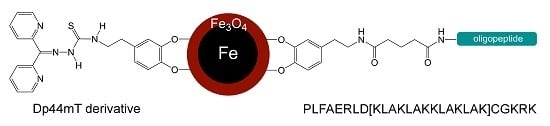
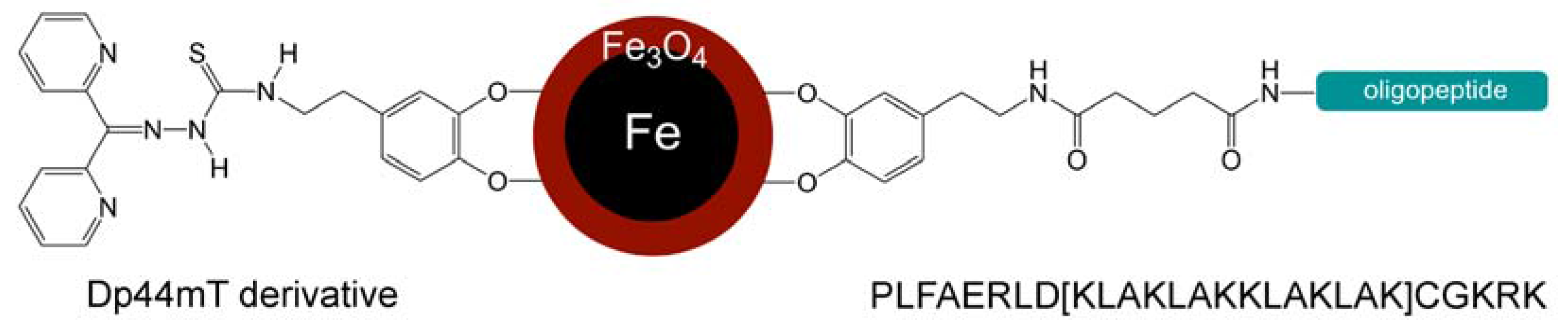
Synergy of Iron Chelators and Therapeutic Peptide Sequences Delivered via a Magnetic Nanocarrier
Abstract
:1. Introduction
2. Results
2.1. Characterization of PLFAERL D[KLAKLAK]2CGKRK by HPLC Purification and MALDI-TOF
2.2. Analysis of the Linking Efficacy of Dopamine-Glutaric Acid-Modified PLFAERL D[KLAKLAK]2CGKRK and the Dp44mT-Derivative to Fe/Fe3O4-nanoparticles via ICP
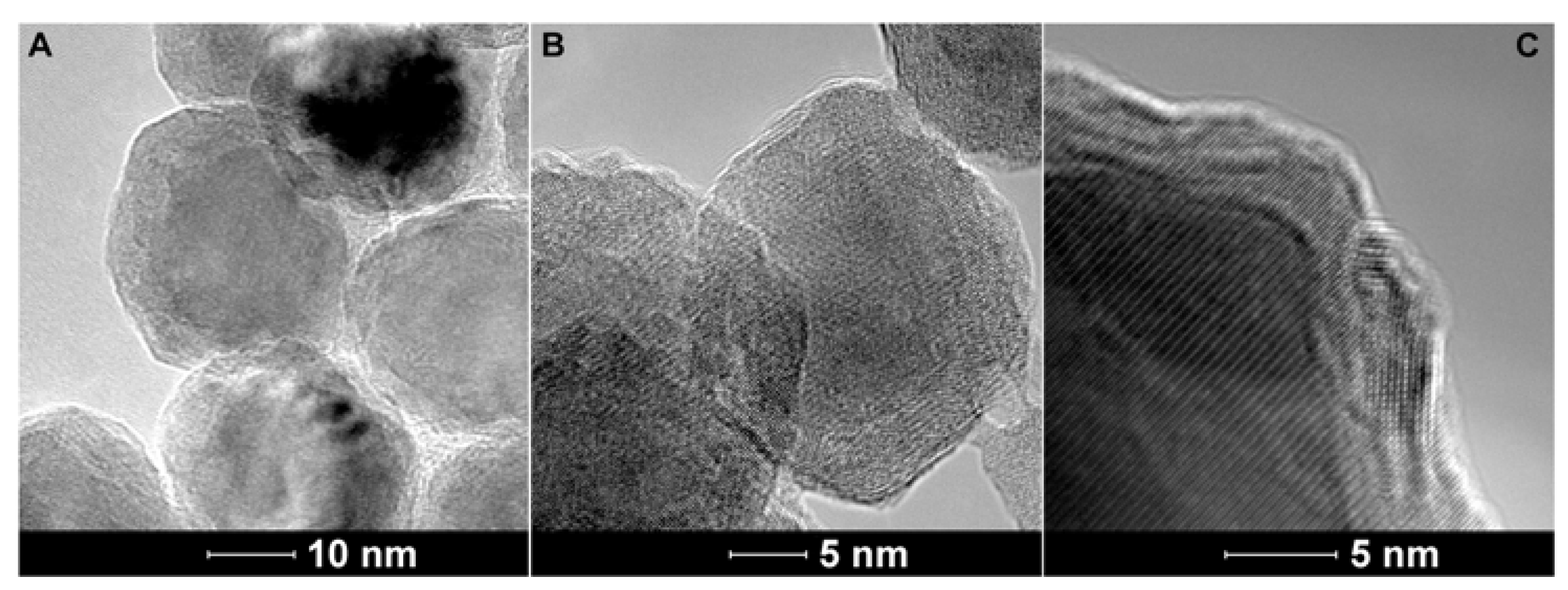
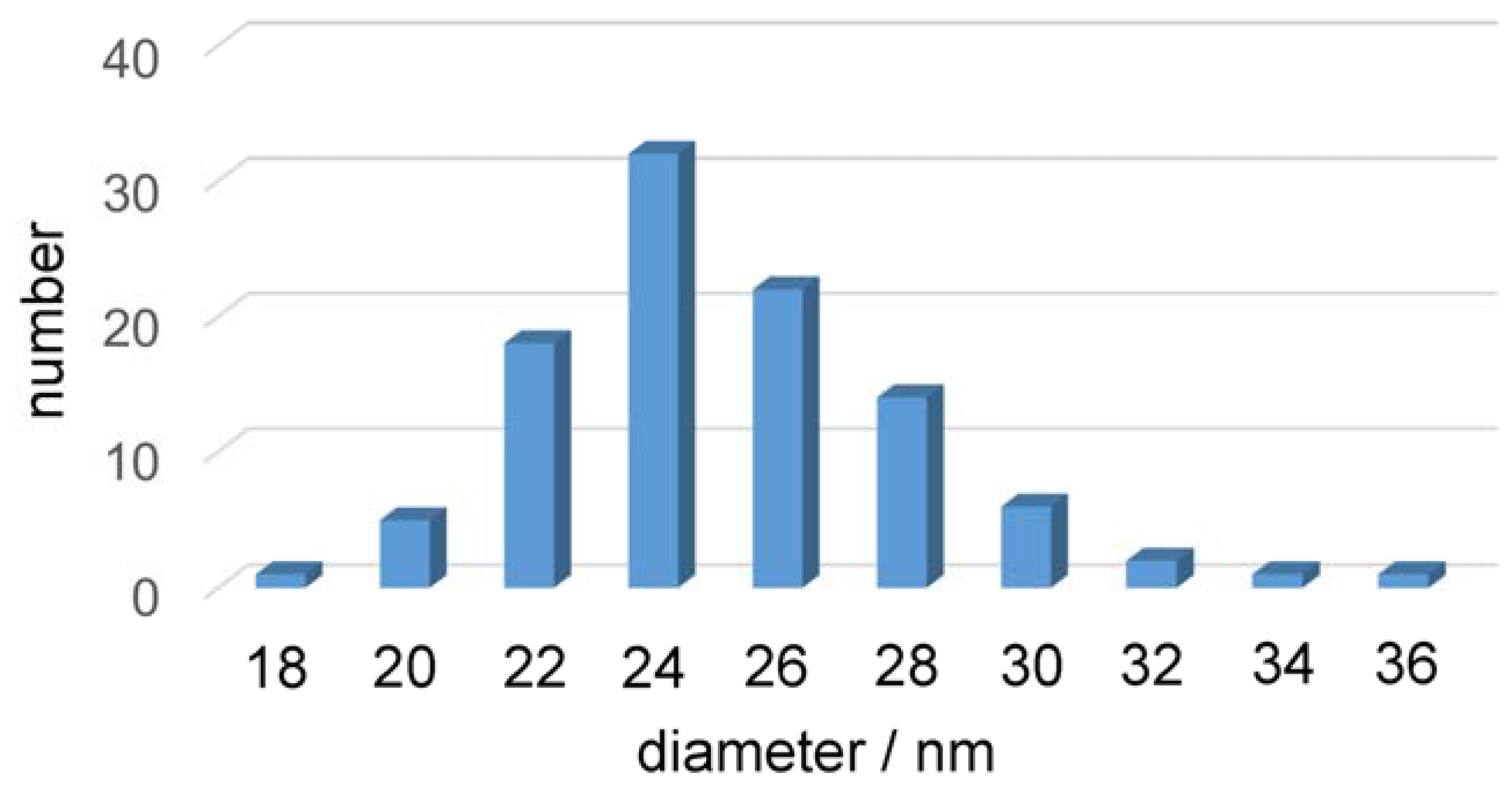
2.3. Transmission Electron Microscopy of the Fe/Fe3O4-Nanocarriers
2.4. Dynamic Light Scattering (DLS) and Zeta-Potential Determination of the Fe/Fe3O4-Nanocarriers
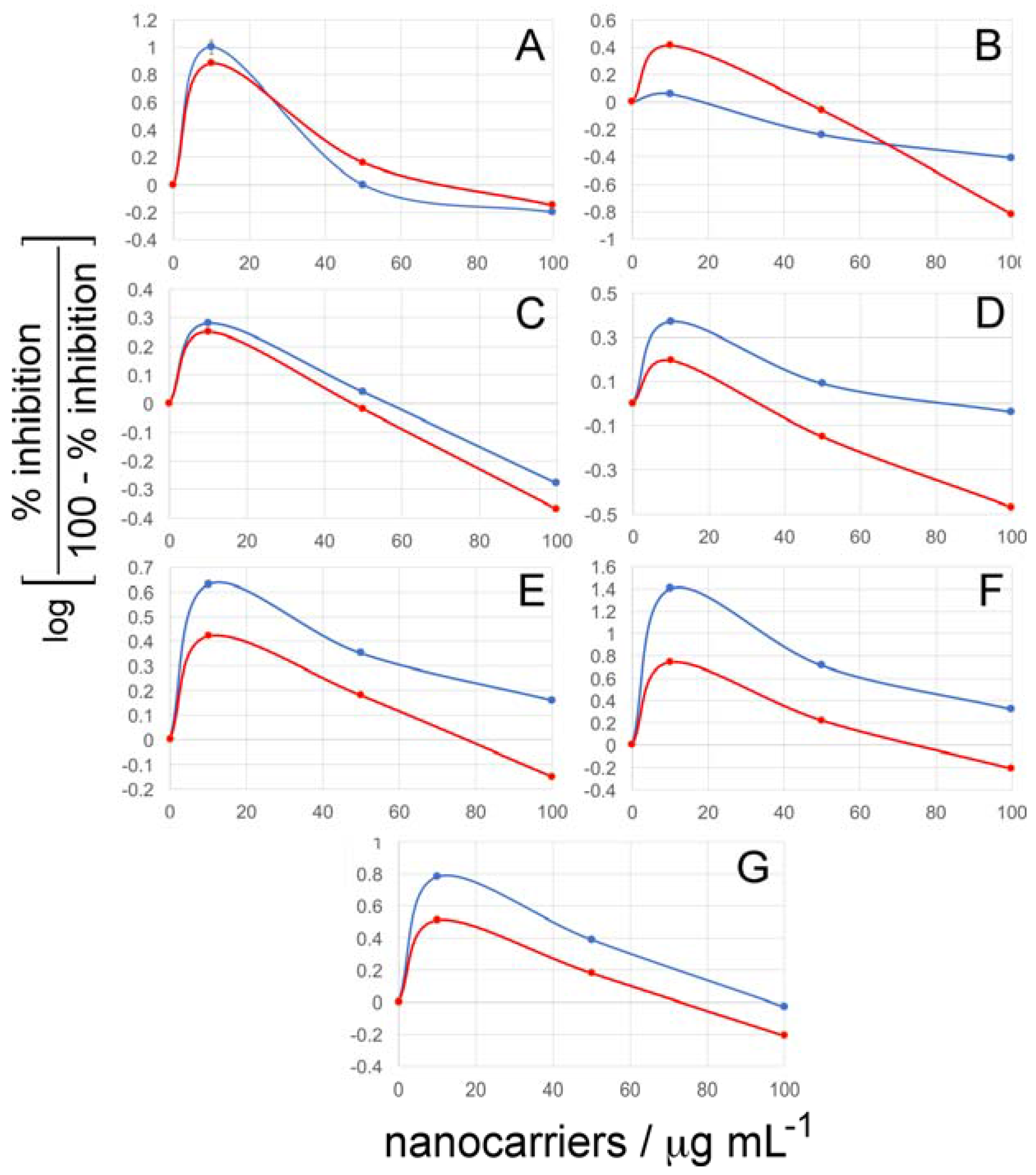
2.5. Cell Viability Assays
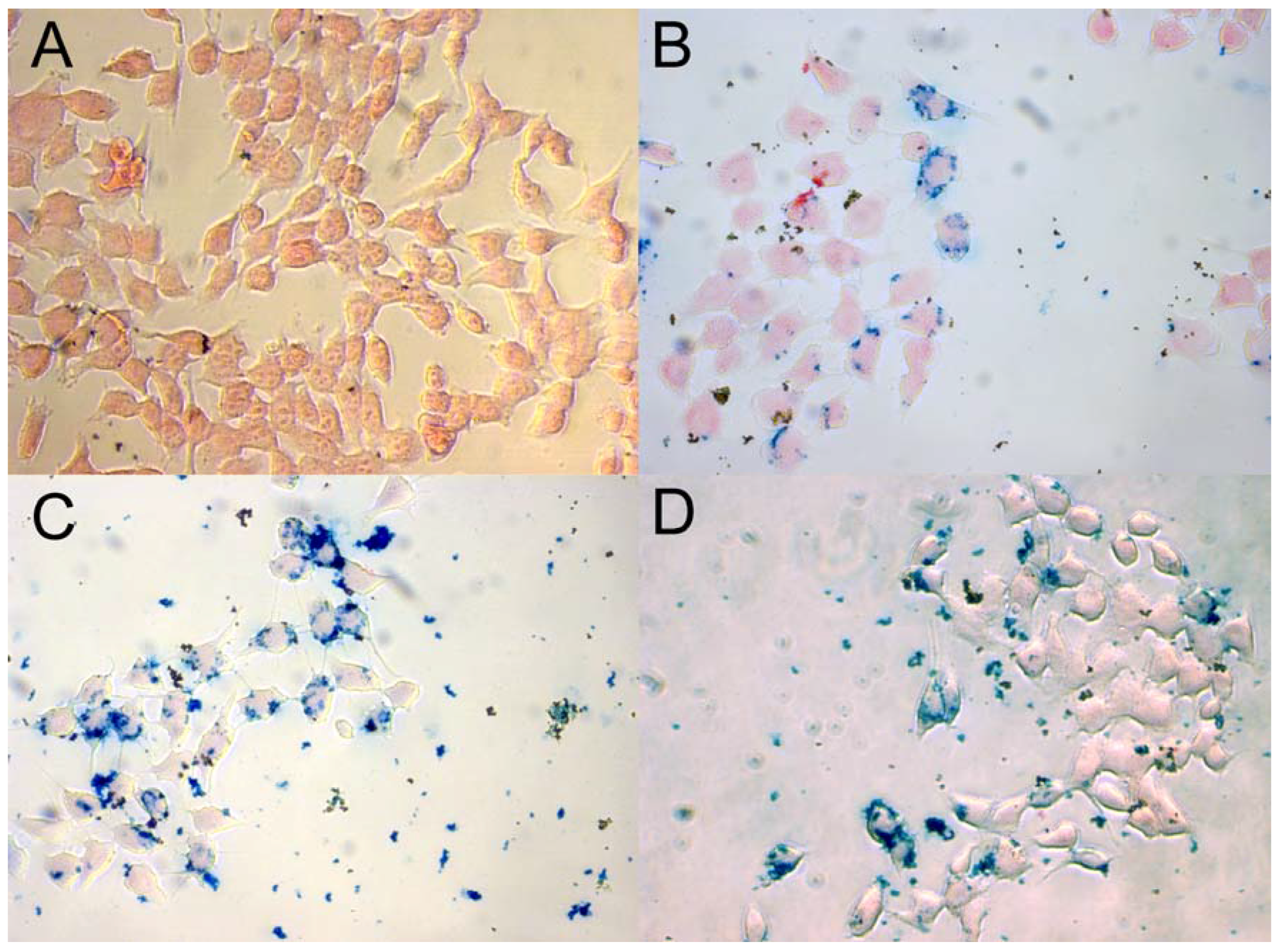
2.6. Prussian Blue Staining
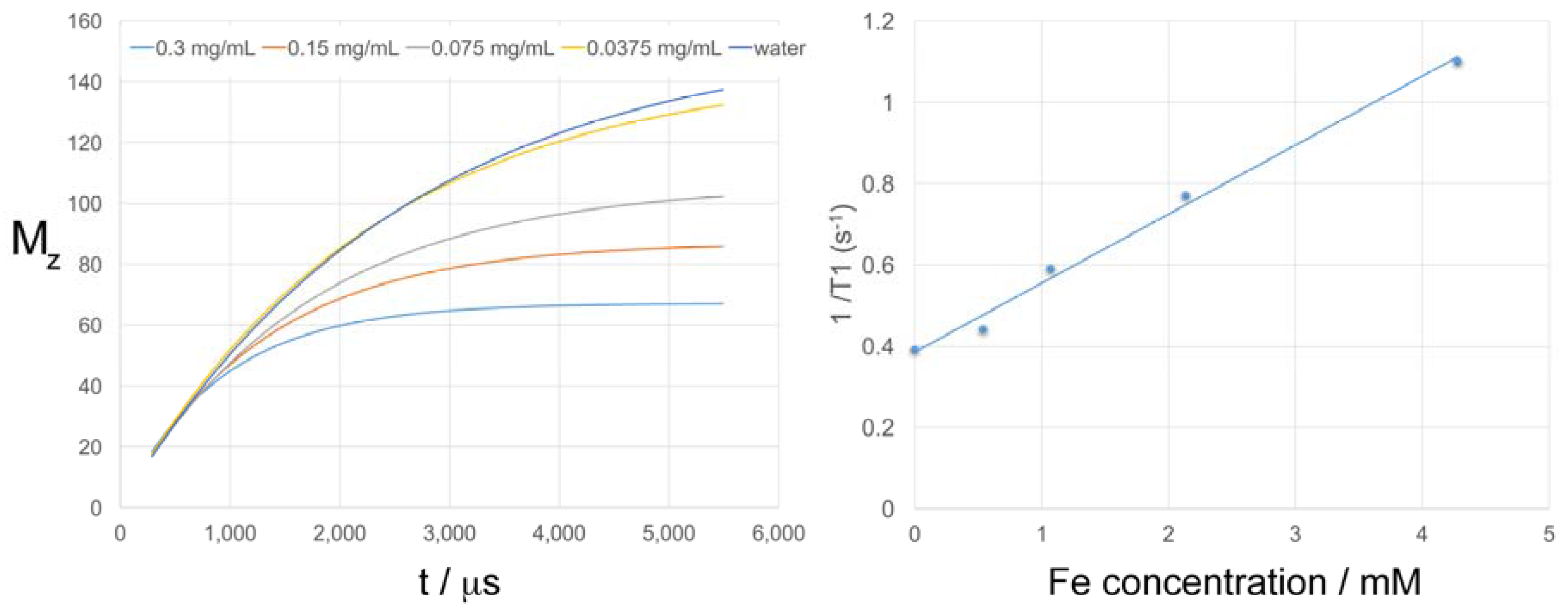
2.7. T1 and T2 Relaxation Times of the Fe/Fe3O4-derived Nanocarriers
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Core/Shell Fe/Fe3O4 Magnetic Nanoparticles (MNPs)
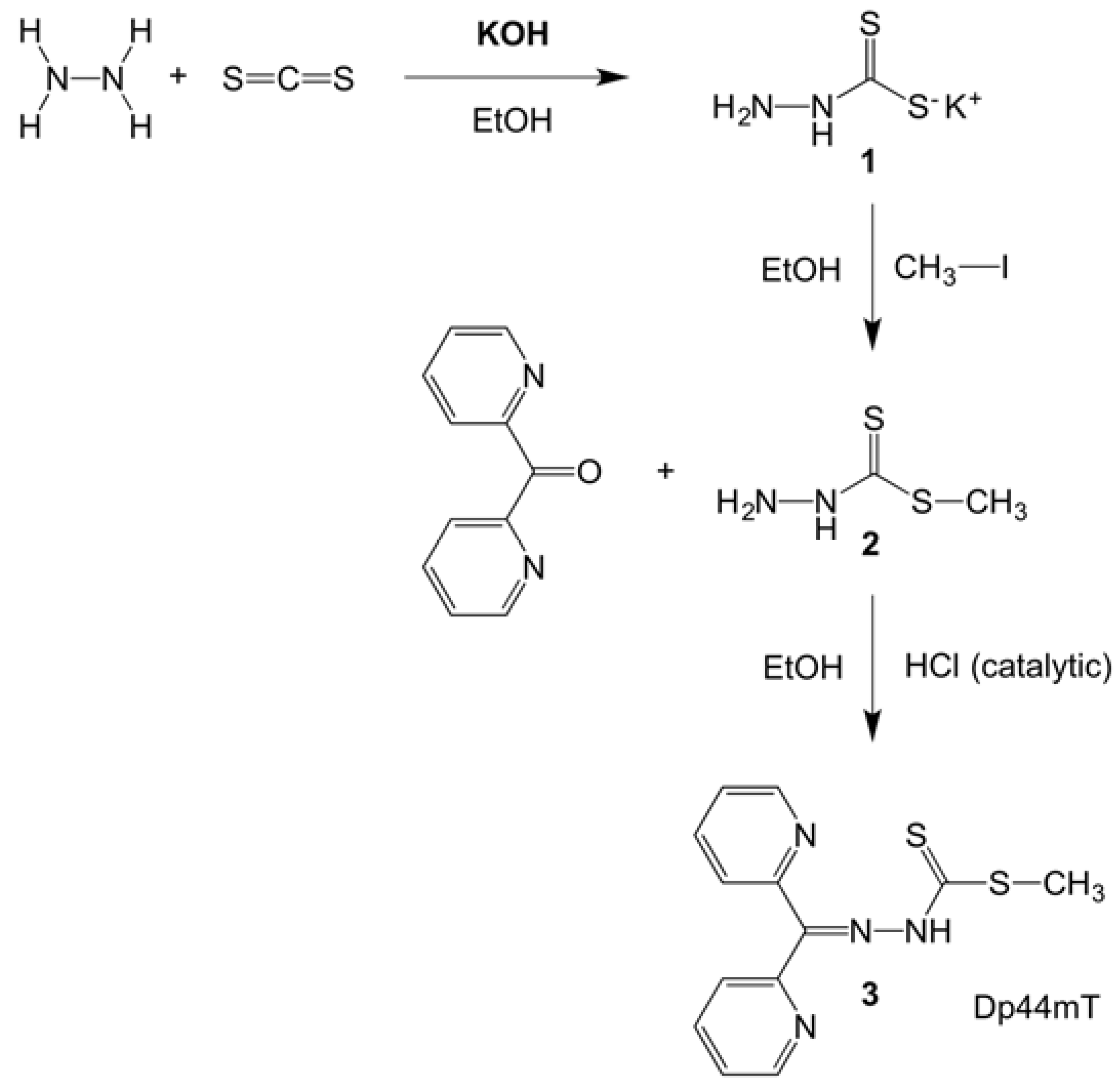
5.2. Synthesis of the Dp44mT Derivative (Methyl-2-(di(pyridine-2-yl)methylene)hydrazinecarbo-dithioate)3
5.3. Synthesis of PLFAERLD[KLAKLAK]2CGKRK
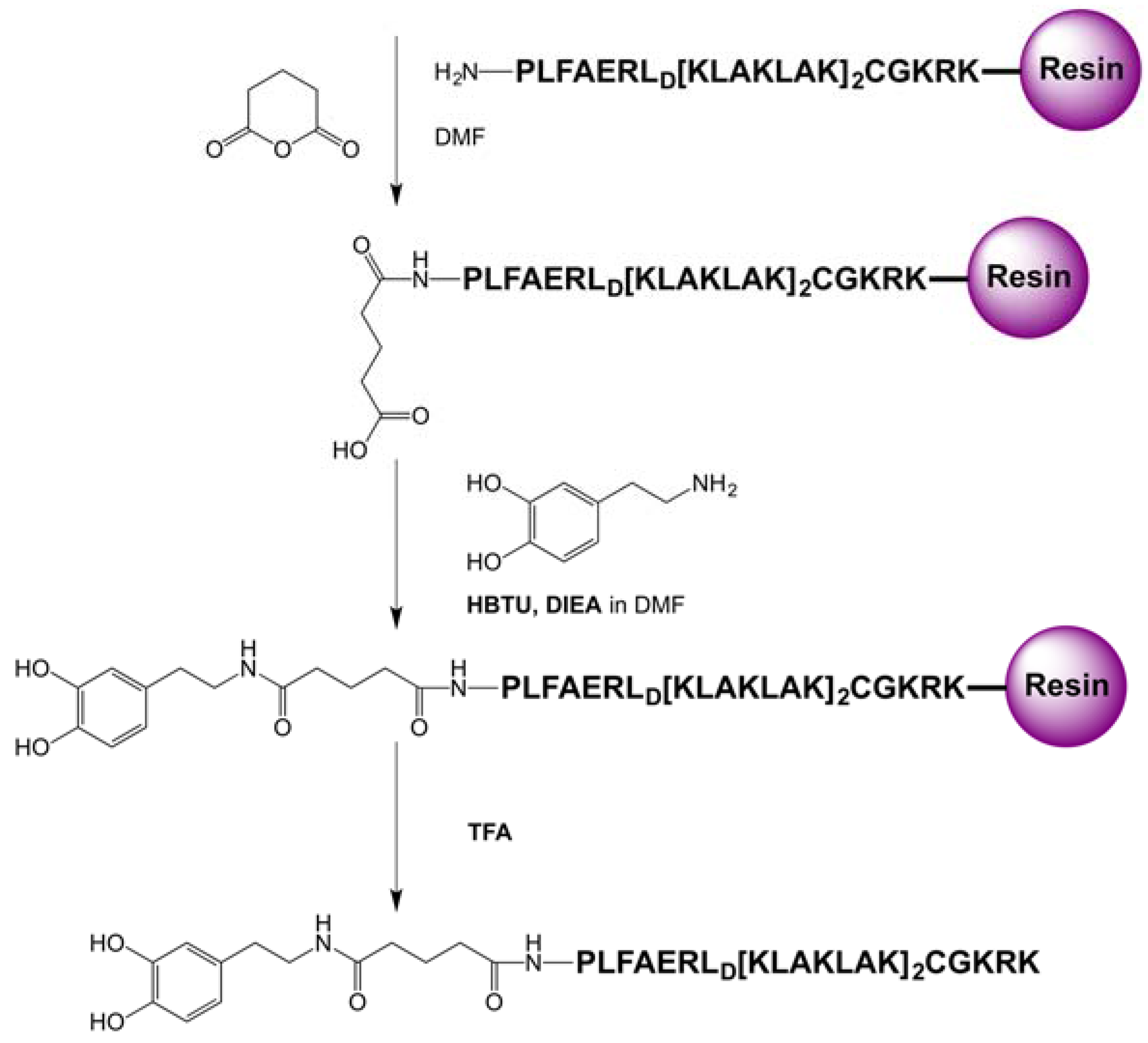
5.4. Synthesis of the Dopamine Peptide Ligand
5.5. HPLC Analysis of the Peptide PLFAERLD[KLAKLAKKLAKLAK]CGKRK
5.6. Ligand Exchange of Fe/Fe3O4 Nanoparticles with the Dopamine Peptide Sequence and Free Dopamine
5.7. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
5.8. Transmission Electron Microscopy (TEM)
5.9. Inductively Coupled Plasma (ICP) Measurements to Determine the Iron and Sulfur Content of the Fe/Fe3O4 Nanocarriers
5.10. Cell Viability Test Measured by MTT Assay
5.11. Test for Cell Uptake of Nanoparticles by Prussian Blue Staining
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Myrberg, H.; Zhang, L.; Maee, M.; Langel, U. Design of a Tumor-Homing Cell-Penetrating Peptide. Bioconjug. Chem. 2008, 19, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, Y.; Wagner, E.; Xie, C.; Lu, W.; Zhu, J.; Shen, J.; Wang, J.; Liu, M. Potent Retro-Inverso d-Peptide for Simultaneous Targeting of Angiogenic Blood Vasculature and Tumor Cells. Bioconjug. Chem. 2013, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Malalasekera, A.P.; Bossmann, S.H.; Zhu, G. Magnetic Nanoformulations for Enhanced Drug Delivery and Retention. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Bossmann, S.H., Wang, H., Eds.; The Royal Society of Chemistry: London, UK, 2017; pp. 228–250. [Google Scholar]
- Agemy, L.; Friedmann-Morvinski, D.; Kotamraju, V.R.; Roth, L.; Sugahara, K.N.; Girard, O.M.; Mattrey, R.F.; Verma, I.M.; Ruoslahti, E. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17450–17455. [Google Scholar] [CrossRef] [PubMed]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Del Rio, G.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. N. Y. 1999, 5, 1032–1038. [Google Scholar]
- Hoffman, J.A.; Giraudo, E.; Singh, M.; Zhang, L.; Inoue, M.; Porkka, K.; Hanahan, D.; Ruoslahti, E. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell 2003, 4, 383–391. [Google Scholar] [CrossRef]
- Rao, V.A.; Klein, S.R.; Agama, K.K.; Toyoda, E.; Adachi, N.; Pommier, Y.; Shacter, E.B. The Iron Chelator Dp44mT Causes DNA Damage and Selective Inhibition of Topoisomerase IIα in Breast Cancer Cells. Cancer Res. 2009, 69, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.J.; Hawkins, C.L.; Lovejoy, D.B.; Richardson, D.R. The iron complex of Dp44mT is redox-active and induces hydroxyl radical formation: An EPR study. J. Inorg. Biochem. 2010, 104, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Krishan, S.; Richardson, D.R.; Sahni, S. The Anticancer Agent, Di-2-Pyridylketone 4,4-Dimethyl-3-Thiosemicarbazone (Dp44mT), Up-Regulates the AMPK-Dependent Energy Homeostasis Pathway in Cancer Cells. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2916–2933. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Shafie, N.H.; Yu, Y.; Richardson, V.; Jansson, P.J.; Sahni, S.; Lane, D.J.R.; Kovacevic, Z.; Kalinowski, D.S.; Richardson, D.R. Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase. Biochem. Pharmacol. (Amst. Neth.) 2016, 109, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.R. Enhanced targeting of mitochondrial peroxide defense by the combined use of thiosemicarbazones and inhibitors of thioredoxin reductase. Free Radical Biol. Med. 2016, 91, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Cuerrier, D.; Moldoveanu, T.; Davies, P.L. Determination of Peptide Substrate Specificity for μ-Calpain by a Peptide Library-based Approach: the importance of primed side interactions. J. Biol. Chem. 2005, 280, 40632–40641. [Google Scholar] [CrossRef] [PubMed]
- Rachakatla, R.S.; Balivada, S.; Seo, G.-M.; Myers, C.B.; Wang, H.; Samarakoon, T.N.; Dani, R.; Pyle, M.; Kroh, F.O.; Walker, B.; et al. Attenuation of Mouse Melanoma by A/C Magnetic Field after Delivery of Bi-Magnetic Nanoparticles by Neural Progenitor Cells. ACS Nano 2010, 4, 7093–7104. [Google Scholar] [CrossRef] [PubMed]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shrestha, T.B.; Basel, M.T.; Dani, R.K.; Seo, G.-M.; Balivada, S.; Pyle, M.M.; Prock, H.; Koper, O.B.; Thapa, P.S.; et al. Magnetic-Fe/Fe3O4-nanoparticle-bound SN38 as carboxylesterase-cleavable prodrug for the delivery to tumors within monocytes/macrophages. Beilstein J. Nanotechnol. 2012, 3, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Udukala, D.N.; Samarakoon, T.N.; Basel, M.T.; Kalita, M.; Abayaweera, G.; Manawadu, H.; Malalasekera, A.; Robinson, C.; Villanueva, D.; et al. Nanoplatforms for highly sensitive fluorescence detection of cancer-related proteases. Photochem. Photobiol. Sci. 2014, 13, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Udukala, D.N.; Wang, H.; Wendel, S.O.; Malalasekera, A.P.; Samarakoon, T.N.; Yapa, A.S.; Abayaweera, G.; Maynez, P.; Ortega, R.; Toledo, Y.; et al. Early breast cancer screening using iron/iron oxide-based nanoplatforms with sub-femtomolar limits of detection. Beilstein J. Nanotechnol. 2016, 7, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Stefani, C.; Toyokuni, S.; Ganz, T.; Anderson, G.J.; Subramaniam, N.V.; Trinder, D.; Olynyk, J.K.; Chua, A.; Jansson, P.J.; et al. Redox cycling metals: Pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as A Robust Anchor to Immobilize Functional Molecules on the Iron Oxide Shell of Magnetic Nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727. [Google Scholar]
- Basha, M.T.; Chartres, J.D.; Pantarat, N.; Akbar Ali, M.; Mirza, A.H.; Kalinowski, D.S.; Richardson, D.R.; Bernhardt, P.V. Heterocyclic dithiocarbazate iron chelators: Fe coordination chemistry and biological activity. Dalton Trans. 2012, 41, 6536–6548. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Albericio, F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef] [PubMed]
- Sittampalam, G.S.; Coussens, N.P.; Brimacombe, K.; Grossman, A.; Arkin, M.; Auld, D.; Austin, C.; Baell, J.; Bejcek, B.; Chung, T.D.Y.; et al. Assay Guidance Manual; Sitaampalam, G.S., Ed.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004.
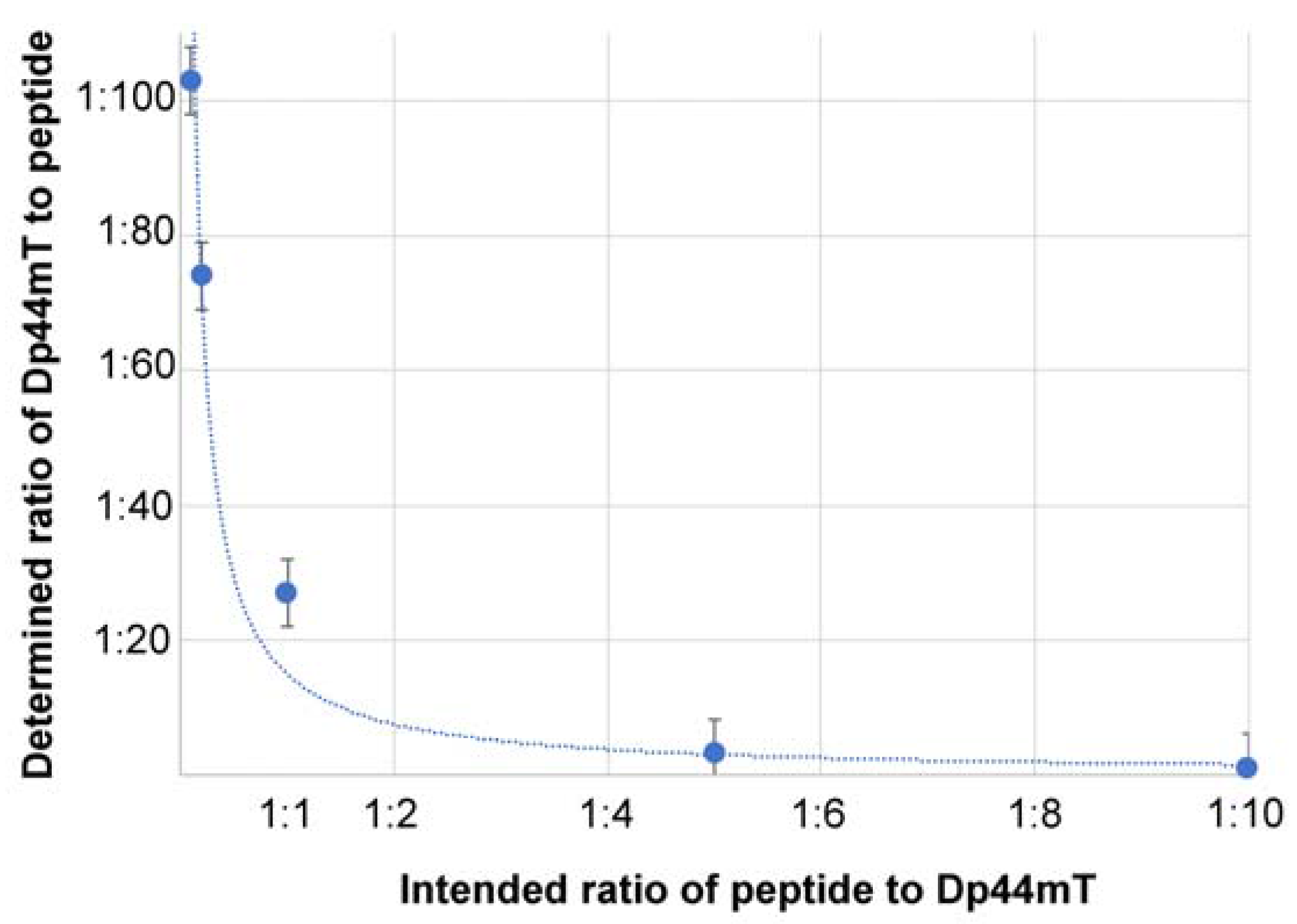



| Nanoparticle System | Fe Percentage of the Nanoparticles | S Percentage of the Nanoparticles | Moles of S Per 1.0 mg of Nanoparticle | Ratio of Therapeutic Peptide/Dp44mT |
|---|---|---|---|---|
| Dop-Fe/Fe3O4 1 | 71.0% | / | / | / |
| Dop-Fe/Fe3O4 Peptide | 72.0% | 0.021% | 6.50 × 10−6 | / |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (10:1) 2 | 70.6% | 0.040% | 1.24 × 10−5 | 1:1.1 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (5:1) 2 | 72.6% | 0.087% | 2.73 × 10−5 | 1:3.2 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (1:1) 2 | 72.8% | 0.58% | 1.86 × 10−4 | 1:27 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (1:5) 2 | 72.8% | 1.56% | 4.87 × 10−4 | 1:74 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (1:10) 2 | 71.9% | 2.06% | 6.77 × 10−4 | 1:103 |
| Nanoparticle System | Hydrodynamic Diameter of Nanoparticles (nm) | Zeta Potential (mV) | Ratio of Therapeutic Peptide/Dp44mT |
|---|---|---|---|
| Dop-Fe/Fe3O4 1 | 475 ± 20 | +30.5 ± 2 | / |
| Dop-Fe/Fe3O4 Peptide | 600 ± 35 | +35.3 ± 3 | / |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (10:1) 2 | 505 ± 27 | +24.4 ± 3 | 1/1.1 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (5:1) 2 | 430 ± 45 | +14.8 ± 3 | 1/3.2 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (1:1) 2 | 530 ± 45 | +37.1 ± 2 | 1/27 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (1:5) 2 | 425 ± 50 | +38.7 ± 3 | 1/74 |
| Dop-Fe/Fe3O4 Peptide/Dp44mT (1:10) 2 | 450 ± 30 | +32.1 ± 3 | 1/103 |
| Fe-conc. (mg/L) | S-conc. (mg/L) |
|---|---|
| 0 | 0 |
| 10 | 5 |
| 20 | 10 |
| 40 | 15 |
| 60 | 20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abayaweera, G.S.; Wang, H.; Shrestha, T.B.; Yu, J.; Angle, K.; Thapa, P.; Malalasekera, A.P.; Maurmann, L.; Troyer, D.L.; Bossmann, S.H. Synergy of Iron Chelators and Therapeutic Peptide Sequences Delivered via a Magnetic Nanocarrier. J. Funct. Biomater. 2017, 8, 23. https://doi.org/10.3390/jfb8030023
Abayaweera GS, Wang H, Shrestha TB, Yu J, Angle K, Thapa P, Malalasekera AP, Maurmann L, Troyer DL, Bossmann SH. Synergy of Iron Chelators and Therapeutic Peptide Sequences Delivered via a Magnetic Nanocarrier. Journal of Functional Biomaterials. 2017; 8(3):23. https://doi.org/10.3390/jfb8030023
Chicago/Turabian StyleAbayaweera, Gayani S., Hongwang Wang, Tej B. Shrestha, Jing Yu, Kyle Angle, Prem Thapa, Aruni P. Malalasekera, Leila Maurmann, Deryl L. Troyer, and Stefan H. Bossmann. 2017. "Synergy of Iron Chelators and Therapeutic Peptide Sequences Delivered via a Magnetic Nanocarrier" Journal of Functional Biomaterials 8, no. 3: 23. https://doi.org/10.3390/jfb8030023
APA StyleAbayaweera, G. S., Wang, H., Shrestha, T. B., Yu, J., Angle, K., Thapa, P., Malalasekera, A. P., Maurmann, L., Troyer, D. L., & Bossmann, S. H. (2017). Synergy of Iron Chelators and Therapeutic Peptide Sequences Delivered via a Magnetic Nanocarrier. Journal of Functional Biomaterials, 8(3), 23. https://doi.org/10.3390/jfb8030023