A New Hyaluronic Emulgel of Hesperetin for Topical Application—An In Vitro Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hesperetin Quantification
2.3. Preformulation Studies
2.3.1. Experimental Design
2.3.2. Preparation of Formulations
2.3.3. Characterization of Emulgels and Stability Assessment
- Physical Evaluation
- Optical Microscopy
- Evaluation of Droplet Size and Zeta Potential
- Temperature Swing Tests
- Long-Term Stability Study
2.4. In Vitro Hesperetin Release and Skin Retention Studies
2.5. Statistical Analysis
3. Results and Discussion
3.1. Experimental Design
- (−1) = 2.0% w/w
- (0) = 3.5% w/w
- (+1) = 5.0% w/w
- (−1) = 0.5% w/w
- (0) = 1.0% w/w
- (+1) = 1.5% w/w
3.2. Physical Evaluation
3.3. Optical Microscopy
3.4. Temperature Swing Test
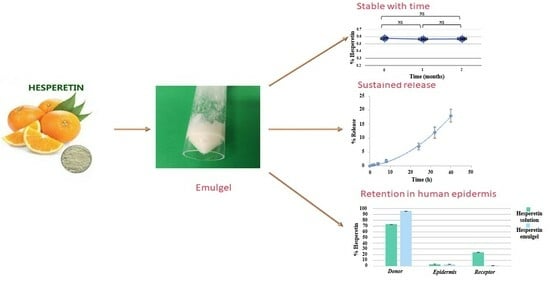
3.5. Long-Term Stability Study
3.6. Release Studies
3.7. Skin Permeation Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathbone, M.J.; Hadgraft, J.; Roberts, M.S. (Eds.) Modified-Release Drug Delivery Technology; Boca Raton CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: Novel strategies for effective transdermal applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Andrade, T.; Heimfarth, L.; dos Santos, D.M.; dos Santos, M.R.; de Albuquerque-Júnior, R.L.; dos Santos-Neto, A.G.; de Araujo, G.R.; Lira, A.A.; Matos, S.S.; Frank, L.A.; et al. Hesperetin-based hydrogels protect the skin against UV radiation-induced damage. AAPS PharmSciTech 2022, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Taléns-Visconti, R.; Perra, M.; Ruiz-Saurí, A.; Nácher, A. New vehiculation systems of mometasone furoate for the treatment of inflammatory skin diseases. Pharmaceutics 2022, 14, 2558. [Google Scholar] [CrossRef] [PubMed]
- Lionberger, D.R.; Brennan, M.J. Topical nonsteroidal anti-inflammatory drugs for the treatment of pain due to soft tissue injury: Diclofenac epolamine topical patch. J. Pain Res. 2010, 3, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, A.; Dandagi, P.; Gadad, A.; Mastiholimath, V. Formulation and characterization of meloxicam loaded emulgel for topical application. Int. J. Pharm. Pharm. Sci. 2015, 7, 216–222. [Google Scholar]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An Effective Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A novel nano carrier as a tool for topical drug delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Padaraju, A.; Dwivedi, F.; Kumar, G. Microemulsions, nanoemulsions and Emulgels as carriers for antifungal antibiotics. Ther. Deliv. 2023, 14, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Cook, M.T. In situ gelling drug delivery systems for topical drug delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef]
- N’Da, D. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef]
- Naga Sravan Kumar Varma, V.; Maheshwari, P.V.; Navya, M.; Reddy, S.C.; Shivakumar, H.G.; Gowda, D.V. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm. J. 2014, 22, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, J.; Jaiswal, S.; Gupta, G. A review on novel approach of antifungal emulgel for topical delivery in fungal infections. Indo Am. J. Pharm. Res. 2016, 6, 6321–6324. [Google Scholar]
- Purushottam, S.S.; Bhaskarrao, G.S.; Ravindra, S. Gellified emulsion: A new born formulation for topical delivery of hydrophobic drugs. Pharm. Tech. Med. 2013, 2, 370–376. [Google Scholar]
- Bae, J.-Y.; Seo, Y.-H.; Oh, S.-W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.; Santos, C.; Fernandes, E. Therapeutic potential of Hesperidin and its Aglycone Hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine 2020, 73, 152887. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-W.; Huang, A.-L.; Zhang, Y.-L.; Li, B.; Huang, C.; Ma, T.; Meng, X.-M.; Li, J. Design, synthesis and biological evaluation of hesperetin derivatives as potent anti-inflammatory agent. Fitoterapia 2017, 121, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Hesperetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/72281 (accessed on 16 February 2024).
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A comparative study of Hesperetin, Hesperidin and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, S.; Zhang, L.; Tian, L.; Li, L.; Liu, Z.; Dong, Q.; Lv, X.; Mu, H.; Zhang, Q.; et al. Hesperetin as an adjuvant augments protective anti-tumour immunity responses in b16f10 melanoma by stimulating cytotoxic CD8+ T cells. Scand. J. Immunol. 2020, 91, e12867. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chiu-Leung, L.C.; Lin, S.; Leung, L.K. The citrus flavonone Hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 210, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Dong, W.; Qu, X.; Huang, C.; Yan, T.; Du, J. Combination of Hesperetin and platinum enhances anticancer effect on lung adenocarcinoma. Biomed. Pharmacother. 2019, 113, 108779. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Taléns-Visconti, R.; Magraner-Pardo, L.; Peris, J.-E. Hesperetin induces melanin production in adult human epidermal melanocytes. Food Chem. Toxicol. 2015, 80, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.; Huang, H.; Liao, Y. The efficacy and safety of an antiaging topical serum containing hesperetin and sodium cyclic lysophosphatidic acid: A single-center clinical trial. J. Cosmet. Dermatol. 2021, 20, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.S.; Milan, T.M.; Tapia-Blácido, D.R. Using response surface methodology (RSM) to optimize 2G bioethanol production: A Review. Biomass Bioenergy 2021, 151, 106166. [Google Scholar] [CrossRef]
- STATISTICA, v 8.0; Stat Soft Inc.: Tulsa, OK, USA, 2007.
- Noboa, G.; Márquez, L.; López, J.C. Tamaño de gota: Factor determinante sobre la velocidad de clarificación de una emulsión o/w. Cienc. E Ing. 2017, 38, 259–264. [Google Scholar]
- Available online: https://www.montana.edu/eal-lres/documents/Mastersizer-3000-user-manual-English-MAN0474-2-1.pdf (accessed on 16 February 2024).
- Chilcott, R.P.; Jenner, J.; Hotchkiss, S.A.; Rice, P. In vitro skin absorption and decontamination of sulphur mustard: Comparison of human and Pig-Ear Skin. J. Appl. Toxicol. 2001, 21, 279–283. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from nonswellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Daneluz, J.; da Silva Favero, J.; dos Santos, V.; Weiss-Angeli, V.; Gomes, L.B.; Mexias, A.S. The influence of different concentrations of a natural clay material as active principle in cosmetic formulations. Mater Res. 2020, 23, e20190572. [Google Scholar] [CrossRef]
- Zanini, M. Gel de ácido tricloroacético—Uma nova técnica para um antigo ácido. Med. Cutánea Ibero Lat. Am. 2007, 35, 14–17. [Google Scholar]
- Alshangiti, D.M.; El-Damhougy, T.K.; Zaher, A.; Madani, M.; Mohamady Ghobashy, M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251–35291. [Google Scholar] [CrossRef]
- Khan, B.A.; Khan, A.; Khan, M.K.; Braga, V.A. Preparation and properties of high sheared poly(vinyl alcohol)/chitosan blended hydrogels films with Lawsonia inermis extract as wound dressing. J. Drug Deliv. Sci. Technol. 2021, 61, 102227. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Release and in vitro skin permeation of polyphenols from cosmetic emulsions. Int. J. Cosmet. Sci. 2013, 35, 491–501. [Google Scholar] [CrossRef]
- Islam, N.; Irfan, M.; Zahoor, A.F.; Iqbal, M.S.; Syed, H.; Khan, I.; Rasul, A.; Khan, S.; Alqahtani, A.M.; Ikram, M.; et al. Development of Transfersomal Oral Films. Improved bioavailability of ebastine through development of transfersomal oral films. Pharmaceutics 2021, 13, 1315. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.; Yew Chieng, C.J.; Choudhury, H.; Pandey, M.; Gorain, B. Tocotrienols-rich naringenin nanoemulgel for the management of diabetic wound: Fabrication, characterization and comparative in vitro evaluations. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100019. [Google Scholar] [CrossRef] [PubMed]










| Ingredient (% w/w) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Hesperetin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Phospholipid | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Polysorbate 80 | 2 | 3.5 | 2 | 2 | 3.5 | 5 | 3.5 | 5 | 5 |
| Cetostearyl alcohol | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Almond oil | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Transcutol GC | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Tocopherol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Propylene glycol | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Methylparaben | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Propylparaben | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Hyaluronic acid | 1.5 | 0.5 | 0.5 | 1 | 1 | 1.5 | 1.5 | 1 | 0.5 |
| Ethanol | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s |
| Water | 68.75 | 68.25 | 69.75 | 69.25 | 67.75 | 65.75 | 67.25 | 66.25 | 66.75 |
| Forced sedimentation test | 30 min at 3500 rpm |
| Temperature swing test | Cold–heat cycle 4 °C to 40 °C |
| Freeze–thaw cycle −20 °C to 25 °C |
| Independent Variable | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| V1: Concentration of Polysorbate 80 | −1 | 0 | −1 | −1 | 0 | +1 | 0 | +1 | +1 |
| V2: Concentration of hyaluronic acid | +1 | −1 | −1 | 0 | 0 | +1 | +1 | 0 | −1 |
| Emulgel | State | Z-Potential | Droplet Size d[4,3] µm | pH | Conductivity (µS) | % Hesperetin |
|---|---|---|---|---|---|---|
| Control | −28.3 ± 1.39 | 2.07 | 5.89 | 487 | - | |
| F7 (selected formula with 0.5% hesperetin) | Initial | −27.8 ± 0.68 | 4.02 | 5.16 | 507 | 0.58 |
| Cycle 4/40 °C | −25.6 ± 2.89 | 6.52 | 5.13 | 564 | 0.56 | |
| Cycle −20/25 °C | −25.8 ± 0.70 | 3.28 | 5.36 | 587 | 0.56 |
| Zero-Order Kinetics | First-Order Kinetics | Square-Root Kinetics | |
|---|---|---|---|
| SS | 1.87 | 64.58 | 86.77 |
| AIC | 5.7S4 | 27.01 | 28.78 |
| Diffusion Model | Parameters | Semi-Solid Vehicle |
|---|---|---|
| Korsmeyer–Peppas | K | 0.10 ± 0.02 |
| n | 1.06 ± 0.06 | |
| r | 0.9990 | |
| Peppas–Shalin | K1 | 0.13 ± 0.08 |
| K2 | 0.002 ± 0.004 | |
| n | 1.05 ± 0.33 | |
| r | 0.9993 |
| Parameter | Solution (Control) | Semi-Solid Vehicle |
|---|---|---|
| P | 648.35 ± 367.67 | 0.374 ± 0.077 |
| D (cm2/h) | 0.075 ± 0.038 | 0.025 ± 0.004 |
| tL (h) | 2.2 ± 0.4 | 6.7 ± 0.7 |
| Kp·10−3 (cm/h) | 48.7 ± 5.9 | 0.010 ± 0.002 |
| J (μg/cm2·h) | 24.2 ± 2.8 | 0.004 ± 0.001 |
| r> | 0.98 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taléns-Visconti, R.; Belarbi, Y.; Díez-Sales, O.; Julián-Ortiz, J.V.d.; Vila-Busó, O.; Nácher, A. A New Hyaluronic Emulgel of Hesperetin for Topical Application—An In Vitro Evaluation. J. Funct. Biomater. 2024, 15, 89. https://doi.org/10.3390/jfb15040089
Taléns-Visconti R, Belarbi Y, Díez-Sales O, Julián-Ortiz JVd, Vila-Busó O, Nácher A. A New Hyaluronic Emulgel of Hesperetin for Topical Application—An In Vitro Evaluation. Journal of Functional Biomaterials. 2024; 15(4):89. https://doi.org/10.3390/jfb15040089
Chicago/Turabian StyleTaléns-Visconti, Raquel, Yousra Belarbi, Octavio Díez-Sales, Jesus Vicente de Julián-Ortiz, Ofelia Vila-Busó, and Amparo Nácher. 2024. "A New Hyaluronic Emulgel of Hesperetin for Topical Application—An In Vitro Evaluation" Journal of Functional Biomaterials 15, no. 4: 89. https://doi.org/10.3390/jfb15040089