Protective Role of Dietary Berries in Cancer
Abstract
:1. Background
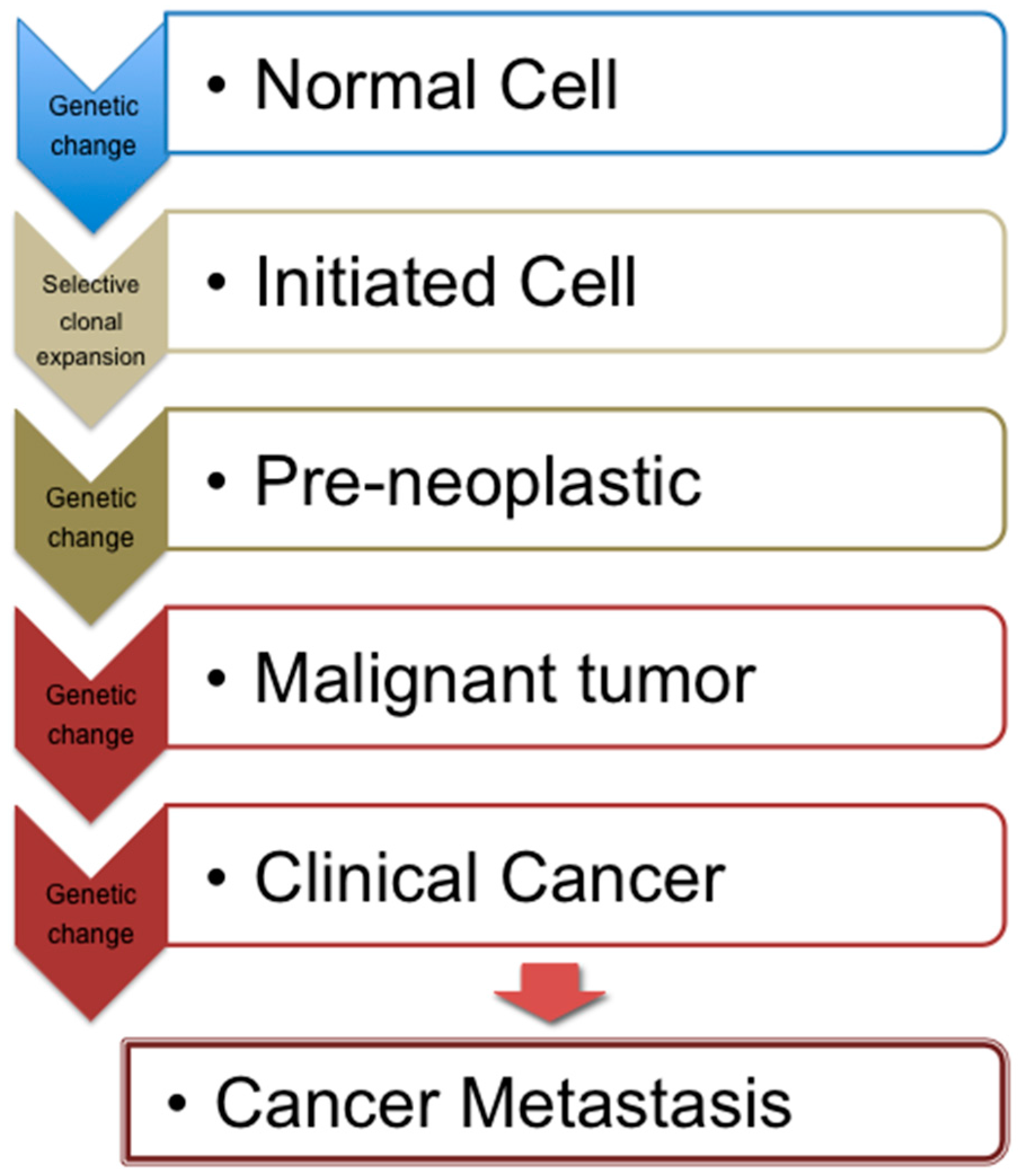
2. Cancer Development and Associated Mechanisms
3. Risk Factors for Cancer
4. Berries and Cancer
4.1. Rationale of the Current Review
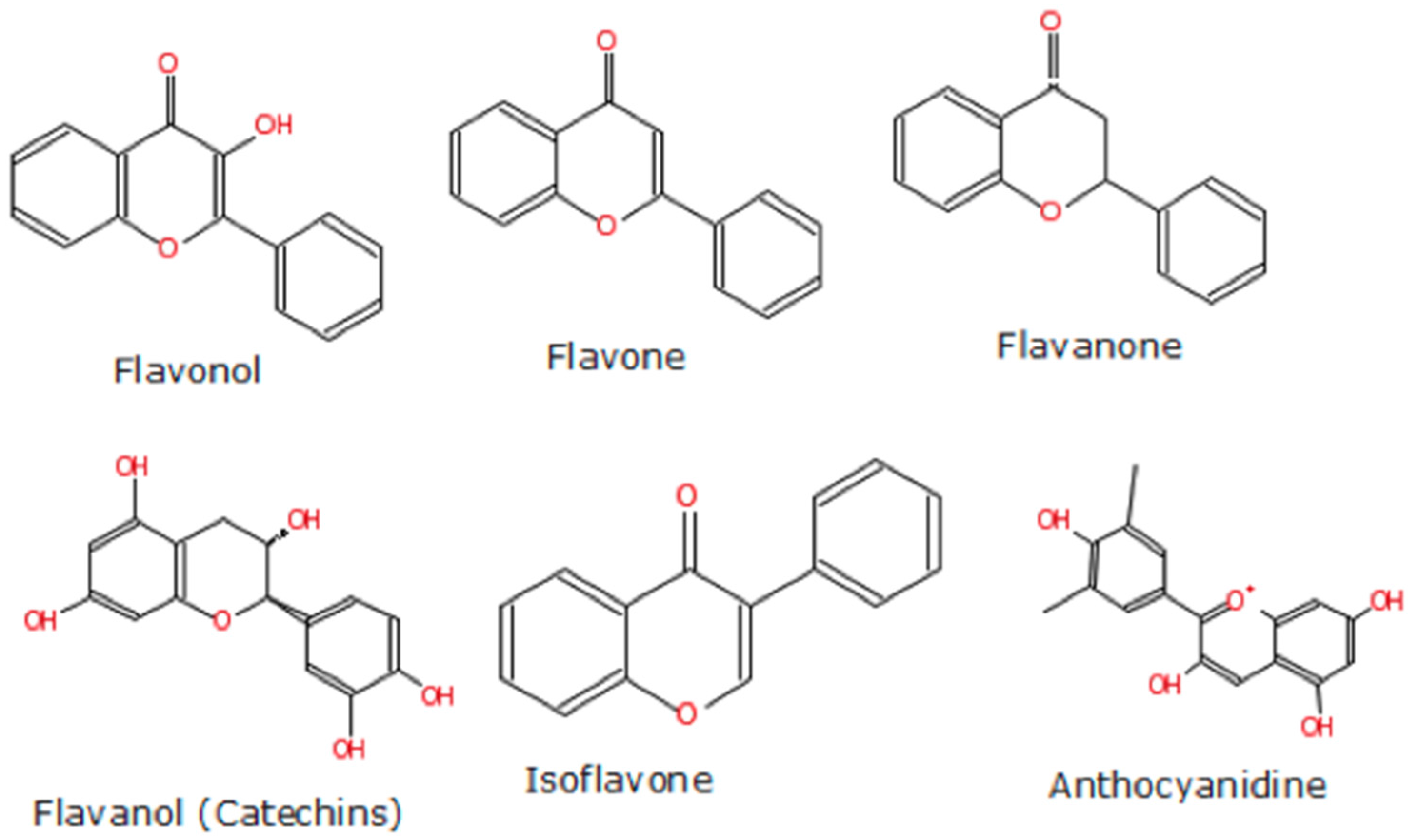
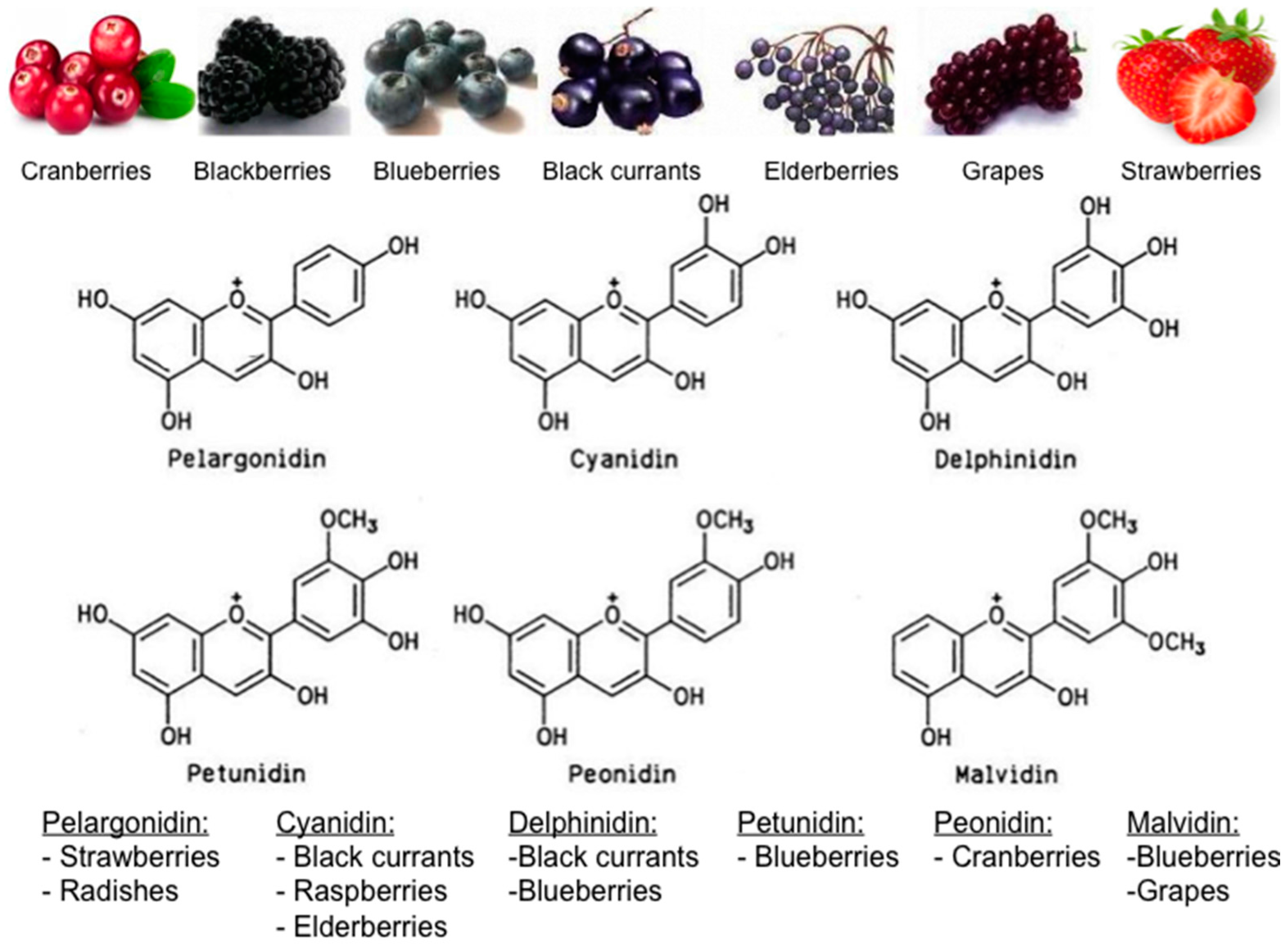
4.2. Berry Types and Composition
4.3. Mechanisms Associated with Berries’ Anticancer Capacity
4.4. Berries’ Potential for Cancer Risk Reduction Due to Specific Constituents
5. The Role of Dietary Berries in Various Types of Cancer
5.1. Cancers of the GI Tract
5.1.1. Diet and Colon Cancer—Berries/Phenolics and Colon Cancer
In Vivo Studies
Human Studies
5.2. Esophageal Cancer
5.3. Breast Cancer
5.4. Miscellaneous Cancers and Berries
6. Conclusions
Supplementary Materials
Conflicts of Interest
References
- Stewart, B.W.; Wild, C.P. (Eds.) World Cancer Report; World Health Organization: Geneva, Switzerland; IARC Publication: Lyon, France, 2014.
- Siegel, R.L.; Miller, K.D.; Ahmedin, J. Cancer Statistics 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Available online: www.ahrq.gov (accessed on 4 April 2016).
- Center for Disease Control. Available online: www.cdc.gov (accessed on 4 April 2016).
- Boivin, D.; Blanchette, M.; Barrette, S.; Moghrabi, A.; Béliveau, R. Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 2007, 27, 937–948. [Google Scholar] [PubMed]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control 1991, 2, 325–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [PubMed]
- Chun, O.K.; Chung, S.J.; Claycombe, K.J.; Song, W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J. Nutr. 2008, 138, 753–760. [Google Scholar] [PubMed]
- Corley, J.; Kyle, J.A.; Starr, J.M.; McNeill, G.; Deary, I.J. Dietary factors and biomarkers of systemic inflammation in older people: The Lothian Birth Cohort 1936. Br. J. Nutr. 2015, 114, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Prasad, S.; Phromnoi, K.; Ravindran, J.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Thiocolchicoside exhibits anticancer effects through downregulation of NF-κB pathway and its regulated gene products linked to inflammation and cancer. Cancer Prev. Res. 2010, 3, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Schetter, A.J.; Heegaard, N.H.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Available online: www.cancer.gov (accessed on 4 April 2016).
- Manson, M.M. Cancer prevention—The potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Hickey, M.; King, C. The Cambridge Illustrated Glossary of Botanical Terms, 1st ed.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Kong, A.N. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.N.; Kuo, W.H.; Chiang, C.L.; Chiou, H.L.; Hsieh, Y.S.; Chu, S.C. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem. Biol. Interact. 2006, 163, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Vellayappan, M.V.; Narasimhan, G.; Supriyanto, E.; Octorina Dewi, D.E.; Narayanan, A.L.; Balaji, A.; Subramanian, A.P.; Yusof, M. Chemopreventive effect of apple and berry fruits against colon cancer. World J. Gastroenterol. 2014, 20, 17029–17036. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, S.; Kautenburger, T.; Habermann, N.; Sauer, J.; Dietrich, H.; Will, F.; Pool-Zobel, B.L. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol. Carcinog. 2006, 45, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Pahlke, G.; Balavenkatraman, K.K.; Böhmer, F.D.; Marko, D. Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J. Agric. Food Chem. 2007, 55, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.K.; Salvi, V.P.; Nair, C.K. Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions. Mol. Cell. Biochem. 2005, 280, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Wang, L.S.; Casto, B.C. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Terabe, K.; Umeda, T. Influence of flavonoids on cell cycle phase as analyzed by flow-cytometry. Cancer Biother. Radiopharm. 1997, 12, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 5, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, A.M.; Szakmary, A.; Schiestl, R.H. Mechanisms of intestinal inflammation and development of associated cancers: Lessons learned from mouse models. Mutat. Res. 2010, 705, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ahn-Jarvis, J.H.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Vodovotz, Y. Characterization of black raspberry functional food products for cancer prevention human clinical trials. J. Agric. Food Chem. 2014, 62, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; McDougall, G.J.; Stewart, D.; Pereira-Caro, G.; González-Barrio, R.; Allsopp, P.; Magee, P.; Crozier, A.; Rowland, I.; Gill, C.I. Persistence of anticancer activity in berry extracts after simulated gastrointestinal digestion and colonic fermentation. PLoS ONE 2012, 7, e49740. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wu, Z.; Huang, L.; Qiu, H.; Wang, L.; Li, L.; Yao, L.; Kang, K.; Qu, J.; Wu, Y.; et al. Mulberry fruit prevents LPS-induced NF-κB/pERK/MAPK signals in macrophages and suppresses acute colitis and colorectal tumorigenesis in mice. Sci. Rep. 2015, 5, 17348. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.; Schwarz, M.; Boocock, D.; Winterhalter, P.; Steward, W.P.; Gescher, A.J.; Marczylo, T.H. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis-relationship with tissue anthocyanin levels. Int. J. Cancer 2006, 119, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A.; et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Fang, W.; Wang, L.S.; Stoner, G.D.; Yang, W. Black raspberries inhibit intestinal tumorigenesis in apc1638+/− and Muc2−/− mouse models of colorectal cancer. Cancer Prev. Res. 2010, 3, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Kuo, C.T.; Huang, T.H.; Yearsley, M.; Oshima, K.; Stoner, G.D.; Yu, J.; Lechner, J.F.; Huang, Y.W. Black raspberries protectively regulate methylation of Wnt pathway genes in precancerous colon tissue. Cancer Prev. Res. 2013, 6, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Skaer, C.W.; Wang, H.T.; Stirdivant, S.M.; Young, M.R.; Oshima, K.; Stoner, G.D.; Lechner, J.F.; Huang, Y.W.; Wang, L.S. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: Relation to metabolite profiles. Carcinogenesis 2015, 36, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; La Perle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Arnold, M.; Huang, Y.W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Skaer, C.W.; Stirdivant, S.M.; Young, M.R.; Stoner, G.D.; Lechner, J.F.; Huang, Y.W.; Wang, L.S. Beneficial Regulation of Metabolic Profiles by Black Raspberries in Human Colorectal Cancer Patients. Cancer Prev. Res. 2015, 8, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Mentor-Marcel, R.A.; Bobe, G.; Sardo, C.; Wang, L.S.; Kuo, C.T.; Stoner, G.; Colburn, N.H. Plasma cytokines as potential response indicators to dietary freeze-dried black raspberries in colorectal cancer patients. Nutr. Cancer 2012, 64, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Kuo, C.T.; Stoner, K.; Yearsley, M.; Oshima, K.; Yu, J.; Huang, T.H.; Rosenberg, D.; Peiffer, D.; Stoner, G.; et al. Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (DSS)-induced ulcerative colitis. Carcinogenesis 2013, 34, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Misikangas, M.; Pajari, A.M.; Päivärinta, E.; Oikarinen, S.I.; Rajakangas, J.; Marttinen, M.; Tanayama, H.; Törrönen, R.; Mutanen, M. Three Nordic berries inhibit intestinal tumorigenesis in multiple intestinal neoplasia/+ mice by modulating beta-catenin signaling in the tumor and transcription in the mucosa. J. Nutr. 2007, 137, 2285–2290. [Google Scholar] [PubMed]
- Rajakangas, J.; Misikangas, M.; Päivärinta, E.; Mutanen, M. Chemoprevention by white currant is mediated by the reduction of nuclear beta-catenin and NF-kappaB levels in Min mice adenomas. Eur. J. Nutr. 2008, 47, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pierini, R.; Gee, J.M.; Belshaw, N.J.; Johnson, I.T. Flavonoids and intestinal cancers. Br. J. Nutr. 2008, 99, ES53–ES59. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutat. Res. 2004, 551, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Wang, L.S.; Zikri, N.; Chen, T.; Hecht, S.S.; Huang, C.; Sardo, C.; Lechner, J.F. Cancer prevention with freeze-dried berries and berry components. Semin. Cancer Biol. 2007, 17, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Liu, Z.; Kruger, M. The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World J. Microbiol. Biotechnol. 2010, 26, 1735–1743. [Google Scholar] [CrossRef]
- Molan, A.L.; Liu, Z.; Plimmer, G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Dombkowski, A.A.; Reen, R.K.; Cukovic, D.; Salagrama, S.; Wang, L.S.; Lechner, J.F. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both phenethyl isothiocyanate and black raspberries. Cancer Res. 2008, 68, 6460–6467. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; McIntyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Wang, L.S.; Zimmerman, N.P.; Ransom, B.W.; Carmella, S.G.; Kuo, C.T.; Chen, J.H.; Oshima, K.; Huang, Y.W.; Hecht, S.S.; et al. Dietary Consumption of Black Raspberries or Their Anthocyanin Constituents Alters Innate Immune Cell Trafficking in Esophageal Cancer. Cancer Immunol. Res. 2016, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Dombkowski, A.A.; Seguin, C.; Rocha, C.; Cukovic, D.; Mukundan, A.; Henry, C.; Stoner, G.D. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carcinogenesis. Mol. Carcinog. 2011, 50, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hwang, H.; Rose, M.E.; Nines, R.G.; Stoner, G.D. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: Down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006, 66, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Rose, M.E.; Hwang, H.; Nines, R.G.; Stoner, G.D. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis 2006, 27, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Reen, R.K.; Nines, R.; Stoner, G.D. Modulation of N-nitrosomethylbenzylamine metabolism by black raspberries in the esophagus and liver of Fischer 344 rats. Nutr. Cancer 2006, 54, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Wang, L.S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Weh, K.M.; Zeyzus-Johns, B.; Perez, L.N.; Howell, A.B. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget 2015, 6, 33438–33455. [Google Scholar] [PubMed]
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Ravoori, S.; Vadhanam, M.V.; Aqil, F.; Gupta, R.C. Inhibition of estrogen-mediated mammary tumorigenesis by blueberry and black raspberry. J. Agric. Food Chem. 2012, 60, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, H.S.; Warri, A.M.; Woode, D.R.; Hilakivi-Clarke, L.; Clarke, R. Influence of berry polyphenols on receptor signaling and cell-death pathways: Implications for breast cancer prevention. J. Agric. Food Chem. 2012, 60, 5693–5708. [Google Scholar] [CrossRef] [PubMed]
- Casto, B.C.; Knobloch, T.J.; Galioto, R.L.; Yu, Z.; Accurso, B.T.; Warner, B.M. Chemoprevention of oral cancer by lyophilized strawberries. Anticancer Res. 2013, 33, 4757–4566. [Google Scholar] [PubMed]
- Somasagara, R.R.; Hegde, M.; Chiruvella, K.K.; Musini, A.; Choudhary, B.; Raghavan, S.C. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE 2012, 7, e47021. [Google Scholar] [CrossRef] [PubMed]
- Balansky, R.; Ganchev, G.; Iltcheva, M.; Kratchanova, M.; Denev, P.; Kratchanov, C.; Polasa, K.; D’Agostini, F.; Steele, V.E.; de Flora, S. Inhibition of lung tumor development by berry extracts in mice exposed to cigarette smoke. Int. J. Cancer 2012, 131, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Carlton, P.S.; Kresty, L.A.; Siglin, J.C.; Morse, M.A.; Lu, J.; Morgan, C.; Stoner, G.D. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis 2001, 22, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, F.; Qian, J.; Guo, M.; Zhang, H.; Tang, X.; Chen, F.; Stoner, G.D.; Wang, X. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev. Res. 2012, 5, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiong, L.; Zhang, X.; Chen, T. Lyophilized strawberries prevent 7,12-dimethylbenz[α]anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters. J. Funct. Foods 2015, 15, 476–486. [Google Scholar] [CrossRef]
- Bishayee, A.; Thoppil, R.J.; Darvesh, A.S.; Ohanyan, V.; Meszaros, J.G.; Bhatia, D. Pomegranate phytoconstituents blunt the inflammatory cascade in a chemically induced rodent model of hepatocellular carcinogenesis. J. Nutr. Biochem. 2013, 24, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, T.J.; Uhrig, L.K.; Pearl, D.K.; Casto, B.C.; Warner, B.M.; Clinton, S.K.; Sardo-Molmenti, C.L.; Ferguson, J.M.; Daly, B.T.; Riedl, K.; et al. Suppression of Proinflammatory and Prosurvival Biomarkers in Oral Cancer Patients Consuming a Black Raspberry Phytochemical-Rich Troche. Cancer Prev. Res. 2016, 9, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.R.; Tong, M.; Shumway, B.S.; Curran, A.E.; Larsen, P.E.; Ness, G.M.; Kennedy, K.S.; Blakey, G.H.; Kushner, G.M.; Vickers, A.M.; et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: Results from a multicentered, placebo-controlled clinical trial. Clin. Cancer Res. 2014, 20, 1910–1924. [Google Scholar] [PubMed]
- Rossi, M.; Rosato, V.; Bosetti, C.; Lagiou, P.; Parpinel, M.; Bertuccio, P.; Negri, E.; La Vecchia, C. Flavonoids, proanthocyanidins, and the risk of stomach cancer. Cancer Causes Control 2010, 21, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Lugo, A.; Lagiou, P.; Zucchetto, A.; Polesel, J.; Serraino, D.; Negri, E.; Trichopoulos, D.; La Vecchia, C. Proanthocyanidins and other flavonoids in relation to pancreatic cancer: A case-control study in Italy. Ann. Oncol. 2012, 23, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr. Nutr. Rep. 2016, 5, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13 (Suppl. 2), S14. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, B.; Padrão, P.; Castro, C.; Ferro, A.; Morais, S.; Lunet, N. Worldwide burden of gastric cancer in 2012 that could have been prevented by increasing fruit and vegetable intake and predictions for 2025. Br. J. Nutr. 2016, 115, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K. Amino Acids and Immune Response: A role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Varamini, B. Roles of hormones and signaling molecules in describing the relationship between obesity and colon cancer. Pathol. Oncol. Res. 2011, 17, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Sikalidis, A.K.; Solis Herruzo, J.A. Colorectal cancer: influence of diet and lifestyle factors. Rev. Esp. Enferm. Dig. 2005, 97, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Fitch, M.D.; Fleming, S.E. Risk of Colonic Cancer is Not Higher in the Obese Lepob Mouse Model Compared to Lean Littermates. Pathol. Oncol. Res. 2013, 19, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Fitch, M.D.; Fleming, S.E. Diet Induced Obesity Increases the Risk of Colonic Tumorigenesis in Mice. Pathol. Oncol. Res. 2013, 19, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Kristo, A.S.; Matthan, N.R.; Lichtenstein, A.H. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: Review of randomized controlled-feeding trials. Nutrients 2013, 5, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Kristo, A.S.; Schuschke, D.A.; Klimis-Zacas, D. Wild blueberry consumption affects arterial vascular function in the obese Zucker rat. Appl. Physiol. Nutr. Metab. 2014, 39, 255–261. [Google Scholar] [CrossRef] [PubMed]


| Type of Cancer | Deaths (Figures in Millions) |
|---|---|
| Lung | 1.590 |
| Liver | 0.745 |
| Stomach | 0.723 |
| Colorectal | 0.694 |
| Breast | 0.521 |
| Esophageal | 0.400 |
| Total | 4.673 |
| Mechanism | Mode of Action | Bioactive Compound * |
|---|---|---|
| Anti-oxidative action | ROS sequestration ↑ GSH | Epigallocatechin-3-gallate (EGCG) |
| Effects on enzymes of phase-I and -II | ↓ CYP1A1 ↓ LDH | Quercetin, kaempferol |
| ↑ UDPGT ↑ NQO | Ellagic acid Chlorogenic acid | |
| Cell-cycle arrest | ↓ cyclin D, E ↓ CDK 1, 2, 4 ↓ PCNA | EGCG |
| ↑ cyclin E ↓ cyclin A, B1 | Ellagic acid | |
| Apoptosis | ↑ ROS in cancer cells ↑ caspase-3, -7, -8 ↑ cytochrome c ↑ Blc-2, Blc-XL | EGCG |
| ↑ caspase-3, -7, -9 ↑ cytochrome c ↑ PARP cleavage | Quercetin | |
| Anti-proliferation/Anti-survival | ↓ GFR/Ras/MAPK & PI3K/Akt ↓ c-fos ↓ erg-1 ↓ PI3K ↓ ERK ↓ Akt phosphorylation ↓ NF-κΒ | EGCG |
| Anti-inflammatory action | ↓ COX-1 ↓ COX-2 | Gallic acid |
| ↓ TNF-α ↓ COX-2 | EGCG | |
| Anti-angiogenesis | ↓ VEGF ↓ PDGF ↓ HIF-1α | EGCG, anthocyanin berry extracts |
| Metastasis inhibition | ↓ MMP-9 ↓ mRNA stabilizing factor HuR | EGCG |
| Cell adhesion and movement inhibition | ↓ MRLC phosphorylation ↓ Vimentin phosphorylation | EGCG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective Role of Dietary Berries in Cancer. Antioxidants 2016, 5, 37. https://doi.org/10.3390/antiox5040037
Kristo AS, Klimis-Zacas D, Sikalidis AK. Protective Role of Dietary Berries in Cancer. Antioxidants. 2016; 5(4):37. https://doi.org/10.3390/antiox5040037
Chicago/Turabian StyleKristo, Aleksandra S., Dorothy Klimis-Zacas, and Angelos K. Sikalidis. 2016. "Protective Role of Dietary Berries in Cancer" Antioxidants 5, no. 4: 37. https://doi.org/10.3390/antiox5040037





