Sono-Photocatalytic Activity of Cloisite 30B/ZnO/Ag2O Nanocomposite for the Simultaneous Degradation of Crystal Violet and Methylene Blue Dyes in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cloisite 30B/ZnO/Ag2O Photocatalyst
2.3. Assessment of Sono-Photocatalytic Activity
3. Results and Discussion
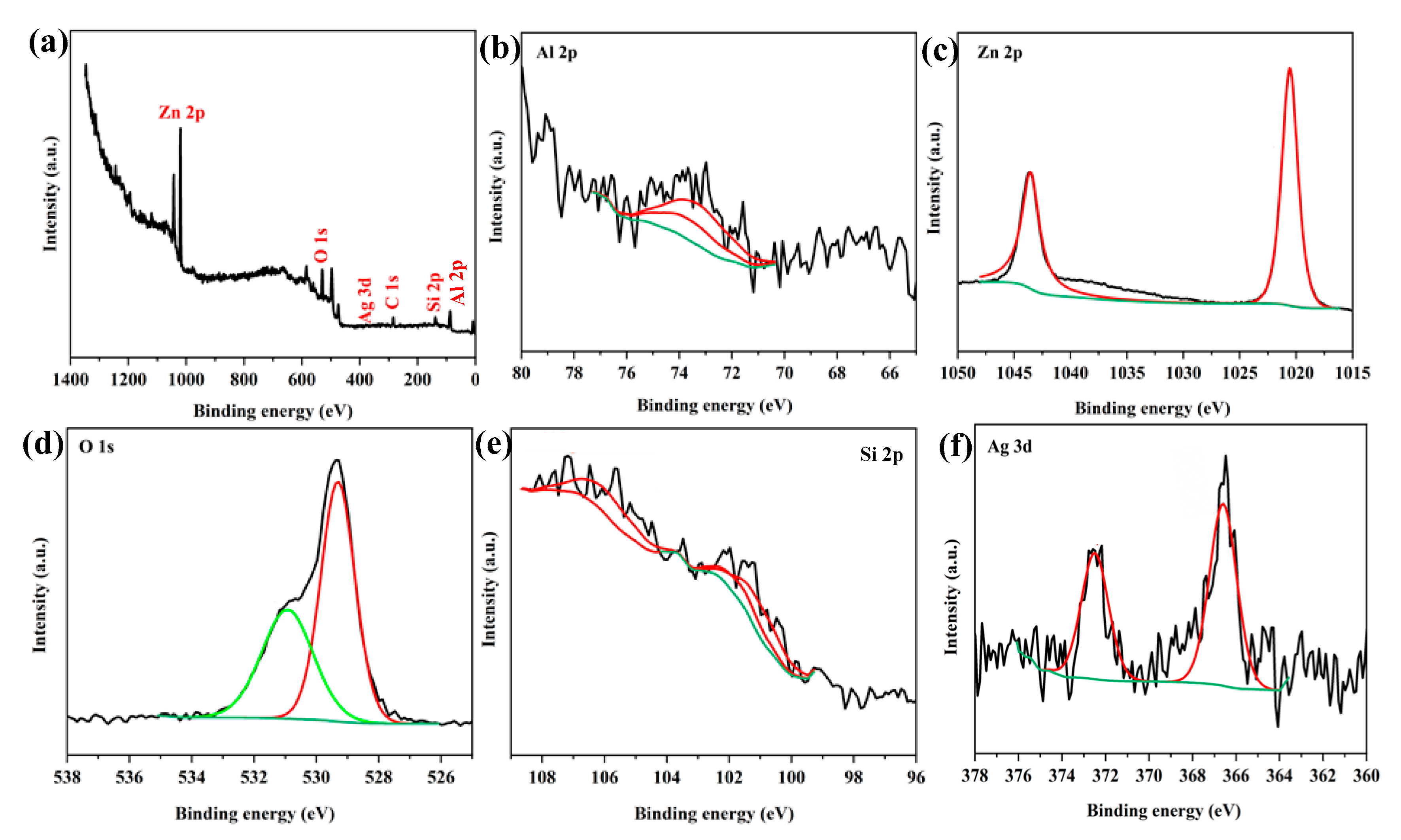
3.1. Properties of the Photocatalyst
3.2. The Effect of pH and the Role of the Catalyst
3.3. The Effect of H2O2 Content, Amount of Photocatalyst, Duration of Reaction, and Dyes Concentration
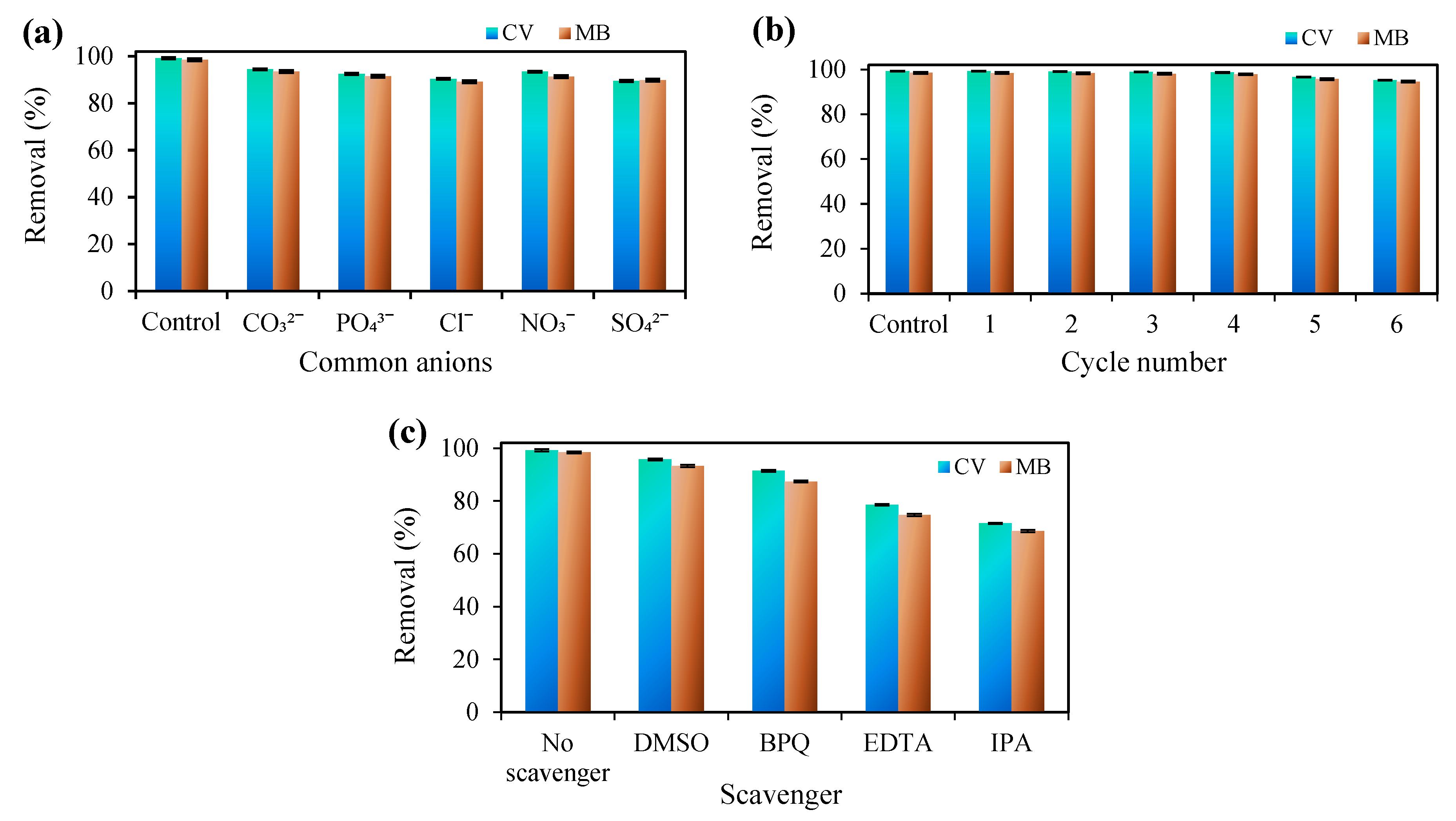
3.4. The Investigation of Common Anions Effect, Scavengers Effect, and Photo-Catalyst Reuse
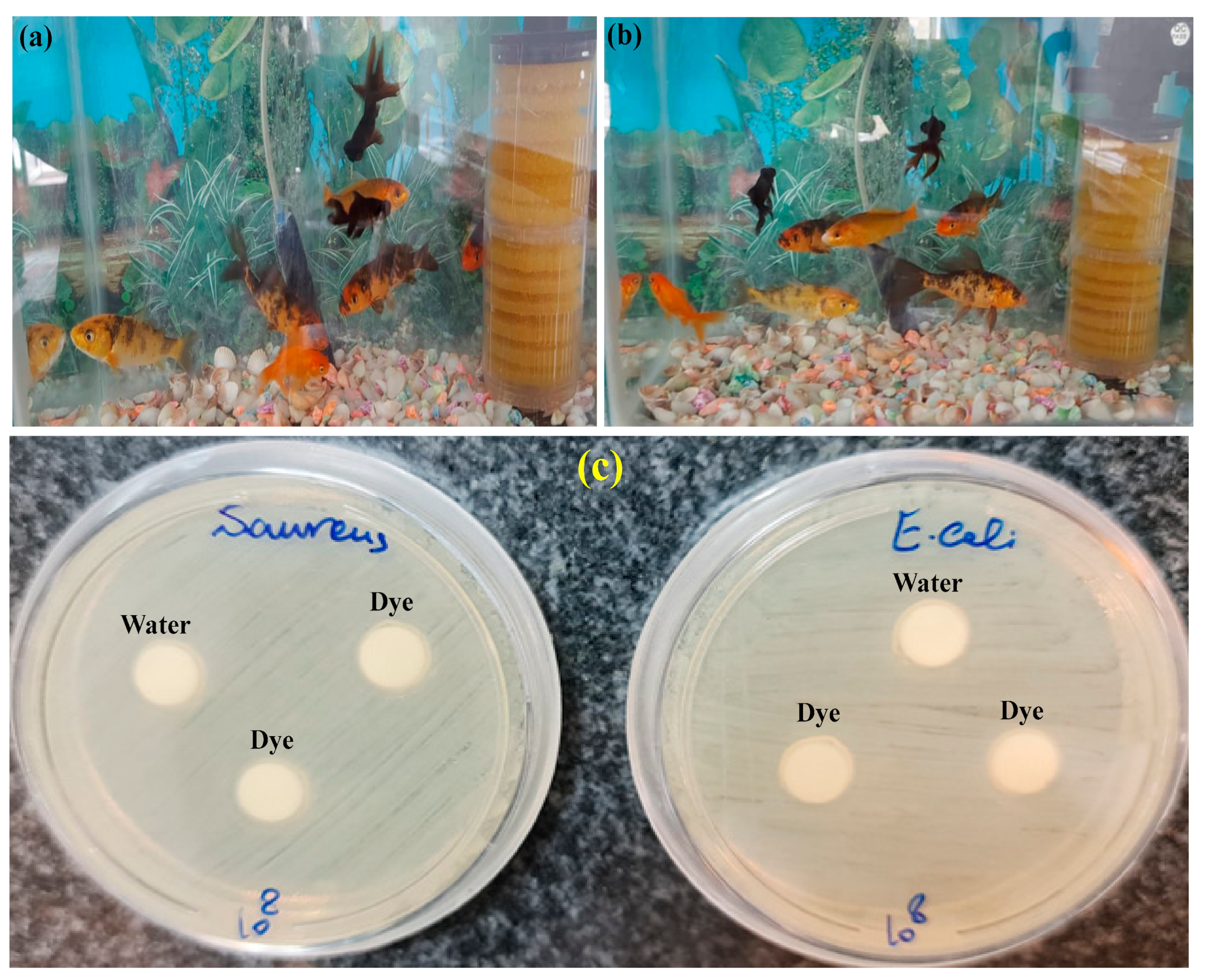
3.5. Evaluation of the Toxicity of the Treated Aqueous Solution
3.6. Kinetic Study and Comparison of Photocatalyst Performance
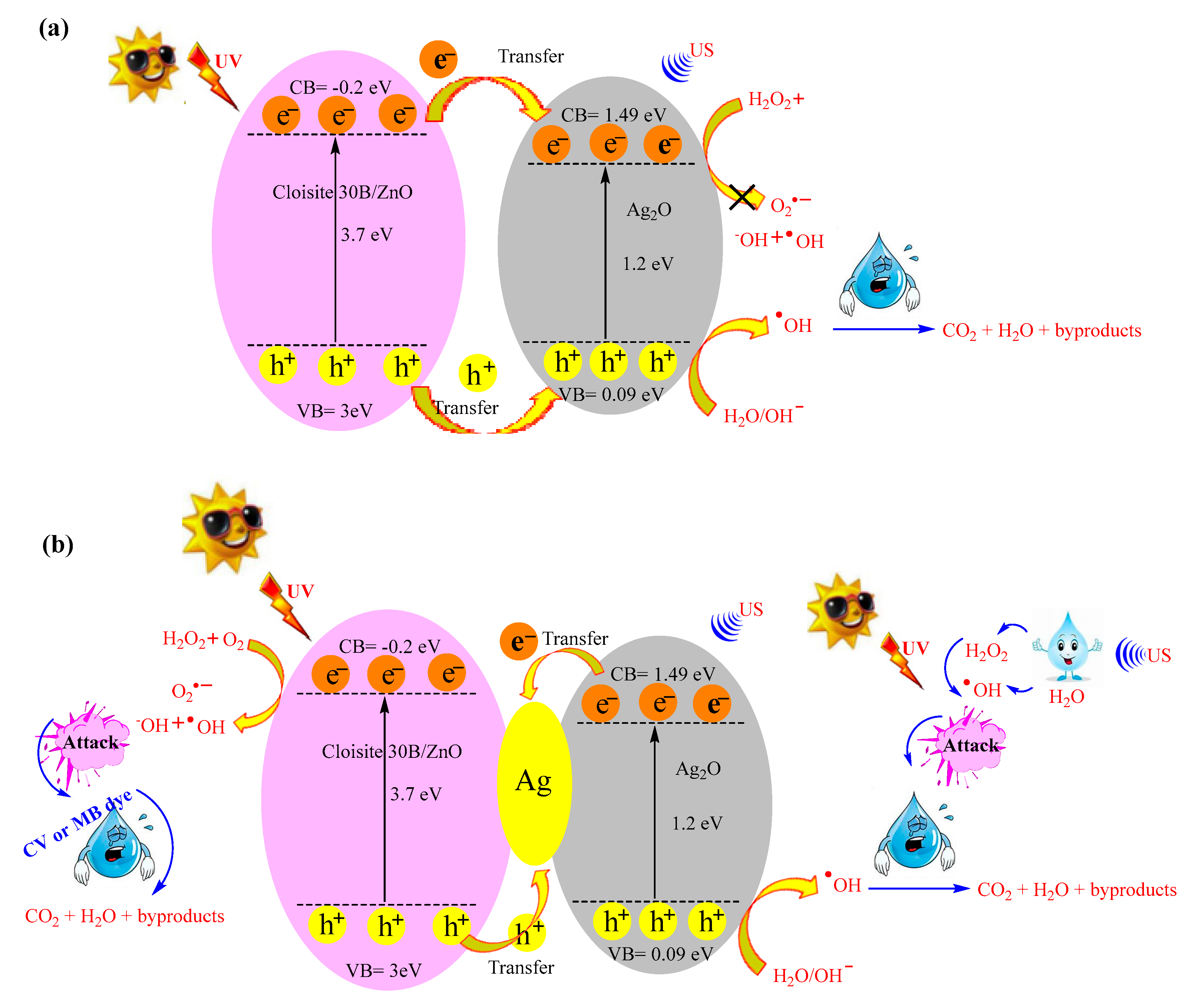
3.7. Proposed Mechanism for Dye Degradation Using Cloisite 30B/ZnO/Ag2O Photocatalyst
3.8. Textile Wastewater Treatment and Dye Fixation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghaedi, S.; Seifpanahi-Shabani, K.; Sillanpää, M. Waste-to-Resource: New application of modified mine silicate waste to remove Pb2+ ion and methylene blue dye, adsorption properties, mechanism of action and recycling. Chemosphere 2022, 292, 133412. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Malloum, A.; Igwegbe, C.A.; Ighalo, J.O.; Ahmadi, S.; Dehghani, M.H.; Othmani, A.; Gökkuş, Ö.; Mubarak, N.M. New generation adsorbents for the removal of fluoride from water and wastewater: A review. J. Mol. Liq. 2022, 346, 118257. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Djellabi, R.; Della Pina, C.; Falletta, E. Doped-polyaniline based sorbents for the simultaneous removal of heavy metals and dyes from water: Unravelling the role of synthesis method and doping agent. Chemosphere 2022, 286, 131941. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kapoor, A.; Prabhakar, S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2022, 424, 127432. [Google Scholar] [CrossRef]
- Ghann, W.; Sharma, V.; Kang, H.; Karim, F.; Richards, B.; Mobin, S.M.; Uddin, J.; Rahman, M.M.; Hossain, F.; Kabir, H.; et al. The synthesis and characterization of carbon dots and their application in dye sensitized solar cell. Int. J. Hydrog. Energy 2019, 44, 14580–14587. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.; Nidheesh, P.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef]
- Li, Y.; Yu, E.; Sun, S.; Liu, W.; Hu, R.; Xu, L. Fast and highly efficient adsorption of cationic dyes by phytic acid crosslinked β-cyclodextrin. Carbohydr. Polym. 2022, 284, 119231. [Google Scholar] [CrossRef]
- Akter, N.; Hossain, M.A.; Hassan, M.J.; Amin, M.K.; Elias, M.; Rahman, M.M.; Asiri, A.M.; Siddiquey, I.A.; Hasnat, M.A. Amine modified tannin gel for adsorptive removal of Brilliant Green dye. J. Environ. Chem. Eng. 2016, 4, 1231–1241. [Google Scholar] [CrossRef]
- Wei, X.; Chen, D.; Wang, L.; Ma, Y.; Yang, W. Carboxylate-functionalized hollow polymer particles modified polyurethane foam for facile and selective removal of cationic dye. Appl. Surf. Sci. 2022, 579, 152153. [Google Scholar] [CrossRef]
- El Gaayda, J.; Titchou, F.E.; Oukhrib, R.; Karmal, I.; Abou Oualid, H.; Berisha, A.; Zazou, H.; Swanson, C.; Hamdani, M.; Akbour, R.A. Removal of cationic dye from coloured water by adsorption onto hematite-humic acid composite: Experimental and theoretical studies. Sep. Purif. Technol. 2022, 288, 120607. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Sohrabi, N.; Sahebi, S.; Farjadfard, S.; Esvandi, Z.; Ramavandi, B. Calcined alluvium of agricultural streams as a recyclable and cleaning tool for cationic dye removal from aqueous media. Environ. Technol. Innov. 2020, 17, 100530. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: A kinetic, equilibrium and thermodynamic study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Safwan, J.A.; Islam, M.S.; Rahman, Z.; Karim, M.R.; Pirzada, T.J.; Samed, A.J.; Rahman, M.M. Electrochemical decolorization of Methylene blue at Pt electrode in KCl solution for environmental remediation. J. Ind. Eng. Chem. 2015, 21, 787–791. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.; Abu-Hamdeh, N.H.; Bezzina, S.; Malekshah, R.E. Molecular dynamics and quantum simulation of different cationic dyes removal from contaminated water using UiO-66 (Zr)-(COOH)2 metal–organic framework. J. Mol. Liq. 2022, 349, 118085. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Peighambardoust, S.J.; Hemmati, S.; Ramavandi, B. Montmorillonite clay/starch/CoFe2O4 nanocomposite as a superior functional material for uptake of cationic dye molecules from water and wastewater. Mater. Chem. Phys. 2022, 284, 126088. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Keerthana, S.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Hong, S.; Velauthapillai, D. Investigation of pure and g-C3N4 loaded CdWO4 photocatalytic activity on reducing toxic pollutants. Chemosphere 2022, 291, 133090. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; McKay, G.; Rajiv, P.; Mengelizadeh, N.; Balarak, D. Efficient sonophotocatalytic degradation of acid blue 113 dye using a hybrid nanocomposite of CoFe2O4 nanoparticles loaded on multi-walled carbon nanotubes. J. Photochem. Photobiol. A Chem. 2022, 424, 113617. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radical’s generation by micro-and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Narzary, S.; Alamelu, K.; Raja, V.; Ali, B.J. Visible light active, magnetically retrievable Fe3O4@ SiO2@ g-C3N4/TiO2 nanocomposite as efficient photocatalyst for removal of dye pollutants. J. Environ. Chem. Eng. 2020, 8, 104373. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Khitab, F. Sonophotocatalytic degradation of textile dyes over Cu impregnated ZnO catalyst in aqueous solution. Process Saf. Environ. Prot. 2018, 116, 149–158. [Google Scholar] [CrossRef]
- Ertugay, N.; Acar, F.N. The degradation of Direct Blue 71 by sono, photo and sonophotocatalytic oxidation in the presence of ZnO nanocatalyst. Appl. Surf. Sci. 2014, 318, 121–126. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Jorfi, S.; Ramezani, H.; Purfadakari, S. Ultrasonically induced ZnO–biosilica nanocomposite for degradation of a textile dye in aqueous phase. Ultrason. Sonochem. 2016, 28, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, G.; Akhtar, M.; Umar, A. Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. J. Alloy. Compd. 2015, 629, 167–172. [Google Scholar] [CrossRef]
- Yang, R.; Wu, Z.; Yang, Y.; Li, Y.; Zhang, L.; Yu, B. Understanding the origin of synergistic catalytic activities for ZnO based sonophotocatalytic degradation of methyl orange. J. Taiwan Inst. Chem. Eng. 2021, 119, 128–135. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Ghanem, M.A.; Khairy, M.; Naguib, E.; Alotaibi, N.H. Zinc oxide incorporated carbon nanotubes or graphene oxide nanohybrids for enhanced sonophotocatalytic degradation of methylene blue dye. Appl. Surf. Sci. 2019, 487, 539–549. [Google Scholar] [CrossRef]
- Sheydaei, M.; Fattahi, M.; Ghalamchi, L.; Vatanpour, V. Systematic comparison of sono-synthesized Ce-, La-and Ho-doped ZnO nanoparticles and using the optimum catalyst in a visible light assisted continuous sono-photocatalytic membrane reactor. Ultrason. Sonochem. 2019, 56, 361–371. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A.; Prakoso, S.P. Fabrication of Ag2O/TiO2 composites on nanographene platelets for the removal of organic pollutants: Influence of oxidants and inorganic anions. Appl. Surf. Sci. 2019, 480, 697–708. [Google Scholar] [CrossRef]
- Subhan, M.A.; Jhuma, S.S.; Saha, P.C.; Ahmed, J.; Asiri, A.M.; Rifat, T.P.; Raihan, T.; Azad, A.K.; Rahman, M.M. Photocatalysis, enhanced anti-bacterial performance and discerning thiourea sensing of Ag2O·SnO2·TiO2 hetero-structure. J. Environ. Chem. Eng. 2020, 8, 104051. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Xie, X.; Niu, J.; Wang, M.; Zhang, X.; Chen, X.; Han, J. Enhanced photocatalytic activity of ferroelectric-based Ag2O/Bi4Ti3O12 hybrids by piezoelectric effect. J. Alloy. Compd. 2021, 882, 160609. [Google Scholar] [CrossRef]
- Rajendran, R.; Vignesh, S.; Sasireka, A.; Suganthi, S.; Raj, V.; Baskaran, P.; Shkir, M.; AlFaify, S. Designing Ag2O modified g-C3N4/TiO2 ternary nanocomposites for photocatalytic organic pollutants degradation performance under visible light: Synergistic mechanism insight. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127472. [Google Scholar] [CrossRef]
- Hossain, M.A.; Elias, M.; Sarker, D.R.; Diba, Z.R.; Mithun, J.M.; Azad, M.A.K.; Siddiquey, I.A.; Rahman, M.M.; Uddin, J.; Uddin, M.N. Synthesis of Fe- or Ag-doped TiO2–MWCNT nanocomposite thin films and their visible-light-induced catalysis of dye degradation and antibacterial activity. Res. Chem. Intermed. 2018, 44, 2667–2683. [Google Scholar] [CrossRef]
- Rizal, M.Y.; Saleh, R.; Prakoso, S.P.; Taufik, A.; Yin, S. Ultraviolet-and visible-light photocatalytic and sonophotocatalytic activities toward Congo red degradation using Ag/Mn3O4 nanocomposites. Mater. Sci. Semicond. Process. 2021, 121, 105371. [Google Scholar] [CrossRef]
- Subhan, M.A.; Rifat, T.P.; Saha, P.C.; Alam, M.; Asiri, A.M.; Rahman, M.M.; Akter, S.; Raihan, T.; Azad, A.; Uddin, J. Enhanced visible light-mediated photocatalysis, antibacterial functions and fabrication of a 3-chlorophenol sensor based on ternary Ag2 O· SrO· CaO. RSC Adv. 2020, 10, 11274–11291. [Google Scholar] [CrossRef]
- Velazquez-Urbina, T.; Espinoza-Gomez, H.; Flores-López, L.Z.; Alonso-Núñez, G. Synthesis and characterization of silver nanoparticles supported on Bivalve mollusk shell for catalytic degradation of commercial dyes. J. Photochem. Photobiol. A Chem. 2021, 419, 113481. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hemmati, S.; Ahmadi, A.; Falletta, E.; Ramavandi, B.; Bianchi, C.L. Zn2+ removal from the aqueous environment using a polydopamine/hydroxyapatite/Fe3O4 magnetic composite under ultrasonic waves. RSC Adv. 2021, 11, 27309–27321. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Peighambardoust, S.J.; Hemmati, S.; Khatooni, H.; Ramavandi, B. Preparation of clinoptilolite/starch/CoFe2O4 magnetic nanocomposite powder and its elimination properties for cationic dyes from water and wastewater. Int. J. Biol. Macromol. 2021, 189, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Günister, E.; Pestreli, D.; Ünlü, C.H.; Atıcı, O.; Güngör, N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carbohydr. Polym. 2007, 67, 358–365. [Google Scholar] [CrossRef]
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of green synthesized MMT/Ag nanocomposite for removal of methylene blue from aqueous solution. Water 2021, 13, 3206. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Aghamohammadi-Bavil, O.; Foroutan, R.; Arsalani, N. Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int. J. Biol. Macromol. 2020, 159, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Mohammadi, R.; MousaKhanloo, F.; Sahebi, S.; Ramavandi, B.; Kumar, P.S.; Vardhan, K.H. Performance of montmorillonite/graphene oxide/CoFe2O4 as a magnetic and recyclable nanocomposite for cleaning methyl violet dye-laden wastewater. Adv. Powder Technol. 2020, 31, 3993–4004. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Application of walnut shell ash/ZnO/K2CO3 as a new composite catalyst for biodiesel generation from Moringa oleifera oil. Fuel 2022, 311, 122624. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Parvin, N.; Tahriri, M. Formation of apatite nano-needles on novel gel derived SiO2-P2O5-CaO-SrO-Ag2O bioactive glasses. Ceram. Int. 2017, 43, 15214–15220. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Salari, H.; Sadeghinia, M. MOF-templated synthesis of nano Ag2O/ZnO/CuO heterostructure for photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 279–287. [Google Scholar] [CrossRef]
- Liu, S.-j.; Li, F.-t.; Li, Y.-l.; Hao, Y.-j.; Wang, X.-j.; Li, B.; Liu, R.-h. Fabrication of ternary g-C3N4/Al2O3/ZnO heterojunctions based on cascade electron transfer toward molecular oxygen activation. Appl. Catal. B Environ. 2017, 212, 115–128. [Google Scholar] [CrossRef]
- Kiziltaş, H.; Tekin, T.; Tekin, D. Synthesis, characterization of Fe3O4@ SiO2@ ZnO composite with a core-shell structure and evaluation of its photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104160. [Google Scholar] [CrossRef]
- Xu, L.; Wei, B.; Liu, W.; Zhang, H.; Su, C.; Che, J. Flower-like ZnO-Ag2O composites: Precipitation synthesis and photocatalytic activity. Nanoscale Res. Lett. 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Sahroni, I.; Kamari, A.; Sagadevan, S.; Doong, R.-A. Nanoflower-like composites of ZnO/SiO2 synthesized using bamboo leaves ash as reusable photocatalyst. Arab. J. Chem. 2021, 14, 102973. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhou, Y.; Zhang, C.; Fang, J.; Sheng, X. Ionic liquid-assisted photochemical synthesis of ZnO/Ag2O heterostructures with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 410, 344–353. [Google Scholar] [CrossRef]
- El-Bindary, A.; Ismail, A.; Eladl, E. Photocatalytic degradation of reactive blue 21 using Ag doped ZnO nanoparticles. J Mater Env. Sci 2019, 10, 1258–1271. [Google Scholar]
- Nas, M.S. AgFe2O4/MWCNT nanoparticles as novel catalyst combined adsorption-sonocatalytic for the degradation of methylene blue under ultrasonic irradiation. J. Environ. Chem. Eng. 2021, 9, 105207. [Google Scholar] [CrossRef]
- Basturk, E.; Karatas, M. Advanced oxidation of reactive blue 181 solution: A comparison between fenton and sono-fenton process. Ultrason. Sonochem. 2014, 21, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, G.; Yu, J.; Si, Y.; Ding, B. Construction of ternary Ag@ ZnO/TiO2 fibrous membranes with hierarchical nanostructures and mechanical flexibility for water purification. Ceram. Int. 2020, 46, 468–475. [Google Scholar] [CrossRef]
- Preethi, M.; Viswanathan, C.; Ponpandian, N. A metal-free, dual catalyst for the removalof Rhodamine B using novel carbon quantum dots from muskmelon peel under sunlight and ultrasonication: A green way to clean the environment. J. Photochem. Photobiol. A Chem. 2022, 426, 113765. [Google Scholar] [CrossRef]
- JothiRamalingam, R.; Periyasami, G.; Ouladsmane, M.; Alothman, Z.A.; Arunachalam, P.; Altalhi, T.; Radhika, T.; Alanazi, A.G. Ultra-sonication assisted metal chalcogenide modified mesoporous Nickel-cobalt doped manganese oxide nanocomposite fabrication for sono-catalytic dye degradation and mechanism insights. J. Alloys Compd. 2021, 875, 160072. [Google Scholar] [CrossRef]
- Saroyan, H.S.; Arampatzidou, A.; Voutsa, D.; Lazaridis, N.K.; Deliyanni, E.A. Activated carbon supported MnO2 for catalytic degradation of reactive black 5. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 166–175. [Google Scholar] [CrossRef]
- Ghodbane, H.; Hamdaoui, O. Decolorization of antraquinonic dye, CI Acid Blue 25, in aqueous solution by direct UV irradiation, UV/H2O2 and UV/Fe (II) processes. Chem. Eng. J. 2010, 160, 226–231. [Google Scholar] [CrossRef]
- Rad, T.S.; Khataee, A.; Arefi-Oskoui, S.; Rad, S.S.; Orooji, Y.; Gengec, E.; Kobya, M. Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere 2022, 286, 131740. [Google Scholar]
- Eshaq, G.; ElMetwally, A.E. Bmim [OAc]-Cu2O/g-C3N4 as a multi-function catalyst for sonophotocatalytic degradation of methylene blue. Ultrason. Sonochem. 2019, 53, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yetim, T.; Tekin, T. Synergistic effect of sonolysis and photocatalysis in the degradation kinetics of effluent solution. Kinet. Catal. 2016, 57, 578–585. [Google Scholar] [CrossRef]
- Belhouchet, N.; Hamdi, B.; Chenchouni, H.; Bessekhouad, Y. Photocatalytic degradation of tetracycline antibiotic using new calcite/titania nanocomposites. J. Photochem. Photobiol. A Chem. 2019, 372, 196–205. [Google Scholar] [CrossRef]
- Jalali, S.; Ardjmand, M.; Ramavandi, B.; Nosratinia, F. Elimination of amoxicillin using zeolite Y-sea salt as a good catalyst for activation of hydrogen peroxide: Investigating degradation pathway and the effect of wastewater chemistry. J. Environ. Manag. 2022, 302, 114045. [Google Scholar] [CrossRef]
- Ghalamchi, L.; Aber, S. An aminated silver orthophosphate/graphitic carbon nitride nanocomposite: An efficient visible light sonophotocatalyst. Mater. Chem. Phys. 2020, 256, 123649. [Google Scholar] [CrossRef]
- Khan, M.F.; Cazzato, G.; Saleemi, H.A.; Macadangdang, R.R., Jr.; Aftab, M.N.; Ismail, M.; Khalid, H.; Ali, S.; Ismail, A.; Zahid, M. Sonophotocatalytic degradation of organic pollutant under visible light over Pt decorated CeO2: Role of ultrasonic waves for unprecedented degradation. J. Mol. Struct. 2022, 1247, 131397. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Bairq, Z.A.S.; Shi, C.; Benzina, M. Evaluation of sono-assisted solar/Fenton process for indigo carmine degradation over magnetic ZnO-Fe3O4 supported Tunisian kaolinite clay. Surf. Interfaces 2021, 26, 101395. [Google Scholar] [CrossRef]
- Talukdar, K.; Saravanakumar, K.; Kim, Y.; Fayyaz, A.; Kim, G.; Yoon, Y.; Park, C.M. Rational construction of CeO2–ZrO2@ MoS2 hybrid nanoflowers for enhanced sonophotocatalytic degradation of naproxen: Mechanisms and degradation pathways. Compos. Part B Eng. 2021, 215, 108780. [Google Scholar] [CrossRef]
- Subaihi, A.; Naglah, A.M. Facile synthesis and characterization of Fe2O3 nanoparticles using L-lysine and L-serine for efficient photocatalytic degradation of methylene blue dye. Arab. J. Chem. 2022, 15, 103613. [Google Scholar] [CrossRef]
- Bezzerrouk, M.A.; Bousmaha, M.; Hassan, M.; Akriche, A.; Kharroubi, B.; Naceur, R. Enhanced methylene blue removal efficiency of SnO2 thin film using sono-photocatalytic processes. Opt. Mater. 2021, 117, 111116. [Google Scholar] [CrossRef]
- Dhanasekar, M.; Ratha, S.; Rout, C.S.; Bhat, S.V. Efficient sono-photocatalytic degradation of methylene blue using nickel molybdate nanosheets under diffused sunlight. J. Environ. Chem. Eng. 2017, 5, 2997–3004. [Google Scholar] [CrossRef]
- Taufik, A.; Muzakki, A.; Saleh, R. Effect of nanographene platelets on adsorption and sonophotocatalytic performances of TiO2/CuO composite for removal of organic pollutants. Mater. Res. Bull. 2018, 99, 109–123. [Google Scholar] [CrossRef]
- Jin, Y.; Li, N.; Liu, H.; Hua, X.; Zhang, Q.; Chen, M.; Teng, F. Highly efficient degradation of dye pollutants by Ce-doped MoO3 catalyst at room temperature. Dalton Trans. 2014, 43, 12860–12870. [Google Scholar] [CrossRef] [PubMed]
- Panahian, Y.; Arsalani, N.; Nasiri, R. Enhanced photo and sono-photo degradation of crystal violet dye in aqueous solution by 3D flower like F-TiO2 (B)/fullerene under visible light. J. Photochem. Photobiol. A: Chem. 2018, 365, 45–51. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.R.; Sadjadi, M.S.; Farhadyar, N.; Sadr, M.H. Synthesis and characterization of a perovskite nanocomposite of CdTiO3@ S with orthorhombic structure: Investigation of photoluminescence properties and its photocatalytic performance for the degradation of congo red and crystal violet under sunlight. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4858–4875. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.R.; Sadjadi, M.S.; Farhadyar, N.; Sadr, M.H. Improving the photocatalytic performance of a perovskite ZnTiO3 through ZnTiO3@S nanocomposites for degradation of Crystal violet and Rhodamine B pollutants under sunlight. Inorg. Chem. Commun. 2020, 119, 108091. [Google Scholar] [CrossRef]
- Su, F.; Li, P.; Huang, J.; Gu, M.; Liu, Z.; Xu, Y. Photocatalytic degradation of organic dye and tetracycline by ternary Ag2O/AgBr–CeO2 photocatalyst under visible-light irradiation. Sci. Rep. 2021, 11, 1–13. [Google Scholar]
- San Martín, S.; Rivero, M.J.; Ortiz, I. Unravelling the mechanisms that drive the performance of photocatalytic hydrogen production. Catalysts 2020, 10, 901. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, F.; Wang, H.; Yu, H.; Zhang, S.; Yang, J.; Zhao, H. Electrodeposition preparation of Ag loaded N-doped TiO2 nanotube arrays with enhanced visible light photocatalytic performance. Catal. Commun. 2011, 12, 689–693. [Google Scholar] [CrossRef]







| The Main Intermediate | Content (µg) | The Main Intermediate | Content (µg) |
|---|---|---|---|
| Trans-1,2-DichloroeEthene | <0.05 | 1,1,2,2-TetrachloroEthane | <0.05 |
| Cis-1,2-DiChloroEthane | <0.05 | Isopropyle Benzene | <0.05 |
| Chloroform | <0.05 | 1,2,3-Trichloropropane | <0.05 |
| 1,1,1-TriChloroEthane | <0.05 | BromoBenzene | <0.05 |
| 1,2-DichloroEthane | <0.05 | 4-ChloroToluene | <0.05 |
| Benzene | <0.05 | n-propylBenzene | <0.05 |
| 1,2-DichloroPropane | <0.05 | 2-chloroToluene | <0.05 |
| Dibromo Methane | <0.05 | 1,2,4TrimethylBenzene | <0.05 |
| BromoDichloroMethane | <0.05 | tert-ButylBenzene | <0.05 |
| TRANS-1,3-DichloroPropene | <0.05 | 1,3,5,trimethylBenzene | <0.05 |
| Toluene | <0.05 | 1,4-DichloroBenzene | <0.05 |
| 1,1,2-TrichloroEthane | <0.05 | Sec-ButylBenzene | <0.05 |
| 1,3-Dichloro Propane | <0.05 | 1,3-DichloroBenzene | <0.05 |
| Dibromochloro Methane | <0.05 | p-Cymene | 0.1 |
| DibromoEthane | <0.05 | 1,2-DichloroBenzene | <0.05 |
| ChloroBenzene | <0.05 | n-ButylBenzene | <0.05 |
| 1,1,1,2-TetrachloroEthane | <0.05 | 1,2-Dibromo-3-ChloroPropane | <0.05 |
| EthylBenzene | <0.05 | 1,2,3-TriChlorBenzene | <0.05 |
| m-Xylene | <0.05 | Naphtalene | <0.05 |
| p-Xylene | <0.05 | HexaChlorobutdiene | <0.05 |
| o-Xylene | <0.05 |
| Photocatalysts | Dye Content | Reaction Time/Photocatalyst Content | Power of the Light Source | Removal Efficiency (%) | Kinetic Rate Constant (1/min) | Ref. |
|---|---|---|---|---|---|---|
| SnO2 | MB: 5 mg/L | 40 min/---- | 7 W UV-LEDs | 88.33 | 0.07 | [70] |
| β-NiMoO4 | MB: 4 mg/L | 150 min/1 g/L | Diffused Sunlight | 98.2 | ---- | [71] |
| ZnO(70)/GO | MB: 20 mg/L | 130 min/0.5 g/L | 160 W Hg Lamp | 93 | 0.01 | [27] |
| Bmim[OAc]-Cu2O/g-C3N4 | MB: 5 mg/L | 30 min/0.1 g/L | 6W UV-C lamp | 100 | 0.151 | [61] |
| TiO2/CuO/NGP | MB:20 mg/L | 120 min/0.3 g/L | 40 W UV-C/40 W Xe Lamp | ---- | 0.09863 | [72] |
| Ce-MoO3 | MB: 5 mg/L | 50 min/0.5 g/L | 500 W Xe Lamp | 65.5% | 0.0171 | [73] |
| α-Fe2O3/Bi2MoO6 | MB:20 mg/L | 240 min/2.5 g/L | 500W Xe Lamp | 90.7 | ---- | [74] |
| F-TiO2(B)/fullerene | CV: 30 mg/L | 120 min/0.1 g/L | UV Filter | 82.93% | ---- | [61] |
| CTO | CV: 5 mg/L | 60 min/10 g/L | Solar | 37.24 | 0.0002 | [75] |
| CTO@S | CV: 5 mg/L | 60 min/10 g/L | Solar | 89 | 0.008 | [75] |
| ZnTiO3@S NC | CV: 10 mg/L | 60 min/1 g/L | Natural Sunlight | 87.81 | 0.0092 | [76] |
| Closite 30B/ZnO/Ag2O | CV: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 99.21 | 0.09 | This study |
| Closite 30B/ZnO/Ag2O | MB: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 98.43 | 0.0693 | This study |
| Closite 30B | CV: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 16% | 0.02 a | This study |
| Closite 30B | MB: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 15% | 0.02 a | This study |
| ZnO | CV: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 25% | 0.031 | This study |
| ZnO | MB: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 25% | 0.030 | This study |
| Ag2O | CV: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 36% | 0.042 | This study |
| Ag2O | MB: 5 mg/L | 50 min/0.5 g/L | 12 W UV Lamp | 35% | 0.041 | This study |
| Sample | Parameters Value | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | COD (mg/L) | BOD5 (mg/L) | TOC (mg/L) | |||||
| Fresh | Treated | Fresh | Treated | Fresh | Treated | Fresh | Treated | |
| Distilled water + MB 1 | 8.0 | 7.8 | 12 | 7 | 26 | 5 | 18 | 7.5 |
| Distilled water + +CV 1 | 8.0 | 7.8 | 13 | 7.8 | 28 | 5 | 19 | 8.3 |
| Textile wastewater | 7.5 | 7.41 | 1305 | 823 | 639 | 330 | 397 | 329 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroutan, R.; Peighambardoust, S.J.; Boffito, D.C.; Ramavandi, B. Sono-Photocatalytic Activity of Cloisite 30B/ZnO/Ag2O Nanocomposite for the Simultaneous Degradation of Crystal Violet and Methylene Blue Dyes in Aqueous Media. Nanomaterials 2022, 12, 3103. https://doi.org/10.3390/nano12183103
Foroutan R, Peighambardoust SJ, Boffito DC, Ramavandi B. Sono-Photocatalytic Activity of Cloisite 30B/ZnO/Ag2O Nanocomposite for the Simultaneous Degradation of Crystal Violet and Methylene Blue Dyes in Aqueous Media. Nanomaterials. 2022; 12(18):3103. https://doi.org/10.3390/nano12183103
Chicago/Turabian StyleForoutan, Rauf, Seyed Jamaleddin Peighambardoust, Daria Camilla Boffito, and Bahman Ramavandi. 2022. "Sono-Photocatalytic Activity of Cloisite 30B/ZnO/Ag2O Nanocomposite for the Simultaneous Degradation of Crystal Violet and Methylene Blue Dyes in Aqueous Media" Nanomaterials 12, no. 18: 3103. https://doi.org/10.3390/nano12183103





