Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control
Abstract
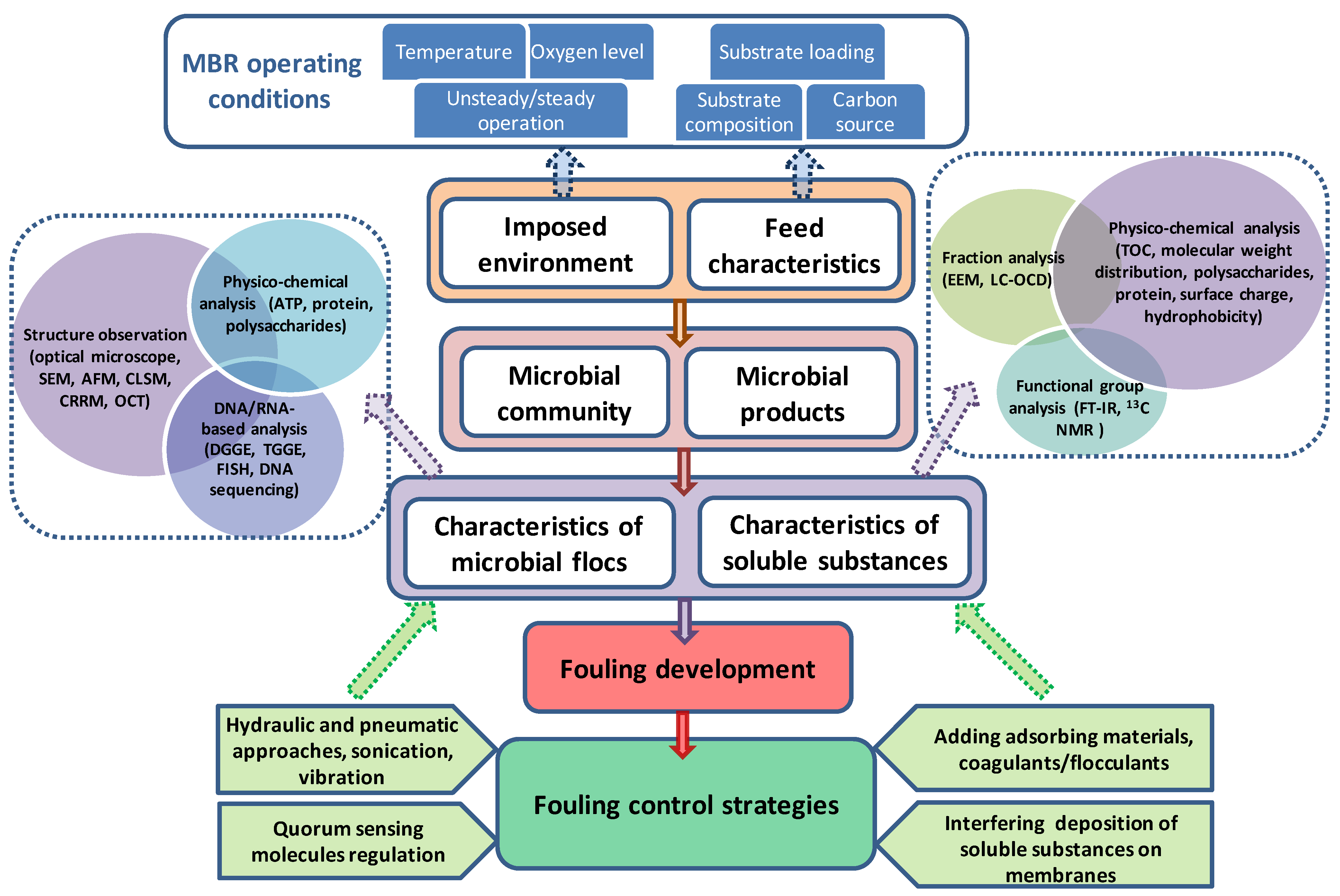
:1. Introduction
1.1. MBRs Application and Development
1.2. Membrane Fouling and Potential Foulants
2. Key Factors Influencing Microbial Behaviors in MBRs
2.1. Feed Characteristics
2.2. Imposed Environment
2.2.1. Oxygen Level
2.2.2. Temperature
2.2.3. Steady-State vs. Unsteady-State Operation of MBRs
3. State-of-the-Art Techniques Characterizing Biofoulants in MBRs
3.1. Microbial Flocs
3.2. Soluble Foulants
4. Effective Strategies Controlling Microbial Relevant Fouling in MBRs
4.1. Microbial Flocs-Dominant Fouling Control
4.2. SMP-Dominant Fouling Control
5. Conclusions
Acknowledgements
References
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef]
- Le-Clech, P. Membrane bioreactors and their uses in wastewater treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Alturki, A.A.; Tadkaew, N.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010, 365, 206–215. [Google Scholar] [CrossRef]
- Kent, F.C.; Farahbakhsh, K.; Mahendran, B.; Jaklewicz, M.; Liss, S.N.; Zhou, H. Water reclamation using reverse osmosis: Analysis of fouling propagation given tertiary membrane filtration and MBR pretreatments. J. Membr. Sci. 2011, 382, 328–338. [Google Scholar] [CrossRef]
- An, Y.; Yang, F.; Chua, H.C.; Wong, F.S.; Wu, B. The integration of methanogenesis with shortcut nitrification and denitrification in a combined UASB with MBR. Bioresour. Technol. 2008, 99, 3714–3720. [Google Scholar] [CrossRef]
- Li, X.; Gao, F.; Hua, Z.; Du, G.; Chen, J. Treatment of synthetic wastewater by a novel MBR with granular sludge developed for controlling membrane fouling. Sep. Purif. Technol. 2005, 46, 19–25. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Fane, A.G.; Pasquier, A.C.S.; Wu, B. A novel membrane bioreactor based on membrane distillation. Desalination 2008, 223, 386–395. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Fane, A.G.; Pasquier, A.C.S.; Wu, B.; Wong, F.S. Experimental study and design of submerged membrane distillation bioreactor. Chem. Eng. Technol. 2009, 32, 38–44. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: a low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Cornelissen, E.R.; Harmsen, D.; de Korte, K.F.; Ruiken, C.J.; Qin, J.-J.; Oo, H.; Wessels, L.P. Membrane fouling and process performance of forward osmosis membranes on activated sludge. J. Membr. Sci. 2009, 319, 158–168. [Google Scholar]
- Lay, W.C.L.; Zhang, J.; Tang, C.Y.; Wang, R.; Liu, Y.; Fane, A.G. Factors affecting flux performance of forward osmosis systems. J. Membr. Sci. 2012, 394–395, 151–168. [Google Scholar]
- Zhang, J.; Lay, W.C.L.; Chou, S.; Tang, C.Y.; Wang, R.; Fane, A.G. Membrane biofouling and scaling in forward osmosis membrane bioreactor. J. Membr. Sci. 2012, 403–404, 8–14. [Google Scholar]
- He, Y.; Xu, P.; Li, C.; Zhang, B. High-concentration food wastewater treatment by an anaerobic membrane bioreactor. Water Res. 2005, 39, 4110–4118. [Google Scholar] [CrossRef]
- Lin, H.J.; Xie, K.; Mahendran, B.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Sludge properties and their effects on membrane fouling in submerged anaerobic membrane bioreactors (SAnMBRs). Water Res. 2009, 43, 3827–3837. [Google Scholar] [CrossRef]
- Padmasiri, S.I.; Zhang, J.; Fitch, M.; Norddahl, B.; Morgenroth, E.; Raskin, L. Methanogenic population dynamics and performance of an anaerobic membrane bioreactor (AnMBR) treating swine manure under high shear conditions. Water Res. 2007, 41, 134–144. [Google Scholar] [CrossRef]
- Wang, Y.K.; Sheng, G.P.; Li, W.W.; Huang, Y.X.; Yu, Y.Y.; Zeng, R.J.; Yu, H.Q. Development of a novel bioelectrochemical membrane reactor for wastewater treatment. Environ. Sci. Technol. 2011, 45, 9256–9261. [Google Scholar]
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar]
- Nady, N.; Franssen, M.C.R.; Zuihof, H.; Eldin, M.S.M.; Boom, R.; Schroen, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.; Wang, H.; Brash, J.; Chen, H. Anti-fouling bioactive surfaces. Acta Biomater. 2011, 7, 1550–1557. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane strucutres: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Rajabi, L.; Zinadini, S.; Derakhshan, A.A. Boehmite nanoparticles as a new nanofilter for preparation of antifouling mixed matrix membranes. J. Membr. Sci. 2012, 401–402, 132–143. [Google Scholar]
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin Z incorporation. Colloids Surf. B Biointerfaces 2012, 94, 333–340. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Chung, T.S.; Jeyaseelan, K.; Armugam, A.; Chen, Z.; Yang, J.; Hong, M. Planar biomimetic aquaporin-incorporated triblock copolymer membranes on porous alumina supports for nanofiltration. J. Membr. Sci. 2012, 409–410, 34–43. [Google Scholar]
- Zhong, P.S.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. Aquaporin-embeded biomimetic membranes for nanofiltration. J. Membr. Sci. 2012, 407–408, 27–33. [Google Scholar]
- Wang, H.; Chung, T.S.; Tong, Y.W.; Meier, W.; Chen, Z.; Hong, M.; Jeyaseelan, K.; Armugam, A. Preparation and characterization of pore-suspending biomimetic membranes embedded with Aquaporin Z on carboxylated polyethylene glycolpolymer cushion. Soft Matter. 2011, 7, 7274–7280. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Interpretation of fouling characteristics of ultrafiltration membranes during the filtration of membrane bioreactror mixed liquor. J. Membr. Sci. 2005, 264, 151–160. [Google Scholar] [CrossRef]
- Defrance, L.; Jaffrin, M.Y.; Gupta, B.; Paullier, P.; Geaugery, V. Contribution of various constituents of activated sludge to membrane bioreactor fouling. Bioresour. Technol. 2000, 73, 105–112. [Google Scholar] [CrossRef]
- Domínguez, L.; Rodríguez, M.; Prats, D. Effect of different extraction methods on bound EPS from MBR sludges. Part I: Influence of extraction methods over three-dimensional EEM fluorescence spectroscopy fingerprint. Desalination 2010, 261, 19–26. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Relation between extraction protocols for activated sludge extracellular polymeric substances (MBR) and MBR complexation properties. Part I: Comparison of the efficiency of eight MBR extractions methods. Enzyme Microb. Technol. 2006, 38, 237–245. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H. Extraction of extracellular polymeric substances (MBR) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar]
- Chang, I.S.; Lee, C.H. Membrane filtration characterisitcs in membrane coupled activated sludge—The effect of physiological states of activated sludge on membrane fouling. Desalination 1998, 120, 221–223. [Google Scholar] [CrossRef]
- Lee, W.; Kang, S.; Shin, H. Sludge characteristics and their contribution to microfiltration in submerged membrane bioreactor. J. Membr. Sci. 2003, 216, 217–227. [Google Scholar] [CrossRef]
- Bouhabila, E.H.; Aim, R.B.; Buisson, H. Fouling characterisation in membrane bioreactors. Sep. Purif. Technol. 2001, 123–132. [Google Scholar]
- Chang, I.S.; Kim, S.N. Water treatment using membrane filtration—Effect of biosolids concentration on cake resistance. Process Biochem. 2005, 40, 1307–1314. [Google Scholar] [CrossRef]
- Wisniewski, C.; Grasmick, A. Floc Size Distribution in a Membrane Bioreactor and Consequences for Membrane Fouling. Colloids Surf. 1998, 138, 403–411. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Liu, Y.; Fane, A.G. A comparison of membrane fouling under constant and variable organic loadings in submerge membrane bioreactors. Water Res. 2010, 44, 5407–5413. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Tang, S. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor. Water Res. 2009, 43, 2504–2512. [Google Scholar] [CrossRef]
- Jarusutthirak, C.; Amy, G. Role of soluble microbial products (SMP) in membrane fouling and flux decline. Environ. Sci. Technol. 2006, 40, 969–974. [Google Scholar] [CrossRef]
- Jiang, T.; Kennedy, M.D.; De Schepper, V.; Nam, S.N.; Nopens, I.; Vanrolleghem, P.A.; Amy, G. Characterization of soluble microbial products and their fouling impacts in membrane bioreactors. Environ. Sci. Technol. 2010, 44, 6642–6648. [Google Scholar]
- Ni, B.J.; Rittmann, B.E.; Yu, H.Q. Soluble microbial products and their implications in mixed culture biotechnology. Trends Biotechnol. 2011, 29, 454–463. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, X.Y. Accumulation of biopolymer clusters in a submerged membrane bioreactor and its effect on membrane fouling. Water Res. 2008, 42, 855–862. [Google Scholar] [CrossRef]
- Sun, F.Y.; Wang, X.M.; Li, X.Y. Effect of biopolymer clusters on the fouling property of sludge from a membrane bioreactor (MBR) and its control by ozonation. Process Biochem. 2011, 46, 162–167. [Google Scholar] [CrossRef]
- Meng, F.; Zhou, Z.; Ni, B.J.; Zheng, X.; Huang, G.; Jia, X.; Li, S.; Xiong, Y.; Kraume, M. Characterization of the size-fractionated biomacromolecules: Tracking their role and fate in a membrane bioreactor. Water Res. 2011, 45, 4661–4671. [Google Scholar] [CrossRef]
- Meng, F.; Drews, A.; Mehrez, R.; Iversen, V.; Ernst, M.; Yang, F.; Jekel, M.; Kraume, M. Occurrence, source, and fate of dissolved organic matter (DOM) in a pilot-scale membrane bioreactor. Environ. Sci. Technol. 2009, 43, 8821–8826. [Google Scholar]
- Wang, Z.; Wu, Z.; Tang, S. Characterization of dissolved organic matter in a submerged membrane bioreactor by using three-dimensional excitation and emission matrix fluorescence spectroscopy. Water Res. 2009, 43, 1533–1540. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Kennedy, M.D.; Amy, G.L.; Schippers, J.C. The fate of Transparent Exopolymer Particles (TEP) in integrated membrane systems: Removal through pre-treatment processes and deposition on reverse osmosis membranes. Water Res. 2009, 43, 5039–5052. [Google Scholar] [CrossRef]
- de la Torre, T.; Lesjean, B.; Drews, A.; Kraume, M. Monitoring of transparent exopolymer particles (TEP) in a membrane bioreactor (MBR) and correlation with other fouling indicators. Water Sci. Technol. 2008, 58, 1903–1909. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Berman-Frank, I.; Liberman, B.; Rahav, E.; Passow, U.; Berman, T. Transparent exopolymer particles: Potential agents for organic fouling and biofilm formation in desalination and water treatment plants. Desalin. Water Treat. 2009, 3, 138–142. [Google Scholar]
- Berman, T.; Mizrahi, R.i.; Dosoretz, C.G. Transparent exopolymer particles (TEP): A critical factor in aquatic biofilm initiation and fouling on filtration membranes. Desalination 2011, 276, 184–190. [Google Scholar] [CrossRef]
- Berman, T.; Passow, U. Transparent Exopolymer Particles (TEP): An overlooked factor in the process of biofilm formation in aquatic environments. Nat. Preced. 2007. Available online: http://dx.doi.org/10.1038/npre.2007.1182.1 (accessed on 14 August 2012). [CrossRef]
- Passow, U.; Alldredge, A.L. A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnol. Oceanogr. 1995, 40, 1326–1335. [Google Scholar] [CrossRef]
- Wu, B.; Yi, S.; Fane, A.G. Microbial community developments and biomass characteristics in membrane bioreactors under different organic loadings. Bioresour. Technol. 2011, 102, 6808–6814. [Google Scholar] [CrossRef]
- Wu, B.; Yi, S.; Fane, A.G. Effect of substrate composition (C/N/P ratio) on microbial community and membrane fouling tendency of biomass in membrane bioreactors. Sep. Sci. Technol. 2012, 47, 440–445. [Google Scholar] [CrossRef]
- Ahmed, Z.; Lim, B.R.; Cho, J.; Song, K.G.; Kim, K.P.; Ahn, K.H. Biological nitrogen and phosphorus removal and changes in microbial community structure in a membrane bioreactor: Effect of different carbon sources. Water Res. 2008, 42, 198–210. [Google Scholar] [CrossRef]
- Chen, R.; LaPara, T.M. Aerobic biological treatment of low-strength synthetic wastewater in membrane-coupled bioreactors: The structure and function of bacterial enrichment cultures as the net growth rate approaches zero. Microb. Ecol. 2006, 51, 99–108. [Google Scholar] [CrossRef]
- LaPara, T.M.; Klatt, C.G.; Chen, R. Adaptations in bacterial catabolic enzyme activity and community structure in membrane-coupled bioreactors fed simple synthetic wastewater. J. Biotechnol. 2006, 121, 368–380. [Google Scholar] [CrossRef]
- Miura, Y.; Hiraiwa, M.N.; Ito, T.; Itonaga, T.; Watanabe, Y.; Okabe, S. Bacterial community structures in MBRs treating municipal wastewater: Relationship between community stability and reactor performance. Water Res. 2007, 41, 627–637. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, N.; Liu, H.; Du, X.; Liu, Y.; Lin, H. The effect of COD/N ratio on process performance and membrane fouling in a submerged bioreactor. Desalination 2012, 285, 232–238. [Google Scholar] [CrossRef]
- Wu, B.; Yi, S.; Fane, A.G. Microbial behaviors involved in cake fouling in membrane bioreactors under different solids retention times. Bioresour. Technol. 2011, 102, 2511–2516. [Google Scholar] [CrossRef]
- Zhang, J.; Chua, H.C.; Zhou, J.; Fane, A.G. Effect of sludge retention time on membrane bio-fouling intensity in a submerged membrane bioreactor. Sep. Sci. Technol. 2006, 41, 1313–1329. [Google Scholar] [CrossRef]
- Malamis, S.; Andreadakis, A. Fractionation of proteins and carbohydrates of extracellular polymeric substances in a membrane bioreactor system. Bioresour. Technol. 2009, 100, 3350–3357. [Google Scholar] [CrossRef]
- Trussell, R.S.; Merlo, R.P.; Hermanowicz, S.W.; Jenkins, D. The effect of organic loading on process performance and membrane fouling in a submerged membrane bioreactor treating municipal wastewater. Water Res. 2006, 40, 2675–2683. [Google Scholar] [CrossRef]
- Gao, D.-W.; Fu, Y.; Tao, Y.; Li, X.-X.; Xing, M.; Gao, X.-H.; Ren, N.-Q. Linking microbial community structure to membrane biofouling associated with varying dissovled oxygen concentrations. Bioresour. Technol. 2011, 102, 5626–5633. [Google Scholar] [CrossRef]
- Ma, B.C.; Lee, Y.N.; Park, J.S.; Lee, C.H.; Lee, S.H.; Chang, I.S.; Ahn, T.S. Correlation between dissolved oxygen concentration, microbial community and membrane permeability in a membrane bioreactor. Process Biochem. 2006, 41, 1165–1172. [Google Scholar] [CrossRef]
- Tocchi, C.; Federici, E.; Fidati, L.; Manzi, R.; Vincigurerra, V.; Petruccioli, M. Aerobic treatment of dairy wastewater in an industrial three-reactor plant: Effect of aeration regime on performances and on protozoan and bacterial communities. Water Res. 2012, 46, 3334–3344. [Google Scholar] [CrossRef]
- Jin, Y.L.; Lee, W.N.; Lee, C.H.; Chang, I.S.; Huang, X.; Swaminathan, T. Effect of DO concentration on biofilm structure and membrane filterability in submerged membrane bioreactor. Water Res. 2006, 40, 2829–2836. [Google Scholar] [CrossRef]
- Molina-Munoz, M.; Poyatos, J.M.; Rodelas, B.; Pozo, C.; Manzanera, M.; Hontoria, E.; Gonzalez-Lopez, J. Microbial enzymatic activities in a pilot-scale MBR experimental plant under different working conditions. Bioresour. Technol. 2010, 101, 696–704. [Google Scholar]
- Molina-Munoz, M.; Poyatos, J.M.; Vilchez, R.; Hontoria, E.; Rodelas, B.; Gonzalez-Lopez, J. Effect of the concentration of suspended solids on the enzymatic activities and biodiversity of a submerged membrane bioreactor for aerobic treatment of domestic wastewater. Appl. Microbiol. Biotechnol. 2007, 73, 1441–1451. [Google Scholar] [CrossRef]
- Calderón, K.; González-Martínez, A.; Montero-Puente, C.; Reboleiro-Rivas, P.; Poyatos, J.M.; Juárez-Jiménez, B.; Martínez-Toledo, M.V.; Rodelas, B. Bacterial community structure and enzyme activites in a membrane bioreactor (MBR) using pure oxygen as an aerobic source. Bioresour. Technol. 2012, 103, 87–94. [Google Scholar] [CrossRef]
- LaPara, T.M.; Konopka, A.; Nakatsu, C.H.; Alleman, J.E. Thermophilic aerobic treatment of a synthetic wastewater in a membrane-coupled bioreactor. J. Indust. Microbiol. Biotechnol. 2001, 26, 203–209. [Google Scholar] [CrossRef]
- Molina-Munoz, M.; Poyatos, J.M.; Sanchez-Peinado, M.; Hontoria, E.; Gonzalez-Lopez, J.; Rodelas, B. Microbial community structure and dynamics in a pilot-scale submerged membrane bioreactor aerobically treating domestic wastewater under real operation conditions. Sci. Total. Environ. 2009, 407, 3994–4003. [Google Scholar] [CrossRef]
- Simstich, B.; Beimfohr, C.; Horn, H. Lab scale experiments using a submerged MBR under thermophilic aerobic conditions for the treatment of paper mill deinking wastewater. Bioresour. Technol. 2012, in press.. [Google Scholar]
- Miyoshi, T.; Tsuyuhara, T.; Ogyu, R.; Kimura, K.; Watanabe, Y. Seasonal variation in membrane fouling in membrane bioreactors (MBRs) treating municipal wastewater. Water Res. 2009, 43, 5109–5118. [Google Scholar] [CrossRef]
- van den Brink, J.C.; Satpradit, O.A.; van Bentem, A.; Zwijnenburg, A.; Temmink, H.; van loosdrecht, M.C. Effect of temperature shocks on membrane fouling in membrane bioreactors. Water Res. 2011, 45, 4491–4500. [Google Scholar]
- Drews, A.; Vocks, M.; Iversen, V.; Lesjean, B.; Kraume, M. Influence of unsteady membrane bioreactor operation on EPS formation and filtration resistance. Desalination 2006, 192, 1–9. [Google Scholar] [CrossRef]
- Wu, B.; Kitade, T.; Chong, T.H.; Uemura, T.; Fane, A.G. Role of initially formed cake layers on limiting membrane fouling in membrane bioreactors. Bioresour. Technol. 2012, 118, 589–593. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Joseph, K. Effect of transient sodium chloride shock loads on the performance of submerged membrane bioreactor. Bioresour. Technol. 2010, 101, 7054–7061. [Google Scholar] [CrossRef]
- Meng, F.; Liao, B.Q.; Liang, S.; Yang, F.; Zhang, H.; Song, L. Morphological visualization, componential characterization and microbiological identification of membrane fouling in membrane bioreactors (MBRs). J. Membr. Sci. 2010, 361, 1–14. [Google Scholar] [CrossRef]
- Kniggendorf, A.K.; Meinhardt-Wollweber, M. Of micropaticles and bacteria identification-(resonance) Raman micro-spectroscopy as a tool for biofilm analysis. Water Res. 2011, 45, 4571–4582. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Adav, S.S.; Lin, J.C.T.; Yang, Z.; Whiteley, C.G.; Lee, D.J.; Peng, X.F.; Zhang, Z.P. Sterological assessment of extracellular polymeric substances, exo-enzymes, and specific bacterial strains in bioaggregates using fluorescence experiments. Biotechnol. Adv. 2010, 28, 255–280. [Google Scholar] [CrossRef]
- Priester, J.H.; Horst, A.M.; Van de Werfhorst, L.C.; Saleta, J.L.; Mertes, L.A.; Holden, P.A. Enhanced visualization of microbial biofilms by staining and environmental scanning electron microscopy. J. Microbiol. Methods 2007, 68, 577–587. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Zhang, S.; Cao, C.; Zhang, S. Correlating membrane fouling with sludge characteristics in membrane bioreactors: An especial interest in EPS and sludge morphology analysis. Bioresour. Technol. 2011, 102, 8820–8827. [Google Scholar]
- Tian, Y.; Chen, L.; Zhang, S.; Zhang, S. A sysmetic study of soluble microbial products and their fouling impacts in membrane bioreactors. Chem. Eng. J. 2011, 168, 1093–1102. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Arayici, S.; Barlas, H. Advanced treatment of pulp and paper mill wastewater by nanofiltration process: Effects of operating conditions on membrane fouling. Sep. Purif. Technol. 2011, 76, 292–302. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Cao, C.Q.; Zhang, J.; Li, Z.N. Interaction energy evaluation of soluble microbial products (SMP) on different membrane surfaces: Role of the reconstructed membrane topology. Water Res. 2012, 46, 2693–2704. [Google Scholar] [CrossRef]
- Sweity, A.; Ying, W.; Ali-Shtayeh, M.S.; Yang, F.; Bick, A.; Oron, G.; Herzberg, M. Relation between EPS adherence, viscoelastic properties, and MBR operation: Biofouling study with QCM-D. Water Res. 2011, 45, 6430–6440. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lee, D.J.; Yang, Z.; Peng, X.F.; Lai, J.Y. Fluorecent staining for study of extracellular polymeric substances in membrane biofouling layers. Environ. Sci. Technol. 2006, 40, 6642–6646. [Google Scholar] [CrossRef]
- Pätzold, R.; Keuntje, M.; Theophile, K.; Muller, J.; Mielcarek, E.; Ngezahayo, A.; Anders-von Ahlften, A. In situ mapping of nitrifiers and anammox bacteria in microbial aggregates by means of confocal resonance Raman microscopy. J. Microbiol. Methods 2008, 72, 241–248. [Google Scholar] [CrossRef]
- Haisch, C.; Niessner, R. Visualisation of transient processes in biofilms by optical coherence tomography. Water Res. 2007, 41, 2467–2472. [Google Scholar] [CrossRef]
- Wagner, M.; Taherzadeh, D.; Haisch, C.; Horn, H. Investigation of the mesoscale structure and volumetric features of biofilms using optical coherence tomography. Biotechnol. Bioeng. 2010, 107, 844–853. [Google Scholar] [CrossRef]
- Magic-Knezev, A.; van der Kooij, D. Optimisation and significance of ATP analysis for measuring active biomass in granular activated carbon filters used in water treatment. Water Res. 2004, 38, 3971–3979. [Google Scholar] [CrossRef]
- Xi, C.; Wu, J. dATP/ATP, a multifunctional nucleotide, stimulates bacterial cell lysis, extracellular DNA release and biofilm development. PLoS One 2010, 5, e13355. [Google Scholar] [CrossRef]
- Duan, L.; Moreno-Andrade, I.; Huang, C.L.; Xia, S.; Hermanowicz, S.W. Effects of short solids retention time on microbial community in a membrane bioreactor. Bioresour. Technol. 2009, 100, 3489–3496. [Google Scholar] [CrossRef]
- Huang, L.N.; De Wever, H.; Diels, L. Diverse and distinct bacterial communities induced biofilm fouling in membrane bioreactors operated under different conditions. Environ. Sci. Technol. 2008, 42, 8360–8366. [Google Scholar] [CrossRef]
- Calderon, K.; Rodelas, B.; Cabirol, N.; Gonzalez-Lopez, J.; Noyola, A. Analysis of microbial communities developed on the fouling layers of a membrane-coupled anaerobic bioreactor applied to wastewater treatment. Bioresour. Technol. 2011, 102, 4618–4627. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—organic carbon detection—organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Filloux, E.; Labanowski, J.; Croue, J.P. Understanding the fouling of UF/MF hollow fibres of biologically treated wastewaters using advanced EfOM characterization and statistical tools. Bioresour. Technol. 2012, in press.. [Google Scholar]
- Ng, T.C.; Ng, H.Y. Characterisation of initial fouling in aerobic submerged membrane bioreactors in relation to physico-chemical characteristics under different flux conditions. Water Res. 2010, 44, 2336–2348. [Google Scholar] [CrossRef]
- Kimura, K.; Naruse, T.; Watanabe, Y. Changes in characteristics of soluble microbial products in membrane bioreactors associated with different solid retention times: Relation to membrane fouling. Water Res. 2009, 43, 1033–1039. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Yin, X.; Tian, L. Membrane fouling in a submerged membrane bioreactor (MBR) under sub-critical flux operation: Membrane foulants and gel layer characterization. J. Membr. Sci. 2008, 325, 238–244. [Google Scholar] [CrossRef]
- Smidt, E.; Parravicini, V. Effect of sewage sludge treatment and additional aerobic post-stabilization revealed by infrared spectroscopy and multivariate data analysis. Bioresour. Technol. 2009, 100, 1775–1780. [Google Scholar] [CrossRef]
- Kimura, K.; Yamato, N.; Yamamura, H.; Watanabe, Y. Membrane fouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater. Environ. Sci. Technol. 2005, 39, 6293–6299. [Google Scholar] [CrossRef]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef]
- Lin, J.C.T.; Lee, D.J.; Huang, C. Membrane fouling mitigation: Membrane cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Genkin, G.; Waite, T.D.; Fane, A.G.; Chang, S. The effect of vibration and coagulant addition on the filtration performance of submerged hollow fibre membranes. J. Membr. Sci. 2006, 281, 726–734. [Google Scholar] [CrossRef]
- Bilad, M.R.; Mezohegyi, G.; Declerck, P.; Vankelecom, I.F. Novel magnetically induced membrane vibration (MMV) for fouling control in membrane bioreactors. Water Res. 2012, 46, 63–72. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl. Microbiol. Biotechnol. 2010, 86, 825–837. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Camilli, A.; Bassler, B.L. Bacterial small-molecule signaling pathways. Science 2006, 311, 1113–1116. [Google Scholar] [CrossRef]
- Hardie, K.R.; Heurlier, K. Establishing bacterial communities by “word of mouth”: LuxS and autoinducer 2 in biofilm development. Nat. Rev. Microbiol. 2008, 6, 635–643. [Google Scholar] [CrossRef]
- Yeon, K.M.; Cheong, W.S.; Oh, H.S.; Lee, W.N.; Hwang, B.K.; Lee, C.H.; Beyenal, H.; Lewandowski, Z. Quorum sensing: A new biofouling control paradigm in a membrane bioreactor for advanced wastewater treatment. Environ. Sci. Technol. 2009, 43, 380–385. [Google Scholar]
- Xu, H.; Liu, Y. Control and cleaning of membrane biofouling by energy uncoupling and cellular communication. Environ. Sci. Technol. 2011, 45, 595–601. [Google Scholar] [CrossRef]
- Yang, X.L.; Song, H.L.; Lu, J.L.; Fu, D.F.; Cheng, B. Influence of diatomite addition on membrane fouling and performance in a submerged membrane bioreactor. Bioresour. Technol. 2010, 101, 9178–9184. [Google Scholar]
- Wu, B.; An, Y.; Li, Y.; Wong, F.S. Effect of adsorption/coagulation on membrane fouling in microfiltration process post-treating anaerobic digestion effluent. Desalination 2009, 242, 183–192. [Google Scholar] [CrossRef]
- Johir, M.A.H.; Aryal, R.; Vigneswaran, S.; Kandasamy, J.; Grasmick, A. Influence of supporting media in suspension on membrane fouling reduction in submerges membrane bioreactor (SMBR). J. Membr. Sci. 2011, 374, 121–128. [Google Scholar] [CrossRef]
- Koseoglu, H.; Yigit, N.O.; Civelekoglu, G.; Harman, B.I.; Kitis, M. Effects of chemical additives on filtration and rheological characteristics of MBR sludge. Bioresour. Technol. 2012, 117, 48–54. [Google Scholar] [CrossRef]
- Wu, J.; Le-Clech, P.; Stuetz, R.M.; Fane, A.G.; Chen, V. Novel filtration mode for fouling limitation in membrane bioreactors. Water Res. 2008, 42, 3677–3684. [Google Scholar] [CrossRef]
- Teychene, B.; Guigui, C.; Cabassud, C. Engineering of an MBR supernatant fouling layer by fine particles addition: A possible way to control cake compressibility. Water Res. 2011, 45, 2060–2072. [Google Scholar] [CrossRef]
- Fane, A.G. Membranes for water production and wastewater reuse. Desalination 1996, 106, 1–9. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, B.; Fane, A.G. Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control. Membranes 2012, 2, 565-584. https://doi.org/10.3390/membranes2030565
Wu B, Fane AG. Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control. Membranes. 2012; 2(3):565-584. https://doi.org/10.3390/membranes2030565
Chicago/Turabian StyleWu, Bing, and Anthony G. Fane. 2012. "Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control" Membranes 2, no. 3: 565-584. https://doi.org/10.3390/membranes2030565
APA StyleWu, B., & Fane, A. G. (2012). Microbial Relevant Fouling in Membrane Bioreactors: Influencing Factors, Characterization, and Fouling Control. Membranes, 2(3), 565-584. https://doi.org/10.3390/membranes2030565



