscharoff, W.J.; Poeggel, G.; Braun, K. Imbalance of immunohistochemically characterized interneuron populationsn in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience 2008, 152, 18–28. [Google Scholar] [CrossRef]
- Alexander, G.E.; Goldman, P.S. Functional development of the dorsolateral prefrontal cortex: An analysis utlizing reversible cryogenic depression. Brain Res. 1978, 143, 233–249. [Google Scholar] [CrossRef]
- Leussis, M.P.; Andersen, S.L. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 2008, 62, 22–30. [Google Scholar] [CrossRef]
- Radley, J.J.; Sisti, H.M.; Hao, J.; Rocher, A.B.; McCall, T.; Hof, P.R.; McEwen, B.S.; Morrison, J.H. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2004, 125, 1–6. [Google Scholar]
- Kikusui, T.; Mori, Y. Behavioral and neurochemical consequences of early weaning in rodents. J. Neuroendocrinol. 2009, 21, 427–431. [Google Scholar]
- Hains, A.B.; Arnsten, A.F. Molecular mechanisms of stress-induced prefrontal cortical impairment: Implications for mental illness. Learn. Mem. 2008, 15, 551–564. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Voorn, P.; Buijs, R.M.; Pool, C.W.; Uylings, H.B. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J. Comp. Neurol. 1988, 269, 58–72. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Volkow, N.; Seamans, J. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron 2005, 45, 647–650. [Google Scholar] [CrossRef]
- Blum, K.; Braverman, E.R.; Holder, J.M.; Lubar, J.F.; Monastra, V.J.; Miller, D.; Lubar, J.O.; Chen, T.J.; Comings, D.E. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs 2000, 32 (Suppl. i–iv), 1–112. [Google Scholar] [CrossRef]
- Bjork, J.M.; Knutson, B.; Fong, G.W.; Caggiano, D.M.; Bennett, S.M.; Hommer, D.W. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. J. Neurosci. 2004, 24, 1793–1802. [Google Scholar]
- Matthews, S.C.; Simmons, A.N.; Lane, S.D.; Paulus, M.P. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport 2004, 15, 2123–2127. [Google Scholar] [CrossRef]
- Piazza, P.V.; Rouge-Pont, F.; Deminiere, J.M.; Kharoubi, M.; Le Moal, M.; Simon, H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991, 567, 169–174. [Google Scholar]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Pierce, R.C.; Cornish, J.; Sorg, B.A. A role for sensitization in craving and relapse in cocaine addiction. J. Psychopharmacol. 1998, 12, 49–53. [Google Scholar]
- Crews, F.; He, J.; Hodge, C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007, 86, 189–199. [Google Scholar] [CrossRef]
- Tseng, K.Y.; O’Donnell, P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb. Cortex 2007, 17, 1235–1240. [Google Scholar]
- Andersen, S.L.; Thompson, A.T.; Rutstein, M.; Hostetter, J.C.; Teicher, M.H. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 2000, 37, 167–169. [Google Scholar] [CrossRef]
- Andersen, S. Changes in the second messenger cyclic amp during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD). Behav. Brain Res. 2002, 130, 197–201. [Google Scholar]
- Alleweireldt, A.T.; Weber, S.M.; Kirschner, K.F.; Bullock, B.L.; Neisewander, J.L. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 2002, 159, 284–293. [Google Scholar]
- Sanchez, C.J.; Bailie, T.M.; Wu, W.R.; Li, N.; Sorg, B.A. Manipulation of dopamine D1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience 2003, 119, 497–505. [Google Scholar] [CrossRef]
- Brenhouse, H.C.; Dumais, K.; Andersen, S.L. Enhancing the salience of dullness: Behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience 2010, 169, 628–636. [Google Scholar] [CrossRef]
- Everitt, B.J.; Wolf, M.E. Psychomotor stimulant addiction: A neural systems perspective. J. Neurosci. 2002, 22, 3312–3320. [Google Scholar]
- Seamans, J.K.; Yang, C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004, 74, 1–58. [Google Scholar]
- Wright, L.D.; Hebert, K.E.; Perrot-Sinal, T.S. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology 2008, 33, 130–142. [Google Scholar] [CrossRef]
- Muhammad, A.; Carroll, C.; Kolb, B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 2012, 216, 103–109. [Google Scholar] [CrossRef]
- See, R.E. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int. J. Neuropsychopharmacol. 2009, 12, 431–436. [Google Scholar] [CrossRef]
- Tseng, K.Y.; O’Donnell, P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse 2007, 61, 843–850. [Google Scholar]
- Kalivas, P.W.; Duffy, P. Dopamine regulation of extracellular glutamate in the nucleus accumbens. Brain Res. 1997, 761, 173–177. [Google Scholar] [CrossRef]
- Tseng, K.Y.; O’Donnell, P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J. Neurosci. 2004, 24, 5131–5139. [Google Scholar]
- Lambe, E.K.; Krimer, L.S.; Goldman-Rakic, P.S. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J. Neurosci. 2000, 20, 8780–8787. [Google Scholar]
- Bordelon-Glausier, J.R.; Khan, Z.U.; Muly, E.C. Quantification of d1 and d5 dopamine receptor localization in layers i, iii, and v of macaca mulatta prefrontal cortical area 9: Coexpression in dendritic spines and axon terminals. J. Comp. Neurol. 2008, 508, 893–905. [Google Scholar] [CrossRef]
- St Onge, J.R.; Stopper, C.M.; Zahm, D.S.; Floresco, S.B. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J. Neurosci. 2012, 32, 2886–2899. [Google Scholar]
- Gladwin, T.E.; Figner, B.; Crone, E.A.; Wiers, R.W. Addiction, adolescence, and the integration of control and motivation. Dev. Cogn. Neurosci. 2011, 1, 364–376. [Google Scholar] [CrossRef]
- Schneider, S.; Peters, J.; Bromberg, U.; Brassen, S.; Miedl, S.F.; Banaschewski, T.; Barker, G.J.; Conrod, P.; Flor, H.; Garavan, H.; et al. Risk taking and the adolescent reward system: A potential common link to substance abuse. Am. J. Psychiatry 2012, 169, 39–46. [Google Scholar]
- Briand, L.A.; Flagel, S.B.; Garcia-Fuster, M.J.; Watson, S.J.; Akil, H.; Sarter, M.; Robinson, T.E. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 2008, 33, 2969–2980. [Google Scholar] [CrossRef]
- Andersen, S.L.; Lyss, P.J.; Dumont, N.L.; Teicher, M.H. Enduring neurochemical effects of early maternal separation on limbic structures. Ann. N. Y. Acad. Sci. 1999, 877, 756–759. [Google Scholar] [CrossRef]
- Plotsky, P.M.; Meaney, M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 1993, 18, 195–200. [Google Scholar] [CrossRef]
- Spear, L.P.; File, S.E. Methodological considerations in neurobehavioral teratology. Pharmacol. Biochem. Behav. 1996, 55, 455–457. [Google Scholar]
- Sherwood, N.; Timeras, P. A Stereotaxic Atlas of the Developing Rat Brain; University of California Press: Los Angeles, CA, USA, 1970. [Google Scholar]
- Cavalieri, B. Geometria degli Indivisibili; Unione Tipografico-Editrice Torinese: Torino, Italy, 1966. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Katz, L.C.; Burkhalter, A.; Dreyer, W.J. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature 1984, 310, 498–500. [Google Scholar] [CrossRef]
- Spear, L. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Laviola, G.; Macri, S.; Morley-Fletcher, S.; Adriani, W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003, 27, 19–32. [Google Scholar] [CrossRef]
- O’Brien, M.S.; Anthony, J.C. Risk of becoming cocaine dependent: Epidemiological estimates for the united states, 2000–2001. Neuropsychopharmacology 2005, 30, 1006–1018. [Google Scholar]
- Dube, S.R.; Felitti, V.J.; Dong, M.; Chapman, D.P.; Giles, W.H.; Anda, R.F. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics 2003, 111, 564–572. [Google Scholar] [CrossRef]
- Andersen, S.L.; Teicher, M.H. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev. 2009, 33, 516–524. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brenhouse, H.C.; Lukkes, J.L.; Andersen, S.L. Early Life Adversity Alters the Developmental Profiles of Addiction-Related Prefrontal Cortex Circuitry. Brain Sci. 2013, 3, 143-158. https://doi.org/10.3390/brainsci3010143
Brenhouse HC, Lukkes JL, Andersen SL. Early Life Adversity Alters the Developmental Profiles of Addiction-Related Prefrontal Cortex Circuitry. Brain Sciences. 2013; 3(1):143-158. https://doi.org/10.3390/brainsci3010143
Chicago/Turabian StyleBrenhouse, Heather C., Jodi L. Lukkes, and Susan L. Andersen. 2013. "Early Life Adversity Alters the Developmental Profiles of Addiction-Related Prefrontal Cortex Circuitry" Brain Sciences 3, no. 1: 143-158. https://doi.org/10.3390/brainsci3010143



