Ginger Polyphenols Reverse Molecular Signature of Amygdala Neuroimmune Signaling and Modulate Microbiome in Male Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Neuropathic Pain Induction
2.3. Animal Treatments
2.4. Assessment of Pain-Related Behavior in Live Animals
2.5. Sample Collection
2.6. RNA Isolation and Gene Expression Profiling Using Neuroinflammation Panel
2.7. RNA Isolation and qRT-PCR
2.8. Gut Microbiota Profiling via 16S rRNA Amplicon Sequencing
2.9. Statistical Analysis
3. Results
3.1. GEG Alleviates Mechanical Hypersensitivity in NP Rats
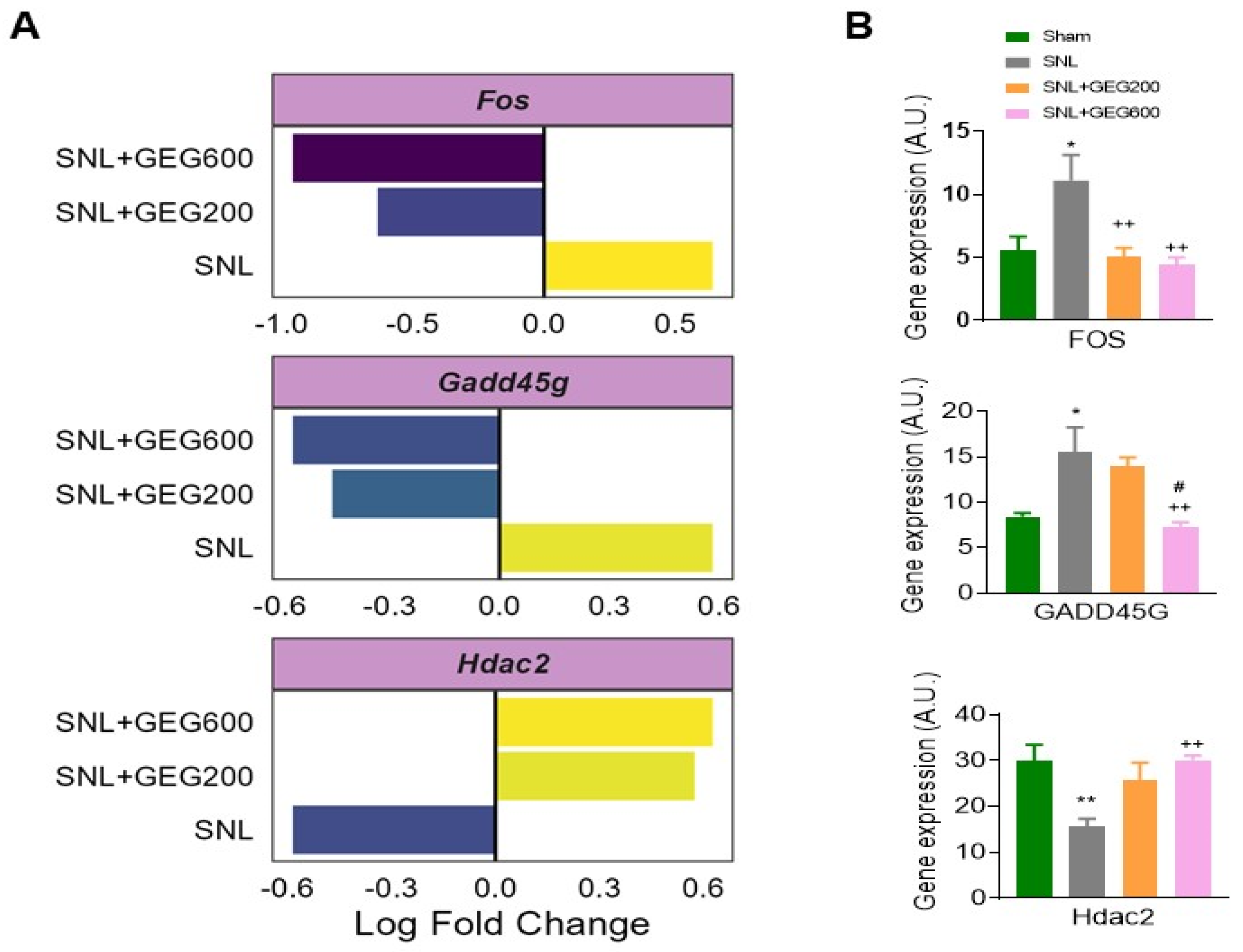
3.2. GEG Reverses the Expression of Neuroinflammatory Markers Associated with NP
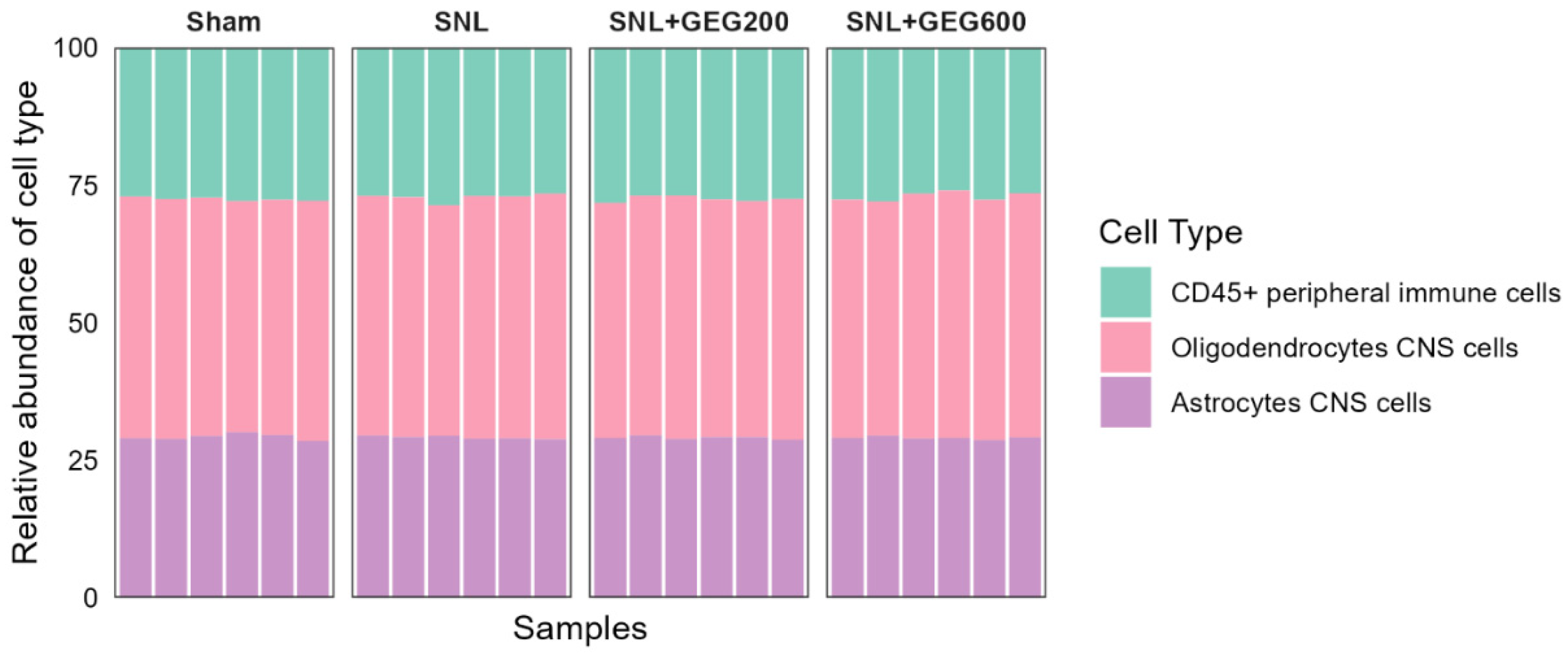
3.3. GEG-Associated Gut Microbiome Changes
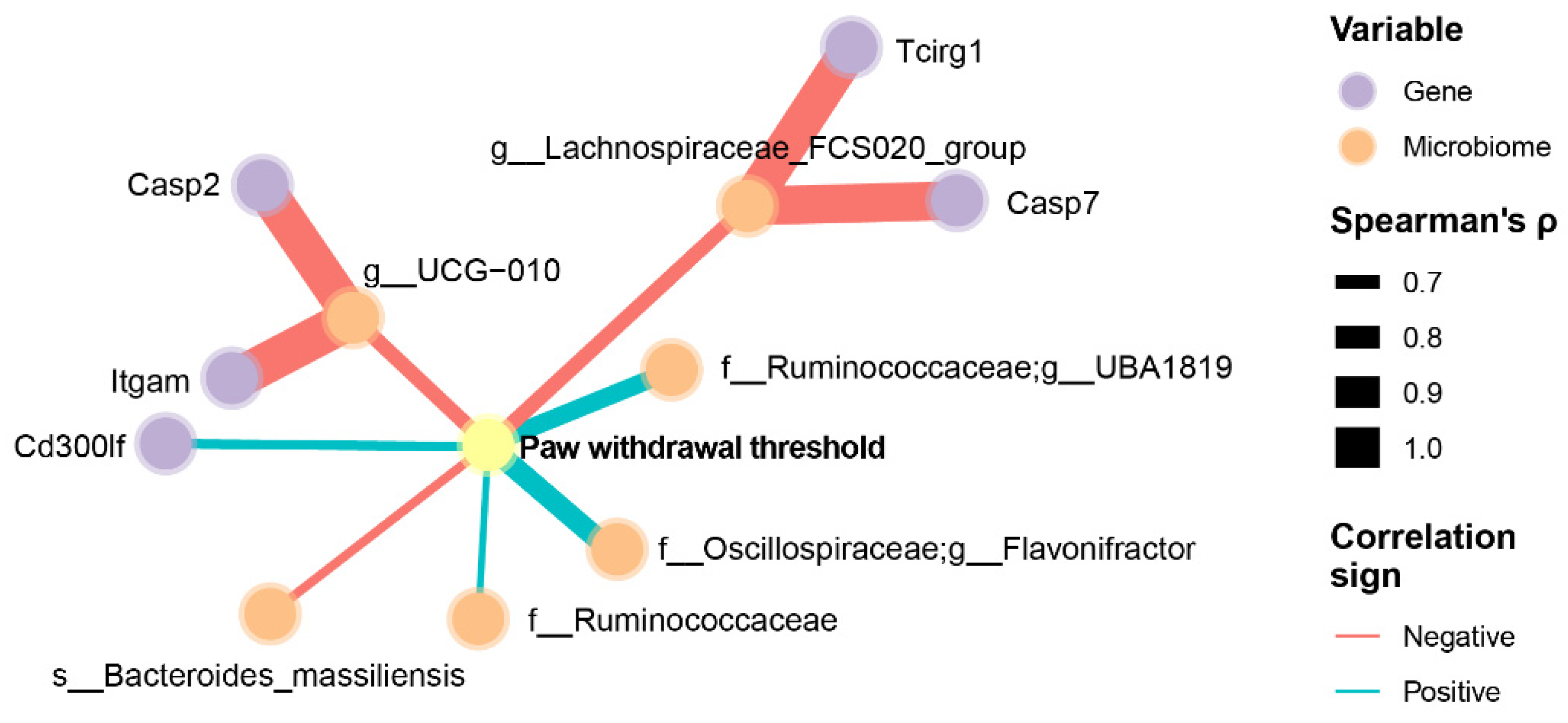
3.4. Integrated Analysis of Pain, Neuroinflammatory Markers, and the Gut Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Yakhnitsa, V.; Kiritoshi, T.; Presto, P.; Neugebauer, V. Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats. Mol. Pain 2018, 14, 1744806918804441. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhou, Z.; Liang, Y.; Cheng, X.; Li, Y.; Teng, W.; Zhao, M.; Liu, C.; Guan, M.; Zhao, C. Targeting strategies for chemotherapy-induced peripheral neuropathy: Does gut microbiota play a role? Crit. Rev. Microbiol. 2019, 45, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Han, W.; Lamine, F.; Eutamene, H.; Fioramonti, J.; Bueno, L.; Theodorou, V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: A possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006, 55, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A novel regulator of pain. J. Neural Transm. 2020, 127, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Hall, J.C.; Wang, L.; Mo, X.; Yu, Z.; Popovich, P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 2016, 213, 2603–2620. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Mostacada, K.; Popovich, P.G. Gut Microbiota Are Disease-Modifying Factors After Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Boccella, S.; Guida, F.; Franzese, M.; Maione, S.; Salvatore, M. Role of gut microbiota in neuropathy and neuropathic pain states: A systematic preclinical review. Neurobiol. Dis. 2020, 170, 105773. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef] [PubMed]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Darcsi, A.; Kery, A.; Riethmuller, E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, R.; Ji, G.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.; Mirzaei, P.; Sang, S.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, R.; Yakhnitsa, V.; Santos, J.M.; Watson, C.; Kiritoshi, T.; Ji, G.; Hamood, A.N.; Neugebauer, V. Gingerol-Enriched Ginger Supplementation Mitigates Neuropathic Pain via Mitigating Intestinal Permeability and Neuroinflammation: Gut-Brain Connection. Front. Pharmacol. 2022, 13, 912609. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V. Amygdala physiology in pain. Handb. Behav. Neurosci. 2020, 26, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Presto, P.; Ji, G.; Ponomareva, O.; Ponomarev, I.; Neugebauer, V. Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 11944. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.S.M.; Hoban, A.E.; Ventura-Silva, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. Bioessays 2018, 40, 1700172. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Kim, H.K.; Chung, K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol. Med. 2004, 99, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Chung, J.M.; Honore, M.; Seltzer, Z. Models of neuropathic pain in the rat. Curr. Protoc. Pharmacol. 2003, 21, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, W.; Mahimainathan, L.; Narasimhan, M.; Kiritoshi, T.; Fan, X.; Wang, J.; Green, T.A.; Neugebauer, V. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci. 2017, 37, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; McGrath, K.C.; Nammi, S.; Heather, A.K.; Roufogalis, B.D. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin. Pharmacol. Toxicol. 2012, 110, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.F.; Abdallah, H.M.I.; Ibrahim, B.M.M.; Hegazy, R.R.; Esmail, R.S.E.; Abdel-Salam, L.O. The Carcinogenic Agent Diethylnitrosamine Induces Early Oxidative Stress, Inflammation and Proliferation in Rat Liver, Stomach and Colon: Protective Effect of Ginger Extract. Asian Pac. J. Cancer Prev. 2019, 20, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.N.; Bobnar, H.J.; Kolber, B.J. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol. 2021, 196, 101891. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, Y.; Gereau, R.W.T. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol. Pain 2008, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Neugebauer, V. Hemispheric lateralization of pain processing by amygdala neurons. J. Neurophysiol. 2009, 102, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.R.; Dawes, J.M.; McMahon, S.B.; Bennett, D.L.; Orengo, C.; Kohl, M. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genom. 2012, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; He, X.T.; Zhou, K.X.; Zhang, C.; Zhao, W.J.; Zhang, T.; Li, J.L.; Deng, J.P.; Dong, Y.L. The analgesic effects of triptolide in the bone cancer pain rats via inhibiting the upregulation of HDACs in spinal glial cells. J. Neuroinflamm. 2017, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale Roscoe Rhizomes Attenuate Oxaliplatin-Induced Neuropathic Pain in Mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Belluscio, L.M.; Alberca, C.D.; Pregi, N.; Canepa, E.T. Altered gene expression in hippocampus and depressive-like behavior in young adult female mice by early protein malnutrition. Genes Brain Behav. 2016, 15, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Morland, R.H.; Novejarque, A.; Spicer, C.; Pheby, T.; Rice, A.S. Enhanced c-Fos expression in the central amygdala correlates with increased thigmotaxis in rats with peripheral nerve injury. Eur. J. Pain. 2016, 20, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Watanabe, Y.; Ikeda, T.; Abe, H.; Ebihara, K.; Matsuo, H.; Nonaka, H.; Hashiguchi, H.; Nishimori, T.; Ishida, Y. Analgesic effect of milnacipran is associated with c-Fos expression in the anterior cingulate cortex in the rat neuropathic pain model. Neurosci. Res. 2009, 64, 380–384. [Google Scholar] [CrossRef]
- Yao, P.W.; Wang, S.K.; Chen, S.X.; Xin, W.J.; Liu, X.G.; Zang, Y. Upregulation of tumor necrosis factor-alpha in the anterior cingulate cortex contributes to neuropathic pain and pain-associated aversion. Neurobiol. Dis. 2019, 130, 104456. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, P.; Fan, S.; Zhai, B.; Li, S.; Li, H.; Zhang, Y.; Li, W.; Sun, G.; Han, R.; et al. miR-30a-3p can inhibit the proliferation and promote the differentiation of chicken primary myoblasts. Br. Poult. Sci. 2022, 63, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S.K.; Balliet, A.G.; Hollander, M.C.; Fornace, A.J.; Hoffman, B.; Liebermann, D.A. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene 2005, 24, 7170–7179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Gupta, M.; Hoffman, B.; Liebermann, D.A. Hematopoietic cells from gadd45a-deficient and gadd45b-deficient mice exhibit impaired stress responses to acute stimulation with cytokines, myeloablation and inflammation. Oncogene 2006, 25, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, D.A.; Hoffman, B. Gadd45 in stress signaling. J. Mol. Signal. 2008, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Cretu, A.; Sha, X.; Tront, J.; Hoffman, B.; Liebermann, D.A. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009, 7, 268–276. [Google Scholar] [PubMed]
- Kawaguchi, K.; Akeda, K.; Yamada, J.; Hasegawa, T.; Takegami, N.; Fujiwara, T.; Sudo, A. Expression of GADD45G and CAPRIN1 in Human Nucleus Pulposus: Implications for Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 5768. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, A.; Akeda, K.; Takebayashi, S.I.; Shimaoka, M.; Okumura, K.; Sudo, A. Genome-wide analysis of DNA methylation profile identifies differentially methylated loci associated with human intervertebral disc degeneration. PLoS ONE 2019, 14, e0222188. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Schafer, A.; Marhold, J.; Stach, D.; Swaminathan, S.K.; Handa, V.; Doderlein, G.; Maltry, N.; Wu, W.; Lyko, F.; et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007, 445, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Guo, J.U.; Ming, G.L.; Song, H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle 2009, 8, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Vairapandi, M.; Balliet, A.G.; Hoffman, B.; Liebermann, D.A. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J. Cell Physiol. 2002, 192, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 1996, 274, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Hsieh, M.C.; Ho, Y.C.; Lee, A.S.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Peng, H.Y. Growth Arrest and DNA-damage-inducible Protein 45beta-mediated DNA Demethylation of Voltage-dependent T-type Calcium Channel 3.2 Subunit Enhances Neuropathic Allodynia after Nerve Injury in Rats. Anesthesiology 2017, 126, 1077–1095. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Liu, D.; Du, J.; Guo, Y.; Deng, Y.; Hei, Z.; Li, X. Early molecular alterations in anterior cingulate cortex and hippocampus in a rodent model of neuropathic pain. Brain Res. Bull. 2021, 166, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Geranton, S.M.; Tochiki, K.K. Regulation of gene expression and pain states by epigenetic mechanisms. Prog. Mol. Biol. Transl. Sci. 2015, 131, 147–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, S.R.; Zhou, M.H.; Jin, D.; Chen, H.; Wang, L.; DePinho, R.A.; Pan, H.L. HDAC2 in Primary Sensory Neurons Constitutively Restrains Chronic Pain by Repressing alpha2delta-1 Expression and Associated NMDA Receptor Activity. J. Neurosci. 2022, 42, 8918–8935. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yamamura, S.; Essilfie-Quaye, S.; Cosio, B.; Ito, M.; Barnes, P.J.; Adcock, I.M. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J. Exp. Med. 2006, 203, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Y.; Ren, X.; Rong, L.; Huang, M.; Cao, J.; Zang, W. HDAC2, but not HDAC1, regulates Kv1.2 expression to mediate neuropathic pain in CCI rats. Neuroscience 2019, 408, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Maiaru, M.; Morgan, O.B.; Tochiki, K.K.; Hobbiger, E.J.; Rajani, K.; Overington, D.W.; Geranton, S.M. Complex regulation of the regulator of synaptic plasticity histone deacetylase 2 in the rodent dorsal horn after peripheral injury. J. Neurochem. 2016, 138, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhou, X.; Ji, T.; Chen, G. NF-kappaB p65-dependent transcriptional regulation of histone deacetylase 2 contributes to the chronic constriction injury-induced neuropathic pain via the microRNA-183/TXNIP/NLRP3 axis. J. Neuroinflamm. 2020, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Chen, D.; Hou, X.; Wang, T.; Wang, J.; Zou, W.; Song, Z.; Huang, C.; Guo, Q.; Weng, Y. Normalizing HDAC2 Levels in the Spinal Cord Alleviates Thermal and Mechanical Hyperalgesia After Peripheral Nerve Injury and Promotes GAD65 and KCC2 Expression. Front. Neurosci. 2019, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Pryce, K.D.; Serafini, R.A.; Ramakrishnan, A.; Nicolais, A.; Giosan, I.M.; Polizu, C.; Torres-Berrio, A.; Vuppala, S.; Kronman, H.; Ruiz, A.; et al. Oxycodone withdrawal induces HDAC1/HDAC2-dependent transcriptional maladaptations in the reward pathway in a mouse model of peripheral nerve injury. Nat. Neurosci. 2023, 26, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, C.; Wan, Y.; Yan, H.; Li, T. Effect of HDAC2/Inpp5f on neuropathic pain and cognitive function through regulating PI3K/Akt/GSK-3beta signal pathway in rats with neuropathic pain. Exp. Ther. Med. 2019, 18, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Shi, X.; Tang, Y.; Yan, Y.; Chen, L.; Chen, Y.; Gao, G.; Lin, C.; Chen, A. Contribution of Amygdala Histone Acetylation in Early Life Stress-Induced Visceral Hypersensitivity and Emotional Comorbidity. Front. Neurosci. 2022, 16, 843396. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Shao, A.; Tang, X.; Feng, M.; Wang, J.; Qiu, Y. MiR-34a affects dexmedetomidine-inhibited chronic inflammatory visceral pain by targeting to HDAC2. BMC Anesthesiol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Wang, J.; Wei, Y.Y.; Zhang, T.; Zhang, Y.; Zuo, Z.F.; Teng, X.Y.; Li, Y.Q. Histone deacetylase 2 is involved in micro-opioid receptor suppression in the spinal dorsal horn in a rat model of chronic pancreatitis pain. Mol. Med. Rep. 2018, 17, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Weng, Y.; Wang, T.; Ouyang, B.; Li, Y.; Song, Z.; Pan, Y.; Zhang, Z.; Zou, W.; Huang, C.; et al. Suppression of HDAC2 in Spinal Cord Alleviates Mechanical Hyperalgesia and Restores KCC2 Expression in a Rat Model of Bone Cancer Pain. Neuroscience 2018, 377, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Brownstone, N.; Reddy, V.; Chan, S.; Thibodeaux, Q.; Truong, A.; Bhutani, T.; Chang, H.W.; Liao, W. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101494. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, X.; Wu, R.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.; Liu, Y.; Ren, B.; Li, J.; et al. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Mathes, T.; Martens, E.C.; Kamada, N.; Nusrat, A.; Inohara, N.; Nunez, G. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci. Immunol. 2019, 4, eaaw4341. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.N.; Sun, Z.F.; Li, X.Y.; Zhang, X.D.; Jin, Z.X.; Zhang, C.; Zhang, Y.; Wang, H.Y.; Huang, N.N.; Jiang, J.H.; et al. Alterations in gut microbiota are related to metabolite profiles in spinal cord injury. Neural Regen. Res. 2023, 18, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Gong, P.; Shi, R.; Chen, W.; Wang, C.; Zhang, C.; Wu, Z. Syringic Acid Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota. J. Agric. Food Chem. 2023, 71, 8458–8470. [Google Scholar] [CrossRef] [PubMed]
- Guzzardi, M.A.; La Rosa, F.; Campani, D.; Cacciato Insilla, A.; De Sena, V.; Panetta, D.; Brunetto, M.R.; Bonino, F.; Collado, M.C.; Iozzo, P. Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet. Nutrients 2021, 13, 3438. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Han, L.; Wang, B.; Shi, R.; Ye, J.; Xia, B.; Zhao, Z.; Zhao, B.; Liu, X. Leucine-Restricted Diet Ameliorates Obesity-Linked Cognitive Deficits: Involvement of the Microbiota-Gut-Brain Axis. J. Agric. Food Chem. 2023, 71, 9404–9418. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, S.; Jin, L.; Zhang, W.; Liu, N.; Wang, H.; Wang, Z.; Wei, P.; Li, F.; Yu, J.; et al. Four-week administration of nicotinemoderately impacts blood metabolic profile and gut microbiota in a diet-dependent manner. Biomed. Pharmacother. 2019, 115, 108945. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.M.; Mendes, M.M.; Oliveira, A.C.; Magalhaes, K.G.; Shivappa, N.; Hebert, J.R.; da Costa, T.H.M.; Botelho, P.B. Dietary inflammatory index and its relationship with gut microbiota in individuals with intestinal constipation: A cross-sectional study. Eur. J. Nutr. 2022, 61, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Keswani, T.; Roland, J.; Herbert, F.; Delcroix-Genete, D.; Bauderlique-Le Roy, H.; Gaayeb, L.; Cazenave, P.A.; Pied, S. Expression of CD300lf by microglia contributes to resistance to cerebral malaria by impeding the neuroinflammation. Genes Immun. 2020, 21, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyotylainen, T.; Hamalainen, A.M.; Peet, A.; Tillmann, V.; Poho, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, Y.N.; Lv, S.Y.; Wu, H.G.; Zhang, L.S.; Cao, Z.; Liu, H.R.; Wang, X.M.; Wu, L.Y. Gut microbiome alterations in colitis rats after moxibustion at bilateral Tianshu acupoints. BMC Gastroenterol. 2022, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a drug target for autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Pellock, S.J.; Redinbo, M.R. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J. Biol. Chem. 2017, 292, 8569–8576. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward | Reverse |
|---|---|---|
| FOS | 5′-ATC GGC AGA AGG GGC AAA GT-3′ | 5′-TCC TCC GAT TCC GGC ACT TG-3′ |
| Gadd45g | 5′-AGT CCG TGG CCA GGA TAC AG-3′ | 5′-TTT GGC GGA CTC GTA GAC GC-3′ |
| HDAC2 | 5′-GCA CCA CGC CAA GAA GTC AG-3′ | 5′-ACG GTC ATC ACG CGA TCT GT-3′ |
| β-actin | 5′-ACA ACC TTC TTG CAG CTC CTC C-3′ | 5′-TGA CCC ATA CCC ACC ATC ACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-L.; Santos, J.M.; Elmassry, M.M.; Bhakta, V.; Driver, Z.; Ji, G.; Yakhnitsa, V.; Kiritoshi, T.; Lovett, J.; Hamood, A.N.; et al. Ginger Polyphenols Reverse Molecular Signature of Amygdala Neuroimmune Signaling and Modulate Microbiome in Male Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Axis. Antioxidants 2024, 13, 502. https://doi.org/10.3390/antiox13050502
Shen C-L, Santos JM, Elmassry MM, Bhakta V, Driver Z, Ji G, Yakhnitsa V, Kiritoshi T, Lovett J, Hamood AN, et al. Ginger Polyphenols Reverse Molecular Signature of Amygdala Neuroimmune Signaling and Modulate Microbiome in Male Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Axis. Antioxidants. 2024; 13(5):502. https://doi.org/10.3390/antiox13050502
Chicago/Turabian StyleShen, Chwan-Li, Julianna Maria Santos, Moamen M. Elmassry, Viren Bhakta, Zarek Driver, Guangchen Ji, Vadim Yakhnitsa, Takaki Kiritoshi, Jacob Lovett, Abdul Naji Hamood, and et al. 2024. "Ginger Polyphenols Reverse Molecular Signature of Amygdala Neuroimmune Signaling and Modulate Microbiome in Male Rats with Neuropathic Pain: Evidence for Microbiota–Gut–Brain Axis" Antioxidants 13, no. 5: 502. https://doi.org/10.3390/antiox13050502






