A Bayesian Reanalysis of the Overall and Sex-Disaggregated Results of the Neonatal Oxygenation Prospective Meta-Analysis (NeOProM)
Abstract
:1. Introduction
2. Materials and Methods
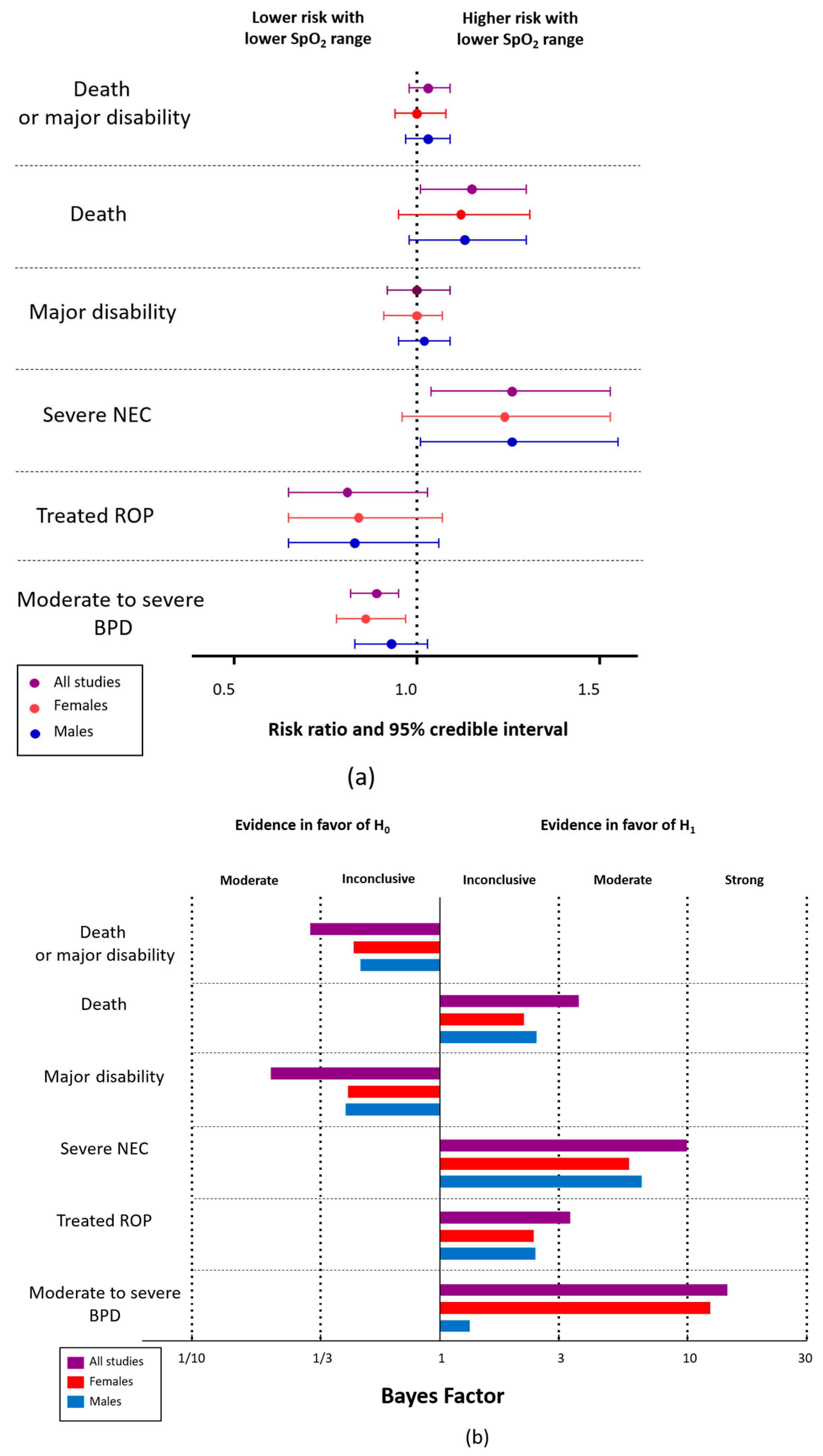
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, K.J. The oxygen paradox, oxidative stress, and ageing. Arch. Biochem. Biophys. 2016, 595, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, A.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Pozo, S.; Parra-Llorca, A.; Lara-Cantón, I.; Solaz, A.; García-Jiménez, J.L.; Pallardó, F.V.; Vento, M. Oxygen in the neonatal period: Oxidative stress, oxygen load and epigenetic changes. In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative stress in preterm newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.Q.; Zhang, L. Gestational hypoxia and developmental plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef]
- Webster, W.S.; Abela, D. The effect of hypoxia in development. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Van Mechelen, K.; van Westering-Kroon, E.; Villamor-Martínez, E.; Villamor, E. Endotypes of prematurity and phenotypes of bronchopulmonary dysplasia: Toward personalized neonatology. J. Pers. Med. 2022, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Claure, N. Oxygenation targets and outcomes in premature infants. JAMA 2013, 309, 2161–2162. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Husain, S. Primary prevention of ROP and the oxygen saturation targeting trials. In Seminars in Perinatology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA 2018, 319, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, Y.E.; Peralta-Carcelen, M.; Finer, N.N.; Carlo, W.A.; Gantz, M.G.; Walsh, M.C.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N. Engl. J. Med. 2012, 367, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N. Engl. J. Med. 2010, 362, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- The BOOST II United Kingdom, Australia, and New Zealand Collaborative Groups. Oxygen saturation and outcomes in preterm infants. N. Engl. J. Med. 2013, 368, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- The BOOST-II Australia and United Kingdom Collaborative Groups. Outcomes of two trials of oxygen-saturation targets in preterm infants. N. Engl. J. Med. 2016, 374, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Marschner, S.L.; Donoghoe, M.; Battin, M.R.; Broadbent, R.S.; Elder, M.J.; Hewson, M.P.; Meyer, M.P.; Ghadge, A.; Graham, P. Randomized controlled trial of oxygen saturation targets in very preterm infants: Two year outcomes. J. Pediatr. 2014, 165, 30–35.e2. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Moddemann, D.; Poets, C.; Rabi, Y.; Solimano, A.; Roberts, R.S.; Canadian Oxygen Trial Group. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: A randomized clinical trial. JAMA 2013, 309, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K.; Alur, P.; Eichenwald, E. The need to address sex as a biological variable in neonatal clinical studies. J. Pediatr. 2023, 255, 17–21. [Google Scholar] [CrossRef] [PubMed]
- van Westering-Kroon, E.; Huizing, M.J.; Villamor-Martínez, E.; Villamor, E. Male disadvantage in oxidative stress-associated complications of prematurity: A systematic review, meta-analysis and meta-regression. Antioxidants 2021, 10, 1490. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.M.; Gulden, S.; Almutairi, M.F.; Bartoš, F.; Cavallaro, G.; Villamor, E. Sex differences in the risk of retinopathy of prematurity: A systematic review, frequentist and Bayesian meta-analysis, and meta-regression. World J. Pediatr. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Werbinski, J.L.; Rojek, M.K.; Cabral, M.D.I. The Need to Integrate Sex and Gender Differences into Pediatric Pedagogy. Adv. Pediatr. 2019, 66, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Lacasse, Y.; Tapp, S.; Tremblay, Y.; Kari, A.; Liu, J.; Fekih, M.; Qublan, H.S.; Amorim, M.M.; Bujold, E. Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: Systematic review and meta-analysis. J. Obstet. Gynaecol. Can. 2011, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Kari, M.; Heinonen, K.; Ikonen, R.; Koivisto, M.; Raivio, K. Dexamethasone treatment in preterm infants at risk for bronchopulmonary dysplasia. Arch. Dis. Child. 1993, 68, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Navarro, C.; Sánchez-Luna, M.; Zeballos-Sarrato, S.; Pescador-Chamorro, I. Antenatal corticosteroids and the influence of sex on morbidity and mortality of preterm infants. J. Matern. Fetal Neonatal Med. 2022, 35, 3438–3445. [Google Scholar] [CrossRef] [PubMed]
- Ment, L.R.; Vohr, B.R.; Makuch, R.W.; Westerveld, M.; Katz, K.H.; Schneider, K.C.; Duncan, C.C.; Ehrenkranz, R.; Oh, W.; Philip, A.G. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J. Pediatr. 2004, 145, 832–834. [Google Scholar] [CrossRef]
- Tarnow-Mordi, W.; Morris, J.; Kirby, A.; Robledo, K.; Askie, L.; Brown, R.; Evans, N.; Finlayson, S.; Fogarty, M.; Gebski, V. Delayed versus immediate cord clamping in preterm infants. N. Engl. J. Med. 2017, 377, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Cubells, E.; Escobar, J.J.; Escrig, R.; Aguar, M.; Brugada, M.; Cernada, M.; Saénz, P.; Izquierdo, I. Oxygen saturation after birth in preterm infants treated with continuous positive airway pressure and air: Assessment of gender differences and comparison with a published nomogram. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F228–F232. [Google Scholar] [CrossRef] [PubMed]
- Kelter, R. Bayesian and frequentist testing for differences between two groups with parametric and nonparametric two-sample tests. Wiley Interdiscip. Rev. Comput. Stat. 2021, 13, e1523. [Google Scholar] [CrossRef]
- Gigerenzer, G. Mindless statistics. J. Socio-Econ. 2004, 33, 587–606. [Google Scholar] [CrossRef]
- Van Zyl, C.J. Frequentist and Bayesian inference: A conceptual primer. New Ideas Psychol. 2018, 51, 44–49. [Google Scholar] [CrossRef]
- Aberson, C. Interpreting Null Results: Improving Presentation and Conclusions with Confidence Intervals. J. Artic. Support Null Hypothesis 2002, 1, 36–42. [Google Scholar]
- Bartoš, F.; Pawel, S.; Wagenmakers, E.-J. When Evidence and Significance Collide. arXiv 2022, arXiv:2206.04435. [Google Scholar]
- Lewis, R.J.; Angus, D.C. Time for clinicians to embrace their inner Bayesian? Reanalysis of results of a clinical trial of extracorporeal membrane oxygenation. JAMA 2018, 320, 2208–2210. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Damiani, L.P.; Bakker, J.; Ospina-Tascon, G.A.; Castro, R.; Cavalcanti, A.B.; Hernandez, G. Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock. A Bayesian reanalysis of the ANDROMEDA-SHOCK trial. Am. J. Respir. Crit. Care Med. 2020, 201, 423–429. [Google Scholar] [CrossRef]
- Albuquerque, A.M.; Tramujas, L.; Sewanan, L.R.; Williams, D.R.; Brophy, J.M. Mortality rates among hospitalized patients with COVID-19 infection treated with tocilizumab and corticosteroids: A Bayesian reanalysis of a previous meta-analysis. JAMA Netw. Open 2022, 5, e220548. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Casey, J.D.; Shankar-Hari, M.; Harrell, F.E., Jr.; Harhay, M.O. Using Bayesian methods to augment the interpretation of critical care trials. An overview of theory and example reanalysis of the alveolar recruitment for acute respiratory distress syndrome trial. Am. J. Respir. Crit. Care Med. 2021, 203, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.N.; Austin, P.C.; Hux, J.E.; Beattie, W.S.; Laupacis, A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J. Clin. Epidemiol. 2009, 62, 13–21.e5. [Google Scholar] [CrossRef] [PubMed]
- Hemming, K.; Melo, P.; Luo, R.; Taljaard, M.; Coomarasamy, A. A re-analysis of 150 women’s health trials to investigate how the Bayesian approach may offer a solution to the misinterpretation of statistical findings. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; van Westering-Kroon, E.; Gonzalez-Luis, G.E.; Bartoš, F.; Abman, S.H.; Huizing, M.J. Patent Ductus Arteriosus and Bronchopulmonary Dysplasia–Associated Pulmonary Hypertension: A Bayesian Meta-Analysis. JAMA Netw. Open 2023, 6, e2345299. [Google Scholar] [CrossRef] [PubMed]
- Gronau, Q.F.; Heck, D.W.; Berkhout, S.W.; Haaf, J.M.; Wagenmakers, E.-J. A primer on Bayesian model-averaged meta-analysis. Adv. Methods Pract. Psychol. Sci. 2021, 4, 25152459211031256. [Google Scholar] [CrossRef]
- Bartoš, F.; Gronau, Q.F.; Timmers, B.; Otte, W.M.; Ly, A.; Wagenmakers, E.J. Bayesian model-averaged meta-analysis in medicine. Stat. Med. 2021, 40, 6743–6761. [Google Scholar] [CrossRef]
- Lavine, M.; Schervish, M.J. Bayes factors: What they are and what they are not. Am. Stat. 1999, 53, 119–122. [Google Scholar] [CrossRef]
- Berger, J.; Pericchi, L. Bayes Factors; Wiley: Hoboken, NJ, USA, 2014; pp. 1–14. [Google Scholar]
- Hoekstra, R.; Monden, R.; van Ravenzwaaij, D.; Wagenmakers, E.-J. Bayesian reanalysis of null results reported in medicine: Strong yet variable evidence for the absence of treatment effects. PLoS ONE 2018, 13, e0195474. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, F.; Maier, M.; Stanley, T.D.; Wagenmakers, E.J. Robust Bayesian Meta-Regression—Model-Averaged Moderation Analysis in the Presence of Publication Bias. PsyArXiv 2023. [Google Scholar] [CrossRef]
- Bartoš, F.; Maier, M. RoBMA: An R Package for Robust Bayesian Meta-Analyses. R Package Version 1.2. 2020. Available online: https://cran.r-project.org/web/packages/RoBMA/RoBMA.pdf (accessed on 10 January 2024).
- Lee, M.; Wagenmakers, E.-J. Bayesian Data Analysis for Cognitive Science: A Practical Course; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Dickey, J.M. The weighted likelihood ratio, linear hypotheses on normal location parameters. Ann. Math. Stat. 1971, 42, 204–223. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Lodewyckx, T.; Kuriyal, H.; Grasman, R. Bayesian hypothesis testing for psychologists: A tutorial on the Savage–Dickey method. Cogn. Psychol. 2010, 60, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, F.; Otte, W.M.; Gronau, Q.F.; Timmers, B.; Ly, A.; Wagenmakers, E.-J. Empirical prior distributions for Bayesian meta-analyses of binary and time to event outcomes. arXiv 2023, arXiv:2306.11468. [Google Scholar]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Manja, V.; Mathew, B.; Suresh, G.K. Oxygen targeting in preterm infants: A physiological interpretation. J. Perinatol. 2015, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sola, A. Oxygen saturation in the newborn and the importance of avoiding hyperoxia-induced damage. NeoReviews 2015, 16, e393–e405. [Google Scholar] [CrossRef]
- Cummings, J.J.; Polin, R.A.; Watterberg, K.L.; Poindexter, B.; Benitz, W.E.; Eichenwald, E.C.; Poindexter, B.B.; Stewart, D.L.; Aucott, S.W.; Goldsmith, J.P. Oxygen targeting in extremely low birth weight infants. Pediatrics 2016, 138, e20161576. [Google Scholar] [CrossRef]
- Whyte, R.K.; Nelson, H.; Roberts, R.S.; Schmidt, B. Benefits of oxygen saturation targeting trials: Oximeter calibration software revision and infant saturations. J. Pediatr. 2017, 182, 382–384. [Google Scholar] [CrossRef]
- Saugstad, O.D. Oxygenation of the immature infant: A commentary and recommendations for oxygen saturation targets and alarm limits. Neonatology 2018, 114, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.J.; Lakshminrusimha, S. Oxygen saturation targeting by pulse oximetry (SpO2) in the Extremely Low Gestational Age Neonate (ELGAN): A quixotic quest. Curr. Opin. Pediatr. 2017, 29, 153. [Google Scholar] [CrossRef] [PubMed]
- Stenson, B.; Brocklehurst, P.; Tarnow-Mordi, W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N. Engl. J. Med. 2011, 364, 1680–1682. [Google Scholar] [CrossRef]
- Manley, B.J.; Kuschel, C.A.; Elder, J.E.; Doyle, L.W.; Davis, P.G. Higher rates of retinopathy of prematurity after increasing oxygen saturation targets for very preterm infants: Experience in a single center. J. Pediatr. 2016, 168, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, M.; Shah, S.P.; Dai, S.; Cartwright, D. Increased risk of retinopathy of prematurity since increased O2 saturation targets: A multi-centre study. J. Paediatr. Child Health 2023, 59, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Taner, A.; Tekle, S.; Hothorn, T.; Adams, M.; Bassler, D.; Gerth-Kahlert, C. Higher incidence of retinopathy of prematurity in extremely preterm infants associated with improved survival rates. Acta Paediatr. 2020, 109, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; VanderVeen, D.K. Impact of higher oxygen saturation levels on postnatal weight gain to predict retinopathy of prematurity. Acta Paediatr. 2021, 110, 2348–2349. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.E.; Carper, B.; Gantz, M.; DeMauro, S.B.; Lakshminrusimha, S.; Walsh, M.; Schmidt, B.; Caplan, M.S.; Laptook, A.R.; Keszler, M. Association between policy changes for oxygen saturation alarm settings and neonatal morbidity and mortality in infants born very preterm. J. Pediatr. 2019, 209, 17–22.e2. [Google Scholar] [CrossRef] [PubMed]
- Willgerodt, N.; Bührer, C.; Rossi, R.; Kühn, T.; Rüdiger, M.; Avenarius, S.; Böttger, R.; Olbertz, D.M.; Proquitte, H.; Bittrich, H.-J. Similar adverse outcome rates with high or low oxygen saturation targets in an area with low background mortality. Front. Pediatr. 2023, 11, 1235877. [Google Scholar] [CrossRef] [PubMed]
- Daitch, V.; Turjeman, A.; Poran, I.; Tau, N.; Ayalon-Dangur, I.; Nashashibi, J.; Yahav, D.; Paul, M.; Leibovici, L. Underrepresentation of women in randomized controlled trials: A systematic review and meta-analysis. Trials 2022, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, S.; Forstmann, B.U.; Wagenmakers, E.-J. Erroneous analyses of interactions in neuroscience: A problem of significance. Nat. Neurosci. 2011, 14, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Palfi, B.; Dienes, Z. Why Bayesian “Evidence for H 1” in One Condition and Bayesian “Evidence for H 0” in Another Condition Does Not Mean Good-Enough Bayesian Evidence for a Difference Between the Conditions. Adv. Methods Pract. Psychol. Sci. 2020, 3, 300–308. [Google Scholar] [CrossRef]
- Hagadorn, J.; Sink, D.; Buus-Frank, M.; Edwards, E.; Morrow, K.; Horbar, J.; Ferrelli, K.; Soll, R. Alarm safety and oxygen saturation targets in the Vermont Oxford Network iNICQ 2015 collaborative. J. Perinatol. 2017, 37, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Vento, M.; Beltempo, M.; Lehtonen, L.; Håkansson, S.; Reichman, B.; Helenius, K.; Sjörs, G.; Sigali, E.; Lee, S. Variations in oxygen saturation targeting, and retinopathy of prematurity screening and treatment criteria in neonatal intensive care units: An international survey. Neonatology 2018, 114, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.J.; Villamor-Martínez, E.; Vento, M.; Villamor, E. Pulse oximeter saturation target limits for preterm infants: A survey among European neonatal intensive care units. Eur. J. Pediatr. 2017, 176, 51–56. [Google Scholar] [CrossRef] [PubMed]
| Outcome | All | Female | Male | BF10 | BFrf | BFmod | BFFemale | BFMale | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | |||||||||
| L | U | L | U | L | U | |||||||||
| Death or major disability (primary analysis) | 1.03 | 0.98 | 1.09 | 1.00 | 0.94 | 1.08 | 1.03 | 0.97 | 1.09 | 0.30 | 0.09 | 0.76 | 0.45 | 0.48 |
| Death or major disability (supportive analysis) | 1.04 | 0.98 | 1.10 | 1.00 | 0.93 | 1.09 | 1.03 | 0.98 | 1.10 | 0.35 | 0.12 | 0.88 | 0.52 | 0.59 |
| Death or major disability (secondary analysis) | 1.05 | 0.97 | 1.14 | 1.02 | 0.93 | 1.14 | 1.04 | 0.96 | 1.14 | 0.51 | 0.16 | 0.75 | 0.53 | 0.55 |
| Death or major disability (trialist defined) | 1.07 | 0.99 | 1.14 | 1.02 | 0.93 | 1.14 | 1.06 | 0.99 | 1.15 | 1.04 | 0.18 | 1.06 | 0.85 | 1.19 |
| Major disability (primary analysis) | 1.00 | 0.92 | 1.09 | 0.98 | 0.91 | 1.07 | 1.02 | 0.95 | 1.09 | 0.21 | 0.17 | 0.84 | 0.43 | 0.42 |
| Major disability (supportive analysis) | 1.01 | 0.93 | 1.10 | 0.98 | 0.91 | 1.07 | 1.02 | 0.95 | 1.10 | 0.21 | 0.17 | 0.92 | 0.46 | 0.46 |
| Major disability (secondary analysis) | 0.97 | 0.85 | 1.11 | 0.98 | 0.86 | 1.10 | 1.00 | 0.88 | 1.11 | 0.38 | 0.35 | 0.89 | 0.54 | 0.53 |
| Major disability (trialist defined) | 1.03 | 0.94 | 1.14 | 0.99 | 0.90 | 1.11 | 1.04 | 0.96 | 1.14 | 0.32 | 0.22 | 1.14 | 0.57 | 0.63 |
| Death prior to 18–24 months’ age corrected for prematurity | 1.15 | 1.01 | 1.30 | 1.12 | 0.95 | 1.31 | 1.13 | 0.98 | 1.30 | 3.60 | 0.50 | 0.84 | 2.17 | 2.45 |
| Death prior to 36 weeks’ postmenstrual age | 1.15 | 1.01 | 1.32 | 1.13 | 0.95 | 1.32 | 1.14 | 0.97 | 1.33 | 3.33 | 0.50 | 0.86 | 2.08 | 2.26 |
| Death prior to discharge | 1.14 | 1.01 | 1.29 | 1.12 | 0.94 | 1.29 | 1.13 | 0.98 | 1.30 | 3.15 | 0.45 | 0.88 | 1.96 | 2.22 |
| Outcome | All | Female | Male | BF10 | BFrf | BFmod | BFFemale | BFMale | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | |||||||||
| L | U | L | U | L | U | |||||||||
| Cerebral palsy with GMFCS ≥ 2 | 1.01 | 0.81 | 1.26 | 1.01 | 0.84 | 1.23 | 1.01 | 0.84 | 1.22 | 0.55 | 0.59 | 0.95 | 0.67 | 0.66 |
| Severe visual impairment (trialist defined) | 1.05 | 0.75 | 1.52 | 1.02 | 0.77 | 1.44 | 1.04 | 0.78 | 1.45 | 0.83 | 1.00 | 1.02 | 0.89 | 0.90 |
| Deafness requiring hearing aids or worse | 1.01 | 0.77 | 1.34 | 1.00 | 0.79 | 1.29 | 1.02 | 0.81 | 1.30 | 0.67 | 0.97 | 1.03 | 0.77 | 0.76 |
| Bayley-III language and/or cognitive scale < 85 | 1.00 | 0.92 | 0.92 | 0.99 | 0.91 | 1.07 | 1.01 | 0.94 | 1.09 | 0.22 | 0.16 | 0.77 | 0.40 | 0.39 |
| Bayley-III cognitive scale < 85 | 1.04 | 0.91 | 1.20 | 1.02 | 0.91 | 1.18 | 1.02 | 0.92 | 1.17 | 0.41 | 0.43 | 0.86 | 0.54 | 0.53 |
| Bayley-III language scale < 85 | 1.03 | 0.94 | 1.13 | 0.99 | 0.91 | 1.11 | 1.03 | 0.95 | 1.12 | 0.30 | 0.30 | 0.93 | 0.54 | 0.51 |
| Bayley-III language or cognitive scale < 70 | 0.96 | 0.81 | 1.12 | 0.98 | 0.82 | 1.11 | 0.98 | 0.83 | 1.11 | 0.48 | 0.51 | 0.91 | 0.58 | 0.58 |
| Bayley-III cognitive scale < 70 | 1.02 | 0.82 | 1.30 | 1.02 | 0.84 | 1.27 | 1.01 | 0.84 | 1.25 | 0.58 | 1.00 | 0.97 | 0.68 | 0.67 |
| Bayley-III language scale < 70 | 1.01 | 0.85 | 1.20 | 1.00 | 0.86 | 1.16 | 1.01 | 0.88 | 1.17 | 0.43 | 0.43 | 0.92 | 0.56 | 0.57 |
| Outcome | All | Female | Male | BF10 | BFrf | BFmod | BFFemale | BFMale | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | |||||||||
| L | U | L | U | L | U | |||||||||
| PDA medically or surgically treated | 1.01 | 0.96 | 1.07 | 1.00 | 0.94 | 1.06 | 1.01 | 0.96 | 1.07 | 0.17 | 0.05 | 0.60 | 0.30 | 0.30 |
| PDA surgically treated | 1.13 | 0.97 | 1.32 | 1.10 | 0.94 | 1.33 | 1.09 | 0.93 | 1.32 | 1.35 | 0.38 | 0.90 | 1.06 | 1.06 |
| Severe NEC | 1.26 | 1.04 | 1.53 | 1.24 | 0.96 | 1.53 | 1.26 | 1.01 | 1.55 | 9.94 | 0.43 | 0.99 | 5.82 | 6.52 |
| Treated ROP | 0.81 | 0.65 | 1.03 | 0.84 | 0.65 | 1.07 | 0.83 | 0.65 | 1.06 | 3.36 | 8.97 | 0.98 | 2.40 | 2.43 |
| Positive airway press with ETT at 36 weeks’ PMA | 0.99 | 0.82 | 1.19 | 0.97 | 0.83 | 1.15 | 1.02 | 0.86 | 1.18 | 0.47 | 0.71 | 1.15 | 0.73 | 0.72 |
| Positive airway press w/o ETT at 36 weeks’ PMA | 1.10 | 0.81 | 1.02 | 0.92 | 0.80 | 1.05 | 0.94 | 0.82 | 1.06 | 1.32 | 0.70 | 0.83 | 1.07 | 0.99 |
| Suppl. O2 w/o positive press. at 36 weeks’ PMA | 0.83 | 0.75 | 0.92 | 0.82 | 0.73 | 0.93 | 0.84 | 0.75 | 0.95 | 99.49 | 0.21 | 0.93 | 49.31 | 31.11 |
| Moderate-to-severe BPD | 0.89 | 0.82 | 0.95 | 0.85 | 0.78 | 0.97 | 0.93 | 0.83 | 1.03 | 14.44 | 1.10 | 3.41 | 12.32 | 1.32 |
| Discharged home on oxygen | 1.01 | 0.90 | 1.13 | 1.00 | 0.91 | 1.11 | 1.01 | 0.92 | 1.12 | 0.30 | 0.38 | 0.83 | 0.46 | 0.46 |
| Readmission to hospital | 1.01 | 0.95 | 1.08 | 0.98 | 0.92 | 1.05 | 1.02 | 0.96 | 1.09 | 0.17 | 0.25 | 0.91 | 0.43 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huizing, M.J.; Hundscheid, T.M.; Bartoš, F.; Villamor, E. A Bayesian Reanalysis of the Overall and Sex-Disaggregated Results of the Neonatal Oxygenation Prospective Meta-Analysis (NeOProM). Antioxidants 2024, 13, 509. https://doi.org/10.3390/antiox13050509
Huizing MJ, Hundscheid TM, Bartoš F, Villamor E. A Bayesian Reanalysis of the Overall and Sex-Disaggregated Results of the Neonatal Oxygenation Prospective Meta-Analysis (NeOProM). Antioxidants. 2024; 13(5):509. https://doi.org/10.3390/antiox13050509
Chicago/Turabian StyleHuizing, Maurice Jacob, Tamara Maria Hundscheid, František Bartoš, and Eduardo Villamor. 2024. "A Bayesian Reanalysis of the Overall and Sex-Disaggregated Results of the Neonatal Oxygenation Prospective Meta-Analysis (NeOProM)" Antioxidants 13, no. 5: 509. https://doi.org/10.3390/antiox13050509






