The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Models
2.2. Human Tissue Samples
2.3. Murine Tissue Sampling
2.4. RNA Isolation and REAL-Time PCR
2.5. Vessel Preparation and Vascular Function Analysis
2.6. Griess Assay for Nitrite
2.7. Serum Analysis
2.8. Statistical Analyses
3. Results
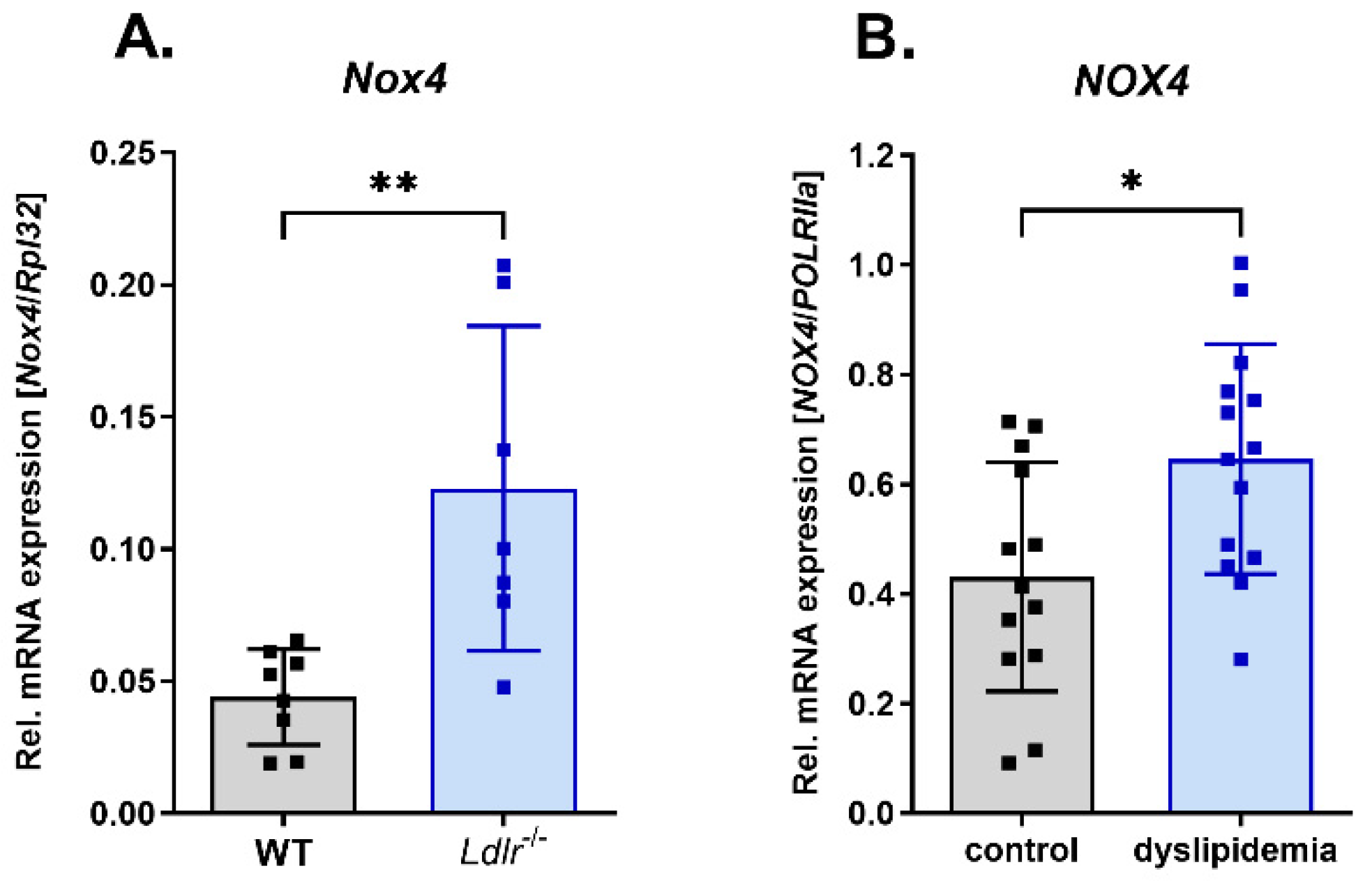
3.1. Increased Nox4 Expression under Dyslipidemia Conditions
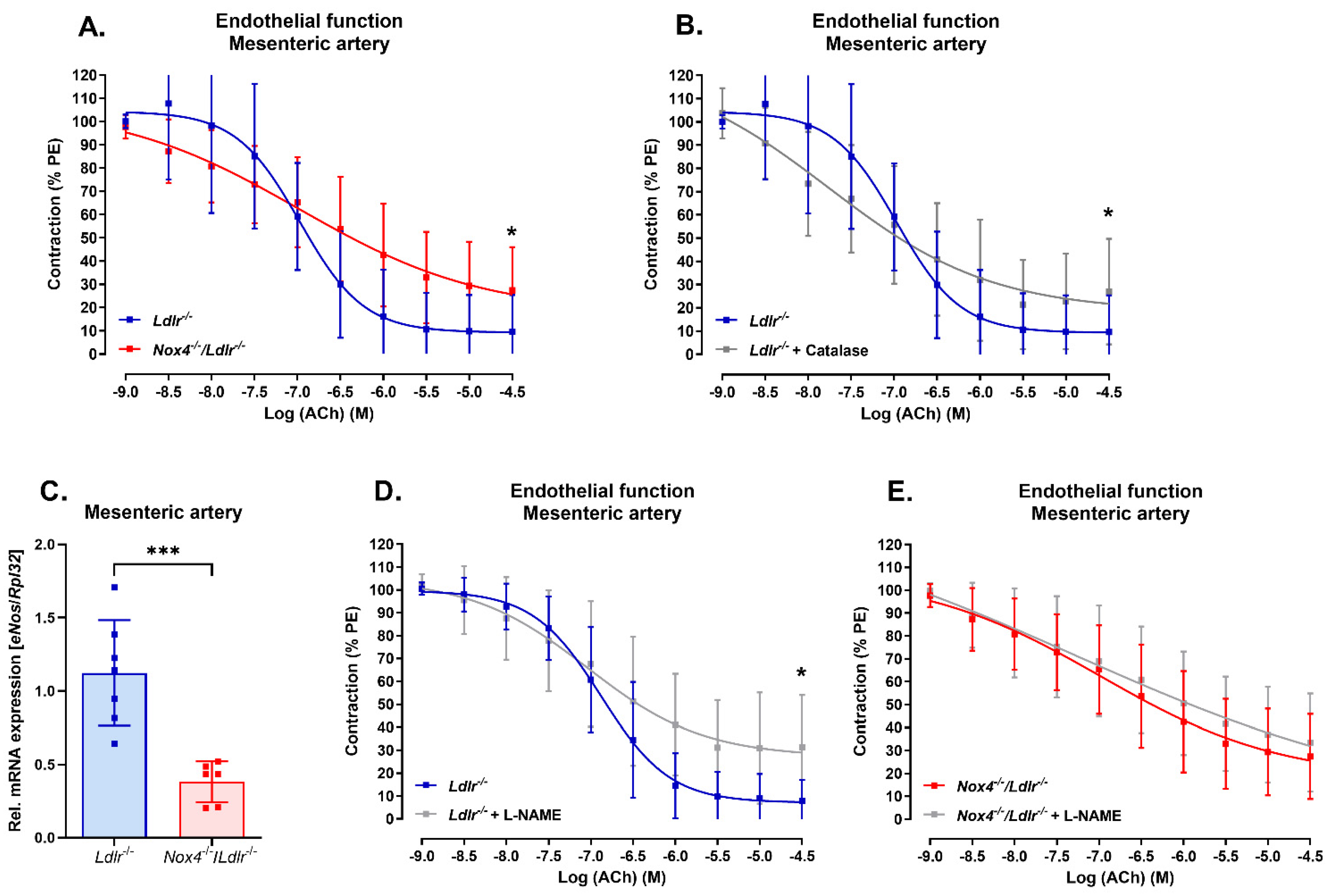
3.2. Nox4 Deletion Led to Endothelial Dysfunction in the Mesenteric Artery
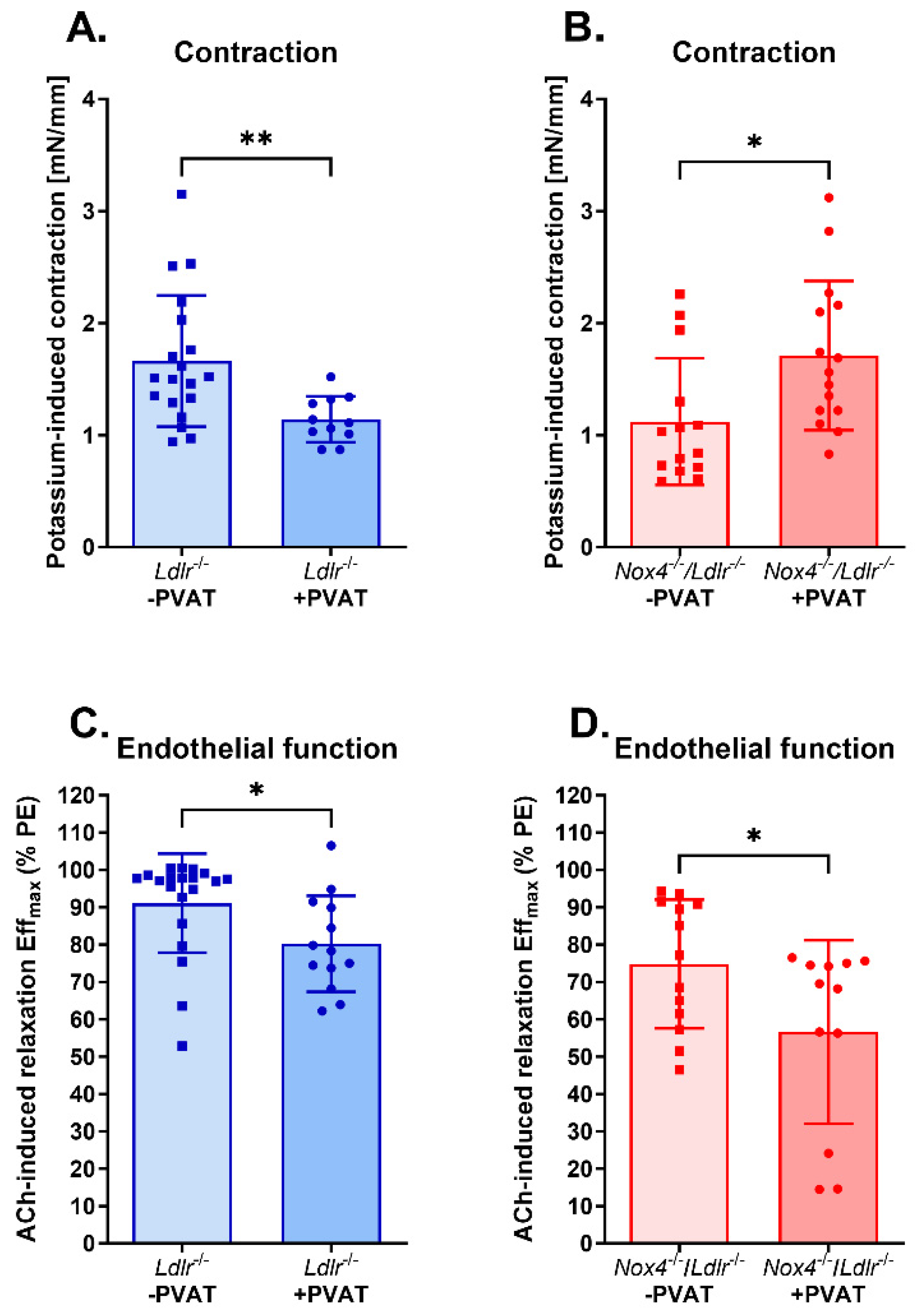
3.3. Anti-Contractile Properties of Perivascular Adipose Tissue on Mesenteric Arteries Were Absent in Nox4-Depleted Mice
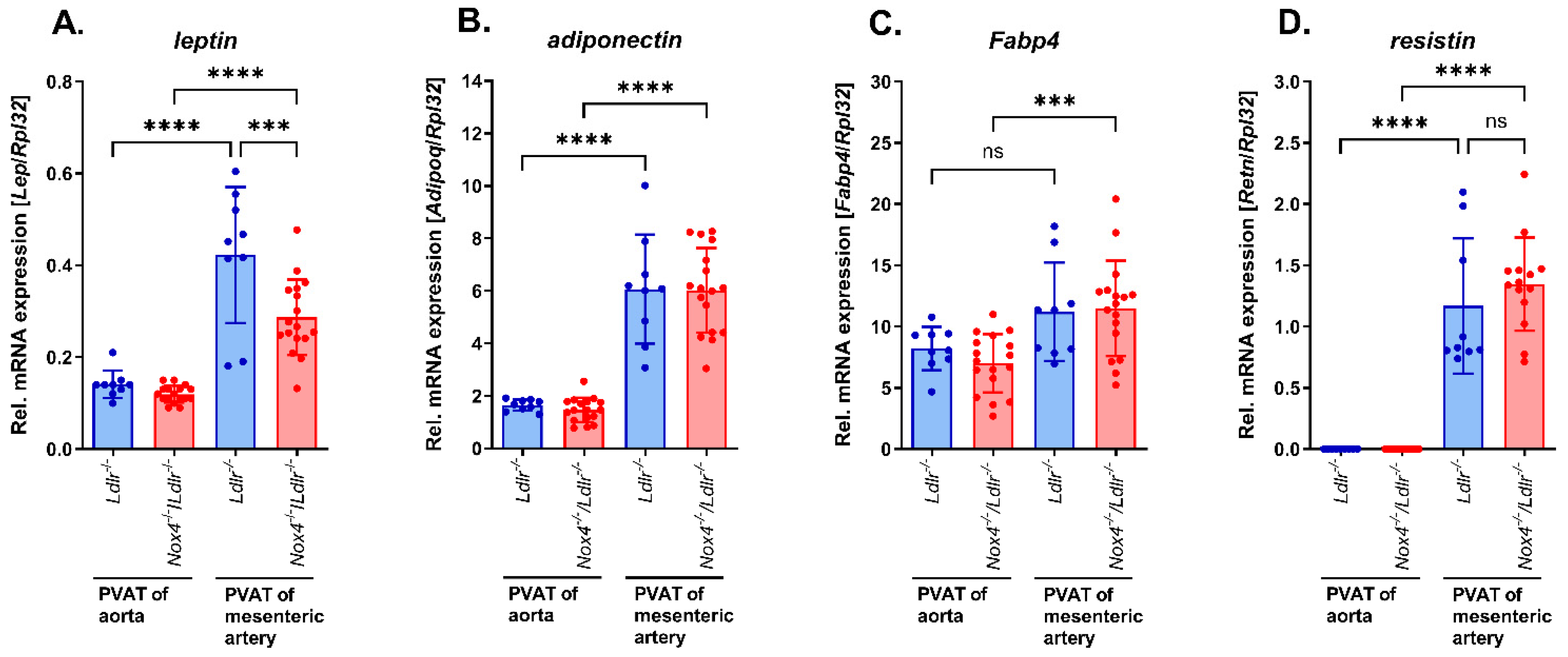
3.4. Expression of Adipokines in Perivascular Adipose Tissue from Mesenteric Arteries of Nox4−/−/Ldlr−/− Mice and Ldlr−/− Mice and Comparison to Perivascular Adipose Tissue from Aortas
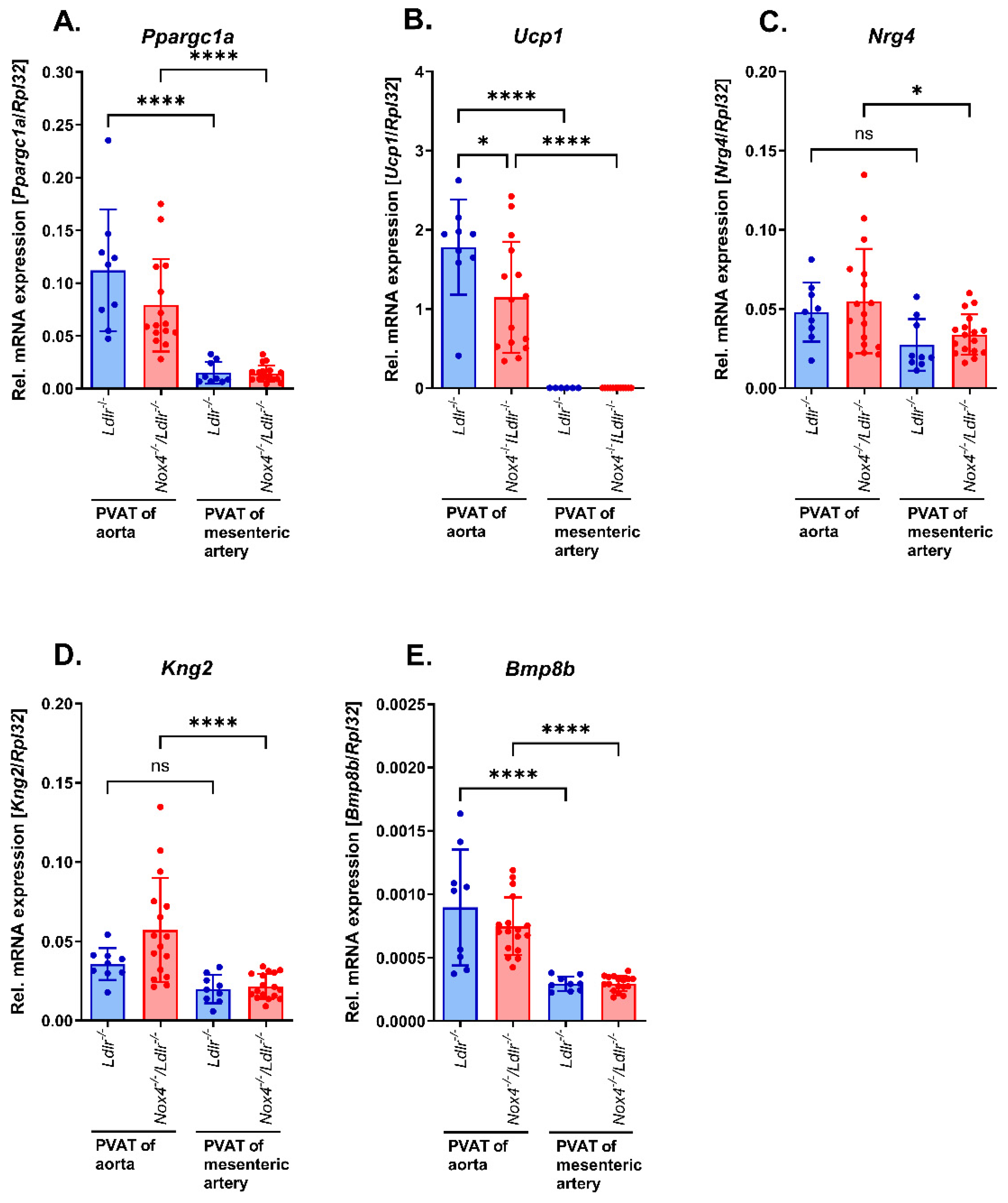
3.5. Marker of Brown/Beige Adipocytes in Aortic and Mesenteric Perivascular Adipose Tissue from Nox4−/−/Ldlr−/− Mice Compared to Ldlr−/− Mice
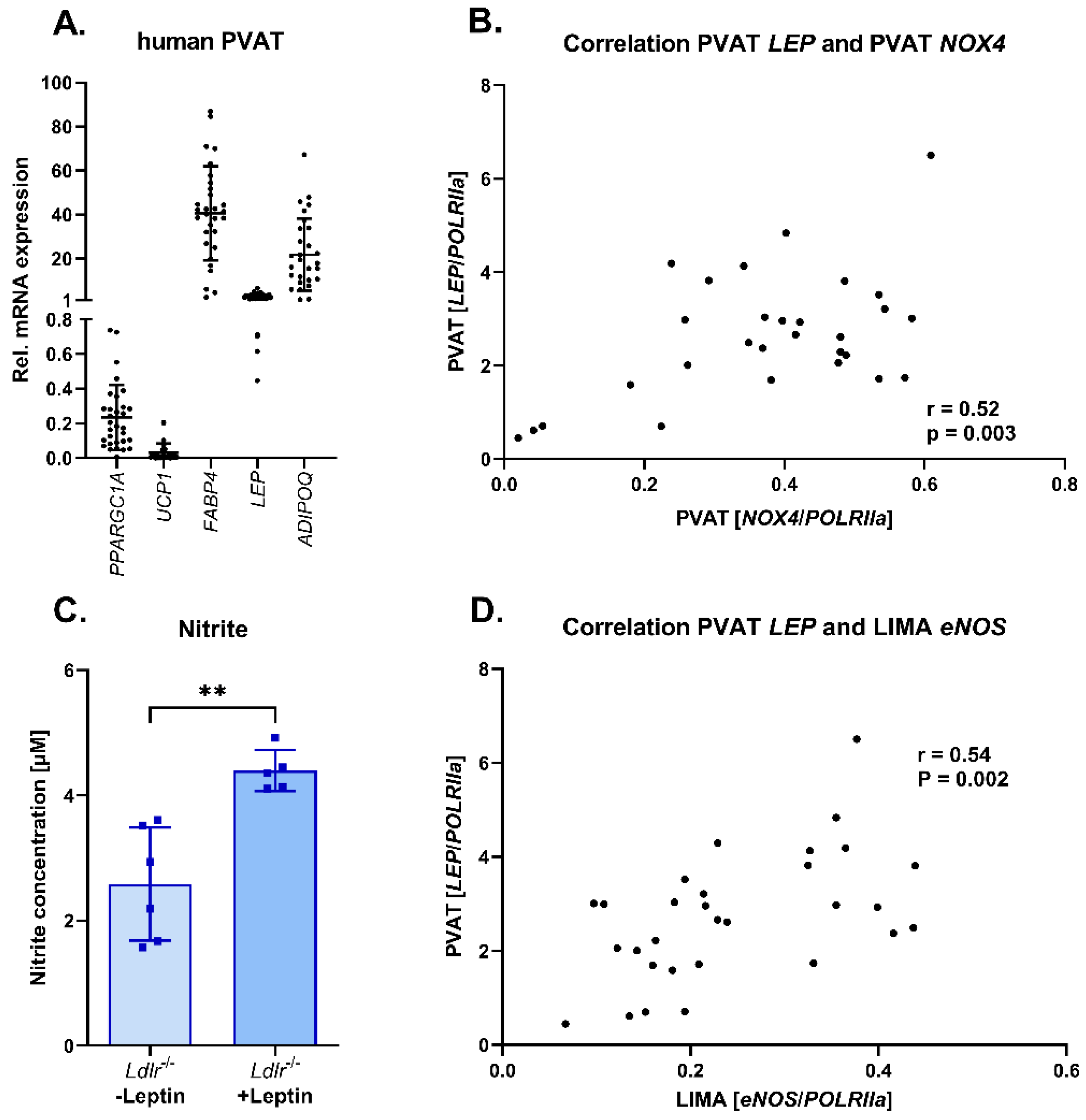
3.6. Adipokine Profile in Human Perivascular Adipose Tissue: Correlation of Leptin and NOX4 Expression in Human Perivascular Adipose Tissue
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Matoba, T.; Shimokawa, H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Pharmacol. Sci. 2003, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, E.; Narkiewicz, K. Hypertension and Dyslipidemia: The Two Partners in Endothelium-Related Crime. Curr. Atheroscler. Rep. 2023, 25, 605–612. [Google Scholar] [CrossRef]
- Félétou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture). Am. J. Physiol. Heart. Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Tajima, E.; Sakuma, M.; Tokoi, S.; Matsumoto, H.; Saito, F.; Watanabe, R.; Toyoda, S.; Abe, S.; Inoue, T. The comparison of endothelial function between conduit artery and microvasculature in patients with coronary artery disease. Cardiol. J. 2020, 27, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Berenji Ardestani, S.; Eftedal, I.; Pedersen, M.; Jeppesen, P.B.; Norregaard, R.; Matchkov, V.V. Endothelial dysfunction in small arteries and early signs of atherosclerosis in ApoE knockout rats. Sci. Rep. 2020, 10, 15296. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Lu, C.; Su, L.-Y.; Sharma, A.M.; Lee, R.M.K.W. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 2007, 151, 323–331. [Google Scholar] [CrossRef]
- Cybularz, M.; Langbein, H.; Zatschler, B.; Brunssen, C.; Deussen, A.; Matschke, K.; Morawietz, H. Endothelial function and gene expression in perivascular adipose tissue from internal mammary arteries of obese patients with coronary artery disease. Atheroscler. Suppl. 2017, 30, 149–158. [Google Scholar] [CrossRef]
- Ahmed, A.; Bibi, A.; Valoti, M.; Fusi, F. Perivascular Adipose Tissue and Vascular Smooth Muscle Tone: Friends or Foes? Cells 2023, 12, 1196. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Costa, R.M.; Neves, K.B.; Tostes, R.C.; Lobato, N.S. Perivascular Adipose Tissue as a Relevant Fat Depot for Cardiovascular Risk in Obesity. Front. Physiol. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed]
- Mellott, E.; Faulkner, J.L. Mechanisms of leptin-induced endothelial dysfunction. Curr. Opin. Nephrol. Hypertens. 2023, 32, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, H.; Brendel, H.; Diaba-Nuhoho, P.; Catar, R.; Perakakis, N.; Wolfrum, C.; Bornstein, S.R. Cross-Talk of NADPH Oxidases and Inflammation in Obesity. Antioxidants 2023, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-T.; Lin, X.-L.; Wu, S.; Liang, Q.; Yang, L.; Gao, Y.-J.; Ge, Z.-Z. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell Signal. 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hahner, F.; Moll, F.; Warwick, T.; Hebchen, D.; Buchmann, G.; Epah, J.; Abplanalp, W.; Schader, T.; Günther, S.; Gilsbach, R.; et al. Nox4 promotes endothelial differentiation through chromatin remodeling. Redox Biol. 2022, 55, 102381. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.-S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox. Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Constentino-Gomes, D.; Lambeth, J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef]
- Craige, S.M.; Chen, K.; Pei, Y.; Li, C.; Huang, X.; Chen, C.; Shibata, R.; Sato, K.; Walsh, K.; Keaney, J.F.; et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 2011, 124, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Murdoch, C.E.; Wang, M.; Santos, C.X.; Zhang, M.; Alom-Ruiz, S.; Anilkumar, N.; Ouattara, A.; Cave, A.C.; Walker, S.J.; et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, C.; Rezende, F.; Kruse, C.; Yasar, Y.; Löwe, O.; Fork, C.; van de Sluis, B.; Bremer, R.; Weissmann, N.; Shah, A.M.; et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Hear. J. 2015, 36, 3447–3456. [Google Scholar] [CrossRef] [PubMed]
- Langbein, H.; Brunssen, C.; Hofmann, A.; Cimalla, P.; Brux, M.; Bornstein, S.R.; Deussen, A.; Koch, E.; Morawietz, H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Hear. J. 2016, 37, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Kavey, R.E.; Daniels, S.R.; Lauer, R.M.; Atkins, D.L.; Hayman, L.L.; Taubert, K.; American Heart Association. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 2003, 107, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Qiao, M.; Schröder, K.; Zhao, Q.; Asmis, R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein–induced macrophage death. Circ. Res. 2010, 106, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-B.; Zhang, Y.-Z.; Liu, W.-Q.; Zhang, J.-J.; Peng, J.; Luo, X.-J.; Ma, Q.-L. Correlation between NADPH oxidase-mediated oxidative stress and dysfunction of endothelial progenitor cell in hyperlipidemic patients. Korean J. Intern. Med. 2018, 33, 313–322. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Wu, X.; Peshavariya, H.M.; Dusting, G.J.; Zhang, M.; Jiang, F. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014, 5, e1010. [Google Scholar] [CrossRef]
- Gálvez-Prieto, B.; Somoza, B.; Gil-Ortega, M.; García-Prieto, C.F.; de las Heras, A.I.; González, M.C.; Arribas, S.; Aranguez, I.; Bolbrinker, J.; Kreutz, R.; et al. Anticontractile Effect of Perivascular Adipose Tissue and Leptin are Reduced in Hypertension. Front. Pharmacol. 2012, 3, 103. [Google Scholar] [CrossRef]
- Matsuda, K.; Teragawa, H.; Fukuda, Y.; Nakagawa, K.; Higashi, Y.; Chayama, K. Leptin causes nitric-oxide independent coronary artery vasodilation in humans. Hypertens. Res. 2003, 26, 147–152. [Google Scholar] [CrossRef]
- Vecchione, C.; Maffei, A.; Colella, S.; Aretini, A.; Poulet, R.; Frati, G.; Gentile, M.T.; Fratta, L.; Trimarco, V.; Trimarco, B.; et al. Leptin effect on Endothelial nitric oxide is mediated through akt–endothelial nitric oxide synthase phosphorylation pathway. Diabetes 2002, 51, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Higashi, Y.; Sasaki, S.; Oshima, T.; Matsuura, H.; Chayama, K. Leptin causes vasodilation in humans. Hypertens. Res. 2002, 25, 161–165. [Google Scholar] [CrossRef]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T.; Tejero, J.; Shiva, S.; Gladwin, M.T.; Loria, A.S.; Spradley, F.T.; Obi, I.E.; et al. Leptin-induced endothelial dysfunction in obesity. Am. J. Physiol. Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arter. Thromb. Vasc. Biol. 2009, 29, 239–245. [Google Scholar] [CrossRef]
- Morawietz, H.; Bornstein, S.R. Leptin, endothelin, NADPH oxidase, and heart failure. Hypertension 2006, 47, e20, author reply e20-1. [Google Scholar] [CrossRef] [PubMed]
- Blanca, A.J.; Ruiz-Armenta, M.V.; Zambrano, S.; Salsoso, R.; Miguel-Carrasco, J.L.; Fortuño, A.; Revilla, E.; Mate, A.; Vázquez, C.M. Leptin Induces Oxidative Stress Through Activation of NADPH Oxidase in Renal Tubular Cells: Antioxidant Effect of L-Carnitine. J. Cell. Biochem. 2016, 117, 2281–2288. [Google Scholar] [CrossRef]
- Mestres-Arenas, A.; Villarroya, J.; Giralt, M.; Villarroya, F.; Peyrou, M. A Differential Pattern of Batokine Expression in Perivascular Adipose Tissue Depots From Mice. Front. Physiol. 2021, 12, 714530. [Google Scholar] [CrossRef] [PubMed]
- Gullicksen, P.; Flatt, W.P.; Dean, R.G.; Hartzell, D.L.; Baile, C.A. Energy metabolism and expression of uncoupling proteins 1, 2, and 3 after 21 days of recovery from intracerebroventricular mouse leptin in rats. Physiol. Behav. 2002, 75, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, W.; Xia, L.; Xia, P.; Yan, Y. Gene expression profiling reveals heterogeneity of perivascular adipose tissues surrounding coronary and internal thoracic arteries. Acta Biochim. Biophys. Sin. 2017, 49, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, H. Cardiovascular protection by Nox4. Cardiovasc. Res. 2018, 114, 353–355. [Google Scholar] [CrossRef] [PubMed]
| Control Group | Patients with Dyslipidemia | p-Value | |
|---|---|---|---|
| Number of patients | 14 | 15 | |
| Dyslipidemia [yes = 1; no = 0] | 0 | 1 | |
| Age [years] | 70.07 ± 8.02 | 71.00 ± 10.13 | 0.79 |
| Sex [% male] | 100 | 100 | |
| BMI [kg/m2] | 28.75 ± 3.74 | 29.60 ± 3.28 | 0.57 |
| Smoking [yes = 1; no = 0] | 0.43 ± 0.51 | 0.47 ± 0.52 | 0.84 |
| Diabetes mellitus type 2 [yes = 1; no = 0] | 0.71 ± 0.47 | 0.67 ± 0.49 | 0.79 |
| Hypertension [yes = 1; no = 0] | 0.93 ± 0.27 | 0.93 ± 0.26 | 0.96 |
| Ejection fraction [%] | 39.67 ± 17.08 | 39.63 ± 13.10 | 0.99 |
| ACE inhibitor [yes = 1; no = 0] | 0.79 ± 0.43 | 0.60 ± 0.51 | 0.30 |
| Beta blocker [yes = 1; no = 0] | 0.86 ± 0.36 | 0.93 ± 0.26 | 0.52 |
| Statin [yes = 1; no = 0] | 0.86 ± 0.36 | 0.87 ± 0.35 | 0.94 |
| Aspirin [yes = 1; no = 0] | 0.86 ± 0.36 | 0.87 ± 0.35 | 0.94 |
| Diuretics [yes = 1; no = 0] | 0.57 ± 0.51 | 0.73 ± 0.46 | 0.38 |
| ARB [yes = 1; no = 0] | 0.14 ± 0.36 | 0.20 ± 0.41 | 0.70 |
| Gene | Primers | Sequence, 5′-3′ | Accession Number |
|---|---|---|---|
| Human ADIPOQ | Forward | TCCTCACTTCCATTCTGACTGC | NM_001177800.1 |
| Reverse | GTAGAACAGCTCCCAGCAACA | ||
| Murine Adipoq | Forward | CAGTGGATCTGACGACACCAA | NM_009605.5 |
| Reverse | ACGTCATCTTCGGCATGACTG | ||
| Murine Bmp8b | Forward | TCCGCCTATTACTGTGCTGG | NM_007559.5 |
| Reverse | TAGGCACACAGCACACCTTG | ||
| Human eNOS | Forward | GAACCTGTGTGACCCTCACC | NM_000603.5, NM_001160109.2, NM_001160110.1, NM_001160111.1 |
| Reverse | TGGCTAGCTGGTAACTGTGC | ||
| Murine eNos | Forward | CTCATGGGCACGGTGATG | NM_008713.4 |
| Reverse | ACCACATCATACTCATCCAT | ||
| Human FABP4 | Forward | GAAAACTGCAGCTTCCTTCTCAC | NM_001442.3 |
| Reverse | CTGGTGGCAAAGCCCACTC | ||
| Murine Fabp4 | Forward | TGGGAACCTGGAAGCTTGTC | NM_001409513.1, NM_001409514.1, NM_024406.4 |
| Reverse | CTTTCCTTGTGGCAAAGCCC | ||
| Murine Kng2 | Forward | CGACTGCAATGCTAACGTGT | NM_001102409.1, NM_001102410.1, NM_201375.2 |
| Reverse | AGGCCTCCTTCGGATAGGAAT | ||
| Human LEP | Forward | CAAGCTGTGCCCATCCAAAAA | NM_000230.2 |
| Reverse | TGAAGTCCAAACCGGTGACT | ||
| Murine Lep | Forward | TGCTGCAGATAGCCAATGAC | NM_008493.3 |
| Reverse | GAGTAGAGTGAGGCTTCCAGGA | ||
| Human NOX4 | Forward | TAACCTCAACTGCAGCCTTATC | NM_001143836.3, NM_001143837.2, NM_001291926.2, NM_001291927.1, NM_001291929.2, NM_001300995.1, NM_016931.5 |
| Reverse | CTTTTATCCAACAATCTCCTGGTTCTC | ||
| Murine Nox4 | Forward | TGTTGGGCCTAGGATTGTGTT | NM_001285833.1, NM_001285835.1, NM_015760.5 |
| Reverse | AGGGACCTTCTGTGATCCTCG | ||
| Murine Nrg4 | Forward | CCTACTATCCCCAGCCCATTCT | NM_032002.3, NM_001425100.1 |
| Reverse | TGCCGACAGATTACTTTCGCT | ||
| Human PPARGC1a | Forward | CTTTGCGCAGGTCAAACGAA | NM_001330753.1, NM_001330752.1, NM_013261.4, NM_001330751.1 |
| Reverse | GGTGGAAGCAGGGTCAAAGT | ||
| Murine Ppargc1a | Forward | AATGCAGCGGTCTTAGCACT | NM_008904.2 |
| Reverse | TCTCGGTCTTAACAATGGCAGG | ||
| Human POLRIIa | Forward | ACCTGCGGTCCACGTTGTGT | NM_000937.4 |
| Reverse | CCACCATTTCCCCGGGATGCG | ||
| Murine Retn | Forward | TGTCCCATCGATGAAGCCAT | NM_001204959.1 |
| Reverse | TGGAGGAGACTGTCCAGCAA | ||
| Murine Rpl32 | Forward | GCGCTGCCTACGAGGTGGCTG | NM_172086.2 |
| Reverse | CTGGCCCTTGAACCTTCTCCGC | ||
| Human UCP1 | Forward | CTAACGAAGGACCAACGGCT | NM_021833.5 |
| Reverse | ACGTTCCAGGATCCAAGTCG | ||
| Murine Ucp1 | Forward | TACCCAAGCGTACCAAGCTG | NM_009463.3 |
| Reverse | ACCCGAGTCGCAGAAAAGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaba-Nuhoho, P.; Mittag, J.; Brunssen, C.; Morawietz, H.; Brendel, H. The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue. Antioxidants 2024, 13, 503. https://doi.org/10.3390/antiox13050503
Diaba-Nuhoho P, Mittag J, Brunssen C, Morawietz H, Brendel H. The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue. Antioxidants. 2024; 13(5):503. https://doi.org/10.3390/antiox13050503
Chicago/Turabian StyleDiaba-Nuhoho, Patrick, Jennifer Mittag, Coy Brunssen, Henning Morawietz, and Heike Brendel. 2024. "The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue" Antioxidants 13, no. 5: 503. https://doi.org/10.3390/antiox13050503





