Pore-Network Modeling of Water and Vapor Transport in the Micro Porous Layer and Gas Diffusion Layer of a Polymer Electrolyte Fuel Cell
Abstract
:1. Introduction
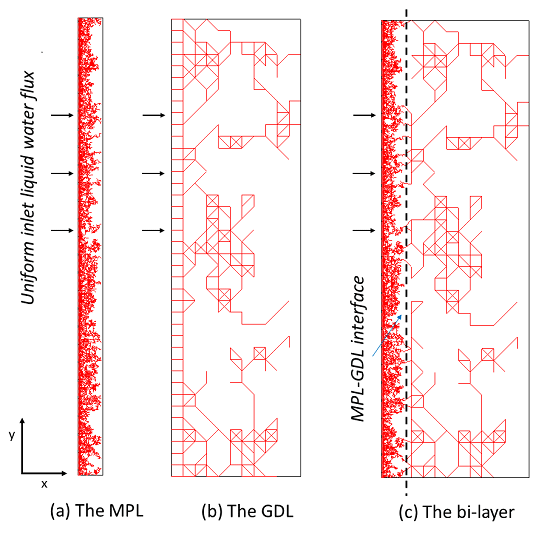
2. Pore-Network Representations of the GDL and MPL
3. Dynamic Pore-Network Model
4. Numerical Implementation and Description of Case Studies
5. Results
5.1. Quasi-Static Modeling of Liquid Water Pathways
5.2. Dynamic Pore-Network Modeling of Water and Vapor Transport
6. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Qin, C.Z. Water transport in the gas diffusion layer of a polymer electrolyte fuel cell: Dynamic pore-network modeling. J. Electrochem. Soc. 2015, 162, F1036–F1046. [Google Scholar] [CrossRef]
- Qin, C.Z.; Rensink, D.; Fell, S.; Hassanizadeh, S.M. Two-phase flow modeling for the cathode side of a polymer electrolyte fuel cell. J. Power Sources 2012, 197, 136–144. [Google Scholar] [CrossRef]
- Qin, C.Z.; Hassanizadeh, S.M. A new approach to modelling water flooding in a polymer eletrolyte fuel cell. Int. J. Hydrog. Energy 2015, 40, 3348–3358. [Google Scholar] [CrossRef]
- Qin, C.Z.; Hassanizadeh, S.M. Multiphase flow through multilayers of thin porous media: General balance equations and constititive relationships for a solid-gas-liquid three-phase system. Int. J. Heat Mass Transf. 2014, 70, 693–708. [Google Scholar] [CrossRef]
- Nam, J.H.; Lee, K.J.; Hwang, G.S.; Kim, C.J.; Kaviany, M. Microporous layer for water morphology control in PEMFC. Int. J. Heat Mass Transf. 2009, 52, 2779–2791. [Google Scholar] [CrossRef]
- Morgan, J.M.; Datta, R. Understanding the gas diffusion layer in proton exchange membrane fuel cells. I. How its structural characteristics affect diffusion and performance. J. Power Sources 2014, 251, 269–278. [Google Scholar] [CrossRef]
- Deevanhxay, P.; Sasabe, T.; Tsushima, S.; Hirai, S. Observation of dynamic liquid water transport in the microporous layer and gas diffusion layer of an operating PEM fuel cell by high-resolution soft X-ray radiography. J. Power Sources 2013, 230, 38–43. [Google Scholar] [CrossRef]
- Lee, J.; Yip, R.; Antonacci, P.; Ge, N.; Kotaka, T.; Tabuchi, Y.; Bazylak, A. Synchrotron investigation of microporous layer thickness on liquid water distribution in a PEM fuel cell. J. Electrochem. Soc. 2015, 162, F669–F676. [Google Scholar] [CrossRef]
- Owejan, J.P.; Owejan, J.E.; Gu, W.; Trabold, T.A.; Tighe, T.W.; Mathias, M.F. Water transport mechanisms in PEMFC gas diffusion layers. J. Electrochem. Soc. 2010, 157, B1456–B1464. [Google Scholar] [CrossRef]
- Atiyeh, H.K.; Karan, K.; Peppley, B.; Phoenix, A.; Halliop, E.; Pharoah, J. Experimental investigation of the role of a microporous layer on the water transport and performance of a PEM fuel cell. J. Power Sources 2007, 170, 111–121. [Google Scholar] [CrossRef]
- Pasaogullari, U.; Wang, C.Y. Two-phase transport and the role of micro-porous layer in polymer electrolyte fuel cells. Electrochim. Acta 2004, 49, 4359–4369. [Google Scholar] [CrossRef]
- Kim, K.N.; Kang, J.H.; Lee, S.G.; Nam, J.H.; Kim, C.J. Lattice Boltzmann simulation of liquid water transport in microporous and gas diffusion layers of polymer electrolyte membrane fuel cells. J. Power Sources 2015, 278, 703–717. [Google Scholar] [CrossRef]
- Wu, R.; Zhu, X.; Liao, Q.; Wang, H.; Ding, Y.D.; Li, J.; Ye, D.D. A pore network study on the role of micro-porous layer in control of liquid water distribution in gas diffusion layer. Int. J. Hydrog. Energy 2010, 35, 7588–7593. [Google Scholar] [CrossRef]
- Spernjak, D.; Prasad, A.K.; Advani, S.G. Experimental investigation of liquid water formation and transport in a transparent single-serpentine PEM fuel cell. J. Power Sources 2007, 170, 334–344. [Google Scholar] [CrossRef]
- Mukherjee, P.P.; Wang, C.Y.; Kang, Q.J. Mesoscopic modeling of two-phase behaviour and flooding phenomena in polymer electrolyte fuel cells. Electrochim. Acta 2009, 54, 6861–6875. [Google Scholar] [CrossRef]
- Lee, K.J.; Kang, J.H.; Nam, J.H. Liquid water distribution in hydrophobic gas-diffusion layers with interconnect rib geometry: An invasion-percolation pore network analysis. Int. J. Hydrog. Energy 2014, 39, 6646–6656. [Google Scholar] [CrossRef]
- Lee, K.J.; Nam, J.H.; Kim, C.J. Steady saturation distribution in hydrophobic gas-diffusion layers of polymer electrolyte membrane fuel cells: A pore-network study. J. Power Sources 2010, 195, 130–141. [Google Scholar] [CrossRef]
- Qin, C.Z.; Rensink, D.; Hassanizadeh, S.M.; Fell, S. Direct Simulation of Liquid Water Dynamics in the Gas Channel of a Polymer Electrolyte Fuel Cell. J. Electrochem. Soc. 2012, 159, B434–B443. [Google Scholar] [CrossRef]
- Médici, E.F.; Allen, J.S. Evaporation, two phase flow, and thermal transport in porous media with application to low-temperature fuel cells. Int. J. Heat Mass Transf. 2013, 65, 779–788. [Google Scholar] [CrossRef]
- Sinha, P.K.; Wang, C.Y. Pore-network modeling of liquid water transport in gas diffusion layer of a polymer electrolyte fuel cell. Electrochim. Acta 2007, 52, 7936–7945. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, G.; Wang, C.Y. Pore-level liquid water transport through composite diffusion media of PEMFC. J. Electrochem. Soc. 2012, 157, B1753–B1761. [Google Scholar] [CrossRef]
- Sadeghi, E.; Djilali, N.; Bahrami, M. A novel approach to determine the in-plane thermal conductivity of gas diffusion layers in proton exchange membrane fuel cells. J. Power Sources 2011, 196, 3565–3571. [Google Scholar] [CrossRef]
- Radhakrishnan, A. Thermal Conductivity Measurement of Gas Diffusion Layer Used in PEMFC. Master’s Thesis, Rochester Institute of Technology, Rochester, NY, USA, 2009. [Google Scholar]
- Hizir, F.E.; Ural, S.O.; Kumbur, E.C.; Mench, M.M. Characterization of interfacial morphology in polymer electrolyte fuel cells: Micro-porous layer and catalyst layer surfaces. J. Power Sources 2010, 195, 3463–3471. [Google Scholar] [CrossRef]
- Médici, E.F.; Allen, J.S. The effect of morphological and wetting properties of porous transport layers on water movement in PEM fuel cells. J. Electrochem. Soc. 2010, 157, B1505–B1514. [Google Scholar] [CrossRef]
- Straubhaar, B.; Pauchet, J.; Prat, M. Water transport in gas diffusion layer of a polymer electrolyte fuel cell in the presence of a temperature gradient. Phase change effect. Int. J. Hydrog. Energy 2015, 40, 11668–11675. [Google Scholar] [CrossRef] [Green Version]
- Gostick, J.T.; Ioannidis, M.A.; Fowler, M.W.; Pritzker, M.D. On the role of the microporous layer in PEMFC operation. Electrochem. Commun. 2009, 11, 576–579. [Google Scholar] [CrossRef]
- Fazeli, M.; Hinebaugh, J.; Bazylak, A. Investigating inlet condition effects on PEMFC GDL liquid water transport through pore network modeling. J. Electrochem. Soc. 2015, 162, F661–F668. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.Y. A nonisothermal, two-phase model for polymer electrolyte fuel cells. J. Electrochem. Soc. 2006, 153, A1193–A1200. [Google Scholar] [CrossRef]










| Parameter | The GDL | The MPL (Crack-Free) |
|---|---|---|
| Pore-network dimensions, x × y × z | 250 µm × 1000 µm × 25 µm | |
| Dimension of the channel area | 500 µm (refer to Figure 1a) | |
| Lattice distance/lattice numbers, x × y × z | 25 µm/10 × 40 × 1 | 2.5 µm/20 × 391 × 10 |
| Number of pore bodies/pore throats (including inlet and outlet) | 480/1077 | 85,712/265,892 |
| Porosity | 0.68 | 0.38 |
| Permeability | 1.1 × 10−11 m2 (through-plane)/1.5 × 10−11 m2 (in-plane) | 1.8 × 10−14 m2 |
| Minimum and maximum sizes of pore bodies (inscribed radius) | 9 µm and 12.5 µm | 0.25 µm and 1.25 µm |
| Mean size of pore bodies | 11 µm | 0.7 µm |
| Average coordination number | 5.4 | 6.8 |
| Parameter | Value + Units | Source |
|---|---|---|
| Operating temperature, | 80 °C | - |
| Current density, | 1.5 A/cm2 | - |
| Operating voltage, | 0.6 V | [10] |
| Thermal conductivity of the GDL, /the MPL, | 1.4/0.2 W/m·K | [24] |
| Contact angle of diffusion layers for liquid water | 180° | [2] |
| Phase change rate coefficent, | 1.0 m/s | - |
| Water vapor diffusivity at reference condition, | 3.0 × 10−5 m2/s | [3] |
| Relative humidity in the GC, | 100% | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.-Z.; Hassanizadeh, S.M.; Van Oosterhout, L.M. Pore-Network Modeling of Water and Vapor Transport in the Micro Porous Layer and Gas Diffusion Layer of a Polymer Electrolyte Fuel Cell. Computation 2016, 4, 21. https://doi.org/10.3390/computation4020021
Qin C-Z, Hassanizadeh SM, Van Oosterhout LM. Pore-Network Modeling of Water and Vapor Transport in the Micro Porous Layer and Gas Diffusion Layer of a Polymer Electrolyte Fuel Cell. Computation. 2016; 4(2):21. https://doi.org/10.3390/computation4020021
Chicago/Turabian StyleQin, Chao-Zhong, S. Majid Hassanizadeh, and Lucas M. Van Oosterhout. 2016. "Pore-Network Modeling of Water and Vapor Transport in the Micro Porous Layer and Gas Diffusion Layer of a Polymer Electrolyte Fuel Cell" Computation 4, no. 2: 21. https://doi.org/10.3390/computation4020021