Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication and Cleaning of Mg and Mg-1.6Li Thin Films for Cell Culture
2.2. Cell Culture
2.3. MTT Assay
2.4. Harvesting Rat Sciatic Nerves
2.5. Preparation of Freeze-Killed Cells and Nerve Extracts
2.6. Treatment of Cells with Injury Stimulants and Thin Films
2.7. Quantification of MCP-1 Release
2.8. Determination of Mg and Li Concentrations
2.9. RNA Extraction and Quantitative RT-PCR
2.10. Statistical Analyses
3. Results
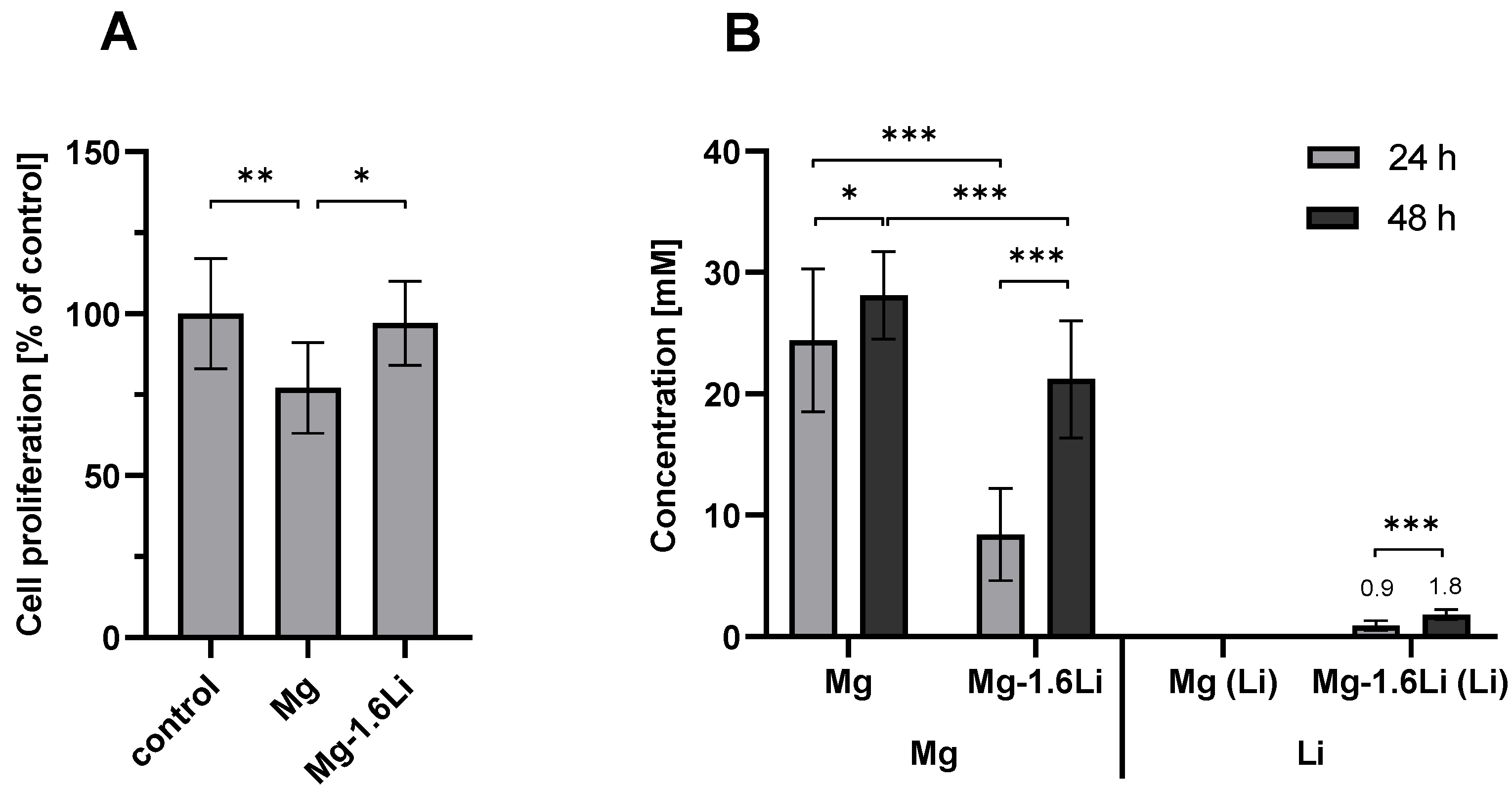
3.1. Mg-Based Extracts Showed Minimal Cytotoxicity with RT4-D6P2T Cells after Short-Term Exposure
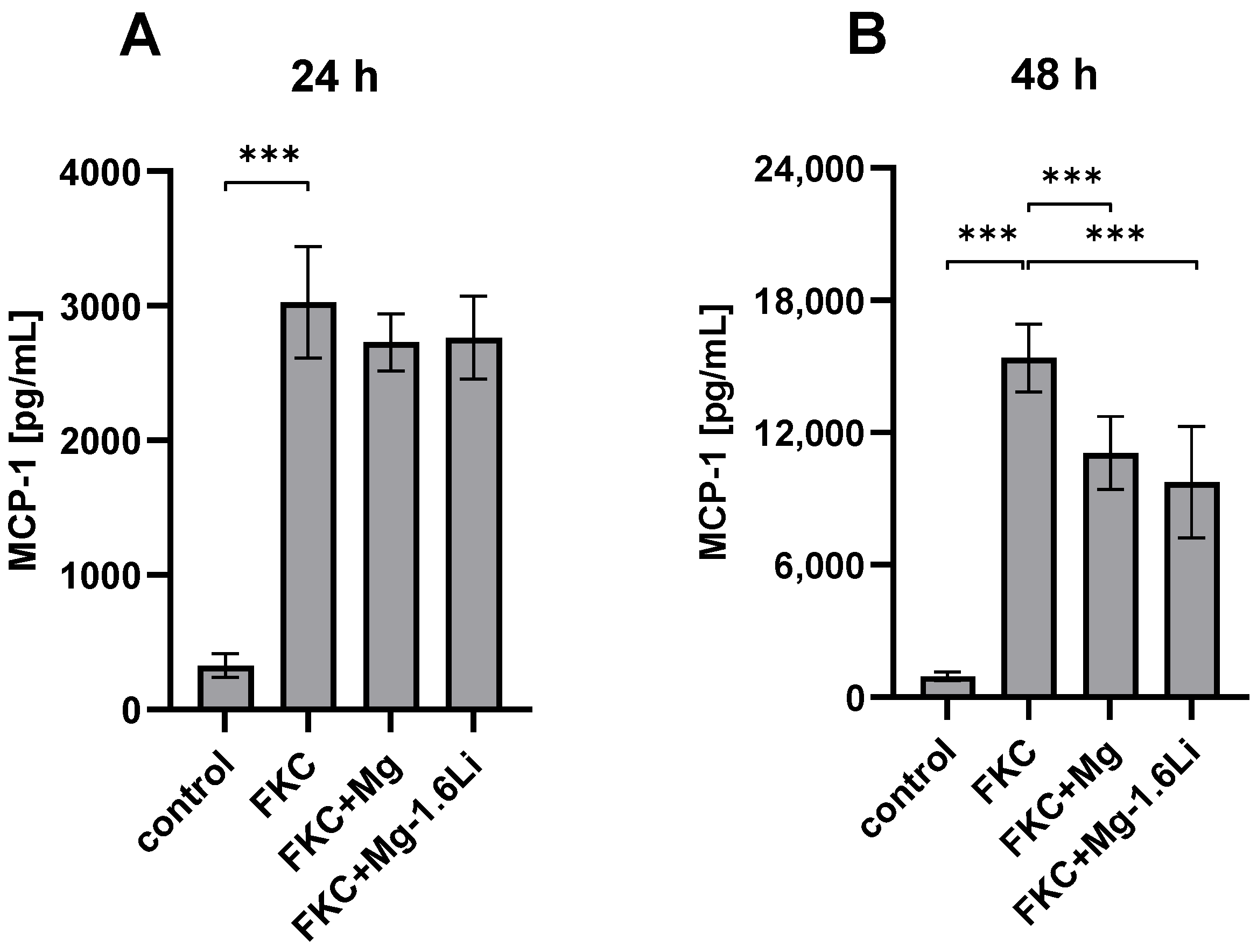
3.2. Extracts from Freeze-Killed Cells and Nerves Triggered MCP-1 Release from RT4-D6P2T Cells
3.3. Influence of Mg-Based Thin Films on RT4-D6P2T Cellular Response to Injury
3.3.1. Mg/Mg-1.6Li Thin Films Reduced MCP-1 Release from FKC-Treated RT4-D6P2T Cells
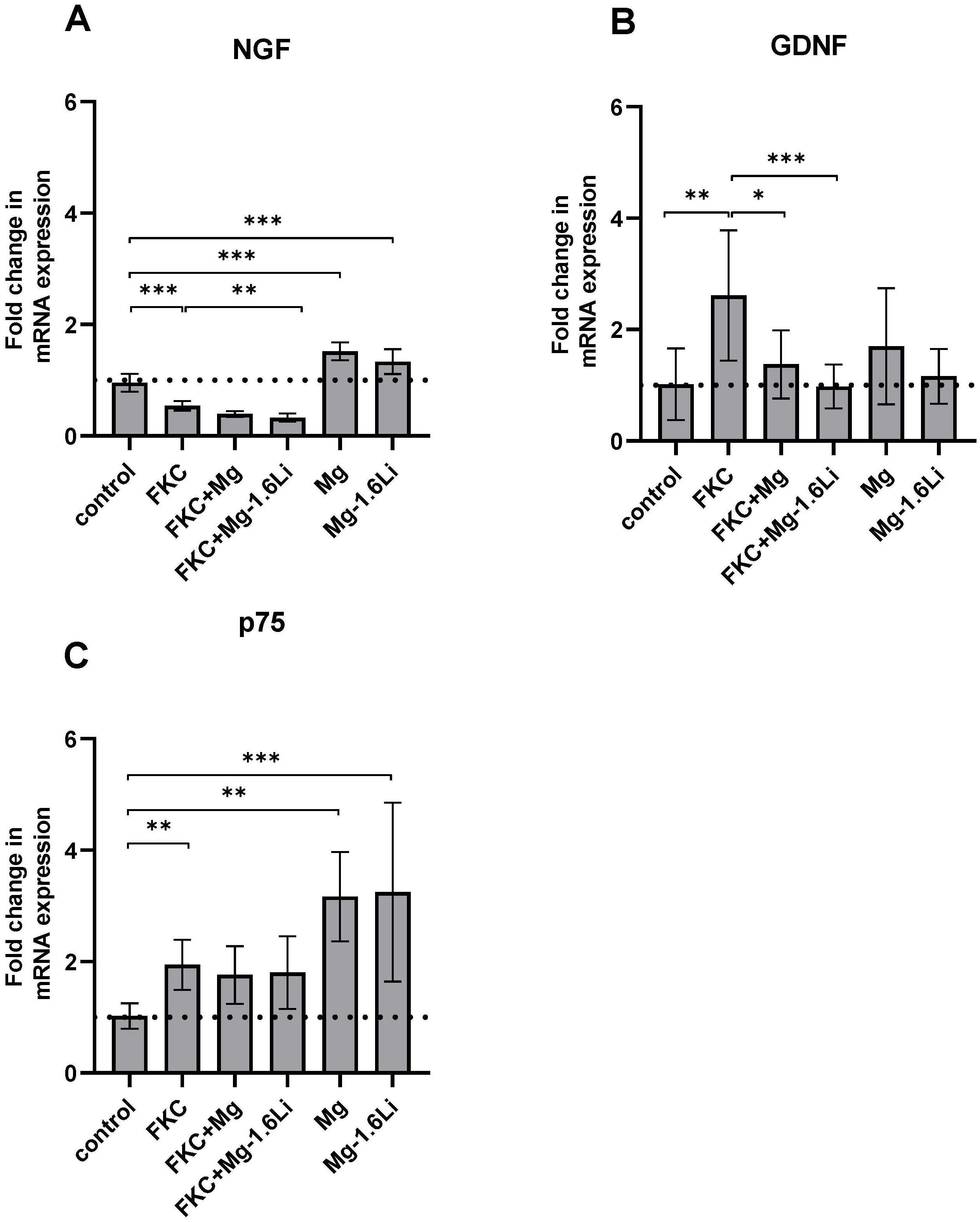
3.3.2. The Gene Expression of Neurotrophins Is Regulated Differently by the Thin Films in the Presence of the Injury Stimulant
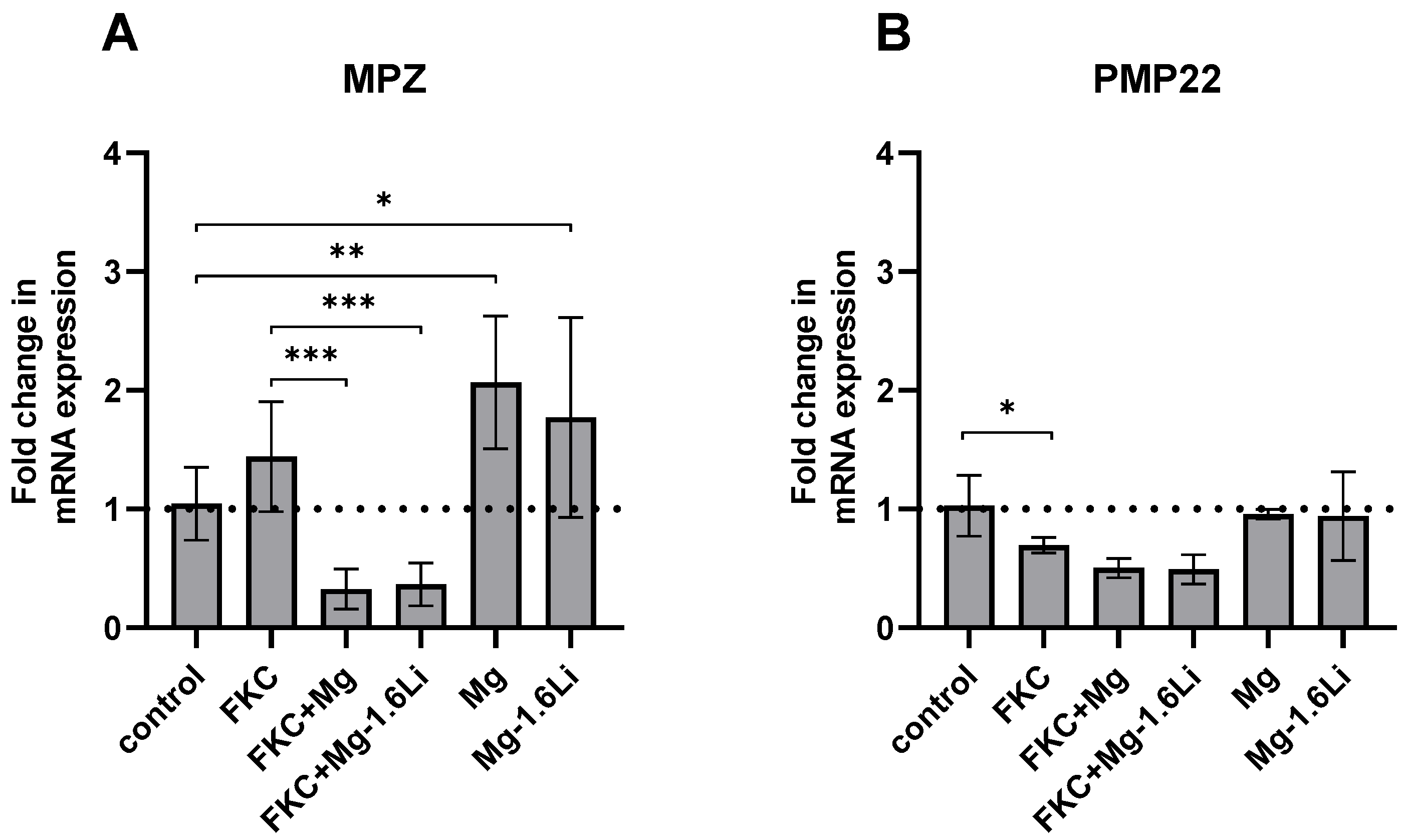
3.3.3. Mg/Mg-1.6Li Thin Films Influenced the Expression of Key Myelin Protein Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflam. 2011, 8, 109. [Google Scholar] [CrossRef]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular Mechanisms Involved in Schwann Cell Plasticity. Front. Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zhang, Q.; Wang, Q.; Wang, X.; Yan, G. Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair. Biomater. Sci. 2023, 21, 7304–7050. [Google Scholar] [CrossRef]
- Manescu, P.V.; Antoniac, I.; Antoniac, A.; Laptoiu, D.; Paltanea, G.; Ciocoiu, R.; Nemoianu, I.V.; Gruionu, L.G.; Dura, H. Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics 2023, 8, 618. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Gao, Y.; Zheng, Y.; Liu, Z.; Zhou, C.; Huang, T.; Gu, X.; Li, A.; Fang, J.; et al. Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2022, 11, 140–153. [Google Scholar] [CrossRef]
- Bhat, K.; Schlotterose, L.; Hanke, L.; Helmholz, H.; Quandt, E.; Hattermann, K.; Willumeit-Römer, R. Magnesium-lithium thin films for neurological applications–An in vitro investigation of glial cytocompatibility and neuroinflammatory response. Acta Biomater. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, J.; Lin, W.; Zhang, S. Magnesium Promotes the Regeneration of the Peripheral Nerve. Front. Cell Dev. Biol. 2021, 9, 717854. [Google Scholar] [CrossRef]
- Gordon, T. Brief Electrical Stimulation Promotes Recovery after Surgical Repair of Injured Peripheral Nerves. Int. J. Mol. Sci. 2024, 25, 665. [Google Scholar] [CrossRef]
- Mathew, A.A.; Panonnummal, R.A. Mini Review on the Various Facets Effecting Brain Delivery of Magnesium and Its Role in Neurological Disorders. Biol. Trace Elem. Res. 2023, 201, 4238–4253. [Google Scholar] [CrossRef]
- Pan, H.C.; Sheu, M.L.; Su, H.L.; Chen, Y.J.; Chen, C.J.; Yang, D.Y.; Chiu, W.T.; Cheng, F.C. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes. Res. 2011, 24, 54–70. [Google Scholar] [CrossRef]
- Li, B.H.; Yang, K.; Wang, X. Biodegradable magnesium wire promotes regeneration of compressed sciatic nerves. Neural Regen. Res. 2016, 11, 2012–2017. [Google Scholar] [CrossRef]
- Yao, Z.; Yuan, W.; Xu, J.; Tong, W.; Mi, J.; Ho, P.C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv. Sci. 2022, 9, 2202102. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Chen, S.; Sun, B.; Guo, Y.; He, C.; Mo, X.; Zhu, B.; You, Z. Molecularly engineered metal-based bioactive soft materials—Neuroactive magnesium ion/polymer hybrids. Acta Biomater. 2019, 85, 310–319. [Google Scholar] [CrossRef]
- Vennemeyer, J.J.; Hopkins, T.; Kuhlmann, J.; Heineman, W.R.; Pixley, S.K. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci. Res. 2014, 84, 72–78. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Zhang, J.; Zhang, M.; Dai, C.; Zhang, Y.; Zhang, L.; Bian, L.; Yang, Y.; Zhang, K.; et al. Advancing neural regeneration via adaptable hydrogels: Enriched with Mg2+ and silk fibroin to facilitate endogenous cell infiltration and macrophage polarization. Bioact. Mater. 2024, 33, 100–113. [Google Scholar] [CrossRef]
- Hopkins, T.M.; Little, K.J.; Vennemeyer, J.J.; Triozzi, J.L.; Turgeon, M.K.; Heilman, A.M.; Minteer, D.; Marra, K.; Hom, D.B.; Pixley, S.K. Short and long gap peripheral nerve repair with magnesium metal filaments. J. Biomed. Mater. Res. A 2017, 105, 3148–3158. [Google Scholar] [CrossRef]
- Vennemeyer, J.J.; Hopkins, T.; Hershcovitch, M.; Little, K.D.; Hagen, M.C.; Minteer, D.; Hom, D.B.; Marra, K.; Pixley, S.K. Initial observations on using magnesium metal in peripheral nerve repair. J. Biomater. Appl. 2014, 29, 1145–1154. [Google Scholar] [CrossRef]
- Tatu, R.; White, L.G.; Yun, Y.; Hopkins, T.; An, X.; Ashraf, A.; Little, K.J.; Hershcovitch, M.; Hom, D.B.; Pixley, S. Effects of Altering Magnesium Metal Surfaces on Degradation In Vitro and In Vivo during Peripheral Nerve Regeneration. Materials 2023, 16, 1195. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, Y.; Liu, Z.; He, D.; Xu, W.; Li, S.; Zhang, C.; Zhang, Z. Li–Mg–Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioact. Mater. 2023, 28, 227–242. [Google Scholar] [CrossRef]
- Monfared, A.; Ghaee, A.; Ebrahimi-Barough, S. Fabrication of tannic acid/poly(N-vinylpyrrolidone) layer-by-layer coating on Mg-based metallic glass for nerve tissue regeneration application. Colloids Surf. B Biointerfaces 2018, 170, 617–626. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Duan, L.; Yao, R.; Yan, Y.; Wang, T.; Wang, J.; Zheng, Z.; Wang, X.; Li, G. Preparation and mechanical optimization of a two-layer silk/magnesium wires braided porous artificial nerve guidance conduit. J. Biomed. Mater. Res. A 2022, 110, 1801–1812. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Manji, H.K.; Zarate, C.A. The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009, 11, 92–109. [Google Scholar] [CrossRef]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a Unified Understanding of Lithium Action in Basic Biology and its Significance for Applied Biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef]
- Wang, F.; Chang, S.; Li, J.; Wang, D.; Li, H.; He, X. Lithium alleviated spinal cord injury (SCI)-induced apoptosis and inflammation in rats via BDNF-AS/miR-9-5p axis. Cell Tissue Res. 2021, 384, 301–312. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Qiao, H.; Liu, D.F.; Li, J.; Li, J.X.; Chang, S.E.; Lu, T.; Li, F.T.; Wang, D.; Li, H.P.; et al. Lithium promotes recovery after spinal cord injury. Neural Regen. Res. 2022, 17, 1324–1333. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, J.; Han, D.; Chen, B.; Ma, M.; Yu, Y.; Li, M.; Liu, Z.; Zhang, P.; Jiang, B. GSK3β inhibition accelerates axon debris clearance and new axon remyelination. Am. J. Transl. Res. 2016, 8, 5410–5420. [Google Scholar]
- Liu, P.; Zhang, Z.; Wang, Q.; Guo, R.; Mei, W. Lithium Chloride Facilitates Autophagy Following Spinal Cord Injury via ERK-dependent Pathway. Neurotox. Res. 2017, 32, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, F.; Zhai, X.; Li, X.H.; He, X.J. Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy. Neural Regen. Res. 2018, 13, 2191–2199. [Google Scholar] [CrossRef]
- Yang, M.; Su, B.; Ma, Z.; Zheng, X.; Liu, Y.; Li, Y.; Ren, J.; Lu, L.; Yang, B.; Yu, X. Renal-friendly Li+-doped carbonized polymer dots activate Schwann cell autophagy for promoting peripheral nerve regeneration. Acta Biomater. 2023, 159, 353–366. [Google Scholar] [CrossRef]
- Ogata, T.; Iijima, S.; Hoshikawa, S.; Miura, T.; Yamamoto, S.; Oda, H.; Nakamura, K.; Tanaka, S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004, 24, 6724–6732. [Google Scholar] [CrossRef]
- Makoukji, J.; Belle, M.; Meffre, D.; Stassart, R.; Grenier, J.; Shackleford, G.; Fledrich, R.; Fonte, C.; Branchu, J.; Goulard, M.; et al. Lithium enhances remyelination of peripheral nerves. Proc. Natl. Acad. Sci. USA 2012, 109, 3973–3978. [Google Scholar] [CrossRef]
- Kocman, A.E.; Dag, I.; Sengel, T.; Söztutar, E. The effect of lithium and lithium-loaded hyaluronic acid hydrogel applications on nerve regeneration and recovery of motor functions in peripheral nerve injury. Rend. Lincei. Sci. Fis. Nat. 2020, 31, 889–904. [Google Scholar] [CrossRef]
- Weng, J.; Wang, Y.H.; Li, M.; Zhang, D.Y.; Jiang, B.G. GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury. Neural Regen. Res. 2018, 13, 324–330. [Google Scholar] [CrossRef]
- Grandjean, E.M.; Aubry, J.M. Lithium: Updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring. CNS Drugs 2009, 23, 331–349. [Google Scholar] [CrossRef]
- Hanke, L.; Jessen, L.K.; Weisheit, F.; Bhat, K.; Westernströer, U.; Garbe-Schönberg, D.; Willumeit-Römer, R.; Quandt, E. Structural characterisation and degradation of Mg–Li thin films for biodegradable implants. Sci. Rep. 2023, 13, 12572. [Google Scholar] [CrossRef]
- Lee, H.; Jo, E.K.; Choi, S.Y.; Oh, S.B.; Park, K.; Kim, J.S.; Lee, S.J. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: Implication in Wallerian degeneration. Biochem. Biophys. Res. Commun. 2006, 350, 742–747. [Google Scholar] [CrossRef]
- Karanth, S.; Yang, G.; Yeh, J.; Richardson, P.M. Nature of signals that initiate the immune response during Wallerian degeneration of peripheral nerves. Exp. Neurol. 2006, 202, 161–166. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Endogenous signals initiating inflammation in the injured nervous system. Glia 2009, 57, 351–361. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- International Organization for Standardization. Part 5: Tests for in vitro cytotoxicity. In ISO 10993 Biological Evaluation of Medical Devices, 3rd ed.; International Organization for Standardization (ISO): Geneva, Switzerland, 2009. [Google Scholar]
- Hai, M.; Muja, N.; DeVries, G.H.; Quarles, R.H.; Patel, P.I. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J. Neurosci. Res. 2002, 69, 497–508. [Google Scholar] [CrossRef]
- Geuna, S.; Raimondo, S.; Fregnan, F.; Haastert-Talini, K.; Grothe, C. In vitro models for peripheral nerve regeneration. Eur. J. Neurosci. 2016, 43, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pereira, C.; Hill, E.E.; Vukcevich, O.; Wang, A. In Vitro, In Vivo and Ex Vivo Models for Peripheral Nerve Injury and Regeneration. Curr. Neuropharmacol. 2022, 20, 344–361. [Google Scholar] [CrossRef]
- Andersen, N.D.; Srinivas, S.; Piñero, G.; Monje, P.V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef] [PubMed]
- Shojapour, M.; Mosayebi, G.; Hajihossein, R.; Noorbakhsh, F.; Mokarizadeh, A.; Ghahremani, M.H. A Simplified Protocol for the Purification of Schwann Cells and Exosome Isolation from C57BL/6 Mice. Rep. Biochem. Mol. Biol. 2018, 7, 9–15. [Google Scholar]
- Amukarimi, S.; Mozafari, M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Thiemicke, A.; Neuert, G. Kinetics of osmotic stress regulate a cell fate switch of cell survival. Sci. Adv. 2021, 7, 1122. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.K.; Li, X.R.; Lu, M.L.; Xu, H. Lithium promotes proliferation and suppresses migration of Schwann cells. Neural Regen. Res. 2020, 15, 1955–1961. [Google Scholar] [CrossRef]
- Piñero, G.; Berg, R.; Andersen, N.D.; Setton-Avruj, P.; Monje, P.V. Lithium Reversibly Inhibits Schwann Cell Proliferation and Differentiation Without Inducing Myelin Loss. Mol. Neurobiol. 2017, 54, 8287–8307. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent progress on coatings of biomedical magnesium alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Albaraghtheh, T.; Willumeit-Römer, R.; Zeller-Plumhoff, B. In silico studies of magnesium-based implants: A review of the current stage and challenges. J. Magnes. Alloy 2022, 11, 2968–2996. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. Investigation on the size and percentage effects of magnesium nanoparticles on thermophysical properties of reinforced calcium phosphate bone cement by molecular dynamic simulation. Heliyon 2023, 9, e18835. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liang, J.; Cui, M.; Zhang, L.; Ren, S.; Zheng, W.; Dong, X.; Zhang, B. Saturated fatty acids activate the inflammatory signalling pathway in Schwann cells: Implication in sciatic nerve injury. Scand. J. Immunol. 2020, 92, 12896. [Google Scholar] [CrossRef] [PubMed]
- Hammel, G.; Zivkovic, S.; Ayazi, M.; Ren, Y. Consequences and mechanisms of myelin debris uptake and processing by cells in the central nervous system. Cell. Immunol. 2022, 380, 104591. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.B.; Laranjeira, S.G.; Eriksson, T.M.; Jessen, K.R.; Mirsky, R.; Quick, T.J.; Phillips, J.B. Characterising cellular and molecular features of human peripheral nerve degeneration. Acta Neuropathol. Commun. 2020, 8, 51. [Google Scholar] [CrossRef]
- Tomita, K.; Kubo, T.; Matsuda, K.; Fujiwara, T.; Yano, K.; Winograd, J.M.; Tohyama, M.; Hosokawa, K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia 2007, 55, 1199–1208. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y.; Yuan, Y.; Yang, S.; Xie, L.; Mao, Y.; Jiang, T.; et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 2020, 10, 1649–1677. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Bessa-Gonçalves, M.; Silva, A.M.; Brás, J.P.; Helmholz, H.; Luthringer-Feyerabend, B.J.C.; Willumeit-Römer, R.; Barbosa, M.A.; Santos, S.G. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020, 114, 471–484. [Google Scholar] [CrossRef]
- Makola, R.T.; Mbazima, V.G.; Mokgotho, M.P.; Gallicchio, V.S.; Matsebatlela, T.M. The Effect of Lithium on Inflammation-Associated Genes in Lipopolysaccharide-Activated Raw 264.7 Macrophages. Int. J. Inflam. 2020, 2020, 8340195. [Google Scholar] [CrossRef] [PubMed]
- Jeub, M.; Siegloch, P.A.; Nitsch, L.; Zimmermann, J.; Mueller, M.M. Reduced inflammatory response and accelerated functional recovery following sciatic nerve crush lesion in CXCR3-deficient mice. NeuroReport 2020, 31, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Talsma, A.D.; Niemi, J.P.; Pachter, J.S.; Zigmond, R.E. The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration. J. Neuroinflam. 2022, 19, 179. [Google Scholar] [CrossRef]
- Niemi, J.P.; DeFrancesco-Lisowitz, A.; Roldán-Hernández, L.; Lindborg, J.A.; Mandell, D.; Zigmond, R.E. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci. 2013, 33, 16236–16248. [Google Scholar] [CrossRef]
- Mutschler, C.; Fazal, S.V.; Schumacher, N.; Loreto, A.; Coleman, M.P.; Arthur-Farraj, P. Schwann cells are axo-protective after injury irrespective of myelination status in mouse Schwann cell–neuron cocultures. J. Cell Sci. 2023, 136, 261557. [Google Scholar] [CrossRef]
- Hongisto, V.; Smeds, N.; Brecht, S.; Herdegen, T.; Courtney, M.J.; Coffey, E.T. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol. Cell. Biol. 2003, 23, 6027–6036. [Google Scholar] [CrossRef]
- Reddy, C.; Albanito, L.; De Marco, P.; Aiello, D.; Maggiolini, M.; Napoli, A.; Musti, A.M. Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons. Cell Death Dis. 2013, 4, e852. [Google Scholar] [CrossRef]
- Altura, B.M.; Kostellow, A.B.; Zhang, A.; Li, W.; Morrill, G.A.; Gupta, R.K.; Altura, B.T. Expression of the nuclear factor-kappaB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: Possible links to hypertension, atherogenesis, and stroke. Am. J. Hypertens. 2003, 16, 701–707. [Google Scholar] [CrossRef]
- Lambuk, L.; Iezhitsa, I.; Agarwal, R.; Agarwal, P.; Peresypkina, A.; Pobeda, A.; Ismail, N.M. Magnesium acetyltaurate prevents retinal damage and visual impairment in rats through suppression of NMDA-induced upregulation of NF-κB, p53 and AP-1 (c-Jun/c-Fos). Neural Regen. Res. 2021, 16, 2330–2344. [Google Scholar] [CrossRef]
- Liao, W.; Jiang, M.; Li, M.; Jin, C.; Xiao, S.; Fan, S.; Fang, W.; Zheng, Y.; Liu, J. Magnesium Elevation Promotes Neuronal Differentiation While Suppressing Glial Differentiation of Primary Cultured Adult Mouse Neural Progenitor Cells through ERK/CREB Activation. Front. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef]
- Kong, Y.; Hu, X.; Zhong, Y.; Xu, K.; Wu, B.; Zheng, J. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res. Ther. 2019, 10, 378. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J. Biomed. Mater. Res. A 2022, 110, 462–487. [Google Scholar] [CrossRef]
- Ulum, M.F.; Caesarendra, W.; Alavi, R.; Hermawan, H. In-Vivo Corrosion Characterization and Assessment of Absorbable Metal Implants. Coatings 2019, 9, 282. [Google Scholar] [CrossRef]


| Gene | Primer Sequence |
|---|---|
| GAPDH (reference gene) | Forward GGCAAGTTCAACGGCACAG Reverse CGCCAGTAGACTCCACGAC |
| NGF | Forward AGCTCACCTCAGTGTCTGG Reverse GCTATCTGTGTACGGTTCTGC |
| GDNF | Forward TCGGGCCACTTGGAGTTAAT Reverse CAGCCACGACATCCCATAAC |
| p75 | Forward CAACCAGACCGTGTGTGAACC Reverse GTCTCCTCGTCCTGGTAGTAGC |
| MPZ | Forward CACCACTCAGTTCCTTGTCC Reverse ACTTCCCTGTCCGTGTAAACC |
| PMP22 | Forward TGTACCACATCCGCCTTGG Reverse CTCATCACACACAGACCAGCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, K.; Hanke, L.; Helmholz, H.; Quandt, E.; Pixley, S.; Willumeit-Römer, R. Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model. J. Funct. Biomater. 2024, 15, 88. https://doi.org/10.3390/jfb15040088
Bhat K, Hanke L, Helmholz H, Quandt E, Pixley S, Willumeit-Römer R. Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model. Journal of Functional Biomaterials. 2024; 15(4):88. https://doi.org/10.3390/jfb15040088
Chicago/Turabian StyleBhat, Krathika, Lisa Hanke, Heike Helmholz, Eckhard Quandt, Sarah Pixley, and Regine Willumeit-Römer. 2024. "Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model" Journal of Functional Biomaterials 15, no. 4: 88. https://doi.org/10.3390/jfb15040088
APA StyleBhat, K., Hanke, L., Helmholz, H., Quandt, E., Pixley, S., & Willumeit-Römer, R. (2024). Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model. Journal of Functional Biomaterials, 15(4), 88. https://doi.org/10.3390/jfb15040088







