Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine
Abstract
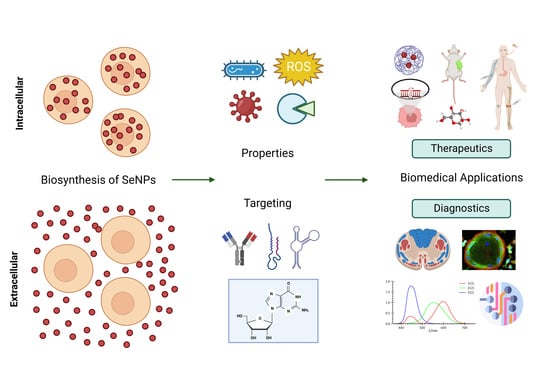
:1. Introduction
2. Selenium and Nanoselenium: General Information
3. Selenium Nanoparticles: Methods of Synthesis
3.1. Physical Methods
3.2. Chemical Methods
3.3. Biological Methods
4. Properties of Selenium Nanoparticles
4.1. Physicochemical Properties
4.2. Optoelectronic Properties
4.3. Catalytic Properties
4.4. Biological Properties
4.4.1. Antioxidant Properties
4.4.2. Scavenging Mechanism of Reactive Oxygen Species
4.4.3. Pro-Oxidant Activity
4.4.4. Production of Reactive Oxygen Species
4.5. Virucidal Activity
5. Pharmacokinetics and Cellular Interactions of Selenium Nanoparticles
5.1. Interaction of Selenium Nanoparticles with Cells and Their Components
5.2. Key Role of Selenoproteins in the Pharmacological Activity of SeNPs
5.3. Pharmacokinetics of Selenium Nanoparticles
6. Green Nanotechnology: A Better Avenue for SeNP Bioapplications
7. Biomedical Applications of Biogenic Selenium Nanoparticles
7.1. Antioxidant Activity
7.2. Antimicrobial Activity
7.2.1. Antibacterial Activity
7.2.2. Antifungal Activity
7.3. Antiparasitic Activity
7.4. Anticancer Activity
7.5. Protective Role of Selenium Nanoparticles against Drug-Induced Toxicity
7.6. Anti-Inflammatory Activity
7.7. Antidiabetic Activity
7.8. Diagnostic Applications
7.8.1. Detection, Biosensing and Diagnostics
7.8.2. Cellular Imaging
8. Translational Nanomedicine: Recent Progress, Emerging Challenges, and Future Prospects for Biogenic Selenium Nanoparticles
9. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Silva, G.A. Introduction to nanotechnology and its applications to medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Morigi, V.; Tocchio, A.; Pellegrini, C.B.; Sakamoto, J.H.; Arnone, M.; Tasciotti, E. Nanotechnology in medicine: From inception to market domination. J. Drug Deliv. 2012, 2012, 389485. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Andraos, C.; Gulumian, M. Intracellular and extracellular targets as mechanisms of cancer therapy by nanomaterials in relation to their physicochemical properties. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1680. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, S.; Chen, X. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef]
- Caster, J.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1416. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, J.; Miao, Q.; Zhu, R.; Wen, L.; Zeng, J.; Gao, M. Biodegradable inorganic nanoparticles for cancer theranostics: Insights into the degradation behavior. Bioconjug. Chem. 2020, 31, 315–331. [Google Scholar] [CrossRef]
- Shete, M.B.; Patil, T.S.; Deshpande, A.S.; Saraogi, G.; Vasdev, N.; Deshpande, M.; Rajpoot, K.; Tekade, R.K. Current trends in theranostic nanomedicines. J. Drug Deliv. Sci. Technol. 2022, 71, 103280. [Google Scholar] [CrossRef]
- Hu, X.; Ha, E.; Ai, F.; Huang, X.; Yan, L.; He, S.; Ruan, S.; Hu, J. Stimulus-responsive inorganic semiconductor nanomaterials for tumor-specific theranostics. Co-ord. Chem. Rev. 2022, 473, 214821. [Google Scholar] [CrossRef]
- Satalkar, P.; Elger, B.S.; Shaw, D.M. Defining nano, nanotechnology and nanomedicine: Why should it matter? Sci. Eng. Ethics 2016, 22, 1255–1276. [Google Scholar] [CrossRef]
- Roco, M.C.; Harthorn, B.; Guston, D.; Shapira, P. Innovative and responsible governance of nanotechnology for societal development. J. Nanopart. Res. 2011, 13, 3557–3590. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2016, 2, 54–88. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pereira, L.; Mehboob, F.; Stams, A.J.M.; Mota, M.M.; Rijnaarts, H.; Alves, M.M. Metallic nanoparticles: Microbial synthesis and unique properties for biotechnological applications, bioavailability and biotransformation. Crit. Rev. Biotechnol. 2015, 35, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Mintri, S.; Menon, A.V.; Lee, H.Y.; Choi, H.S.; Kim, J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 2015, 7, 18848–18862. [Google Scholar] [CrossRef] [Green Version]
- Laroui, H.; Rakhya, P.; Xiao, B.; Viennois, E.; Merlin, D. Nanotechnology in diagnostics and therapeutics for gastrointestinal disorders. Dig. Liver Dis. 2013, 45, 995–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.A.; Chow, J.C.L. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- AlShehri, S.; Imam, S.S.; Rizwanullah; Akhter, S.; Mahdi, W.; Kazi, M.; Ahmad, J. Progress of Cancer Nanotechnology as Diagnostics, Therapeutics, and Theranostics Nanomedicine: Preclinical Promise and Translational Challenges. Pharmaceutics 2020, 13, 24. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rizwanullah; Ahmad, J.; Alasmary, M.Y.; Akhter, H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 602–623. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Y.; Zhou, F.; Ding, J.; Zhou, W. Macrophage-targeted nanomedicine for chronic diseases immunotherapy. Chin. Chem. Lett. 2022, 33, 597–612. [Google Scholar] [CrossRef]
- Emerich, D.F. Nanomedicine—Prospective therapeutic and diagnostic applications. Expert Opin. Biol. Ther. 2005, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mei, C.; Deng, X.; Lin, W.; He, L.; Chen, T. Rapid visualizing and pathological grading of bladder tumor tissues by simple nanodiagnostics. Biomaterials 2021, 264, 120434. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.-H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Health Mater. 2018, 7, e1701392. [Google Scholar] [CrossRef] [PubMed]
- Korany, M.; Mahmoud, B.; Ayoub, S.M.; Sakr, T.M.; Ahmed, S.A. Synthesis and radiolabeling of vitamin C-stabilized selenium nanoparticles as a promising approach in diagnosis of solid tumors. J. Radioanal. Nucl. Chem. 2020, 325, 237–244. [Google Scholar] [CrossRef]
- Liu, T.; Xu, L.; He, L.; Zhao, J.; Zhang, Z.; Chen, Q.; Chen, T. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today 2020, 35, 100975. [Google Scholar] [CrossRef]
- Mukherjee, A.; Paul, M.; Mukherjee, S. Recent Progress in the Theranostics Application of Nanomedicine in Lung Cancer. Cancers 2019, 11, 597. [Google Scholar] [CrossRef] [Green Version]
- Augustine, R.; Hasan, A. Emerging applications of biocompatible phytosynthesized metal/metal oxide nanoparticles in healthcare. J. Drug Deliv. Sci. Technol. 2020, 56, 101516. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 2015, 24, 119–129. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-B.; Cheng, Y.-Y.; Wu, C.; Li, W.-W.; Li, N.; Yang, Z.-C.; Tong, Z.-H.; Yu, H.-Q. Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci. Rep. 2014, 4, 3735. [Google Scholar] [CrossRef] [Green Version]
- Biswas, K.C.; Barton, L.L.; Tsui, W.L.; Shuman, K.; Gillespie, J.; Eze, C.S. A novel method for the measurement of elemental selenium produced by bacterial reduction of selenite. J. Microbiol. Methods 2011, 86, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Zhang, J.S.; Wang, X.F.; Xu, T.W. Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Wang, T.; Jiang, M.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Construction of arabinogalactans/selenium nanoparticles composites for enhancement of the antitumor activity. Int. J. Biol. Macromol. 2019, 128, 444–451. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Liu, W.; Chen, T.; Li, Y.; Zheng, W.; Man, C.W.-Y.; Wong, M.-K.; Wong, K.-H. Surface decoration of selenium nanoparticles by mushroom polysaccharides–protein complexes to achieve enhanced cellular uptake and antiproliferative activity. J. Mater. Chem. 2012, 22, 9602–9610. [Google Scholar] [CrossRef]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Moneim, A.E.A.; Al-Quraishy, S.; Dkhil, M.A. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulmalek, S.A.; Balbaa, M. Synergistic effect of nano-selenium and metformin on type 2 diabetic rat model: Diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS ONE 2019, 14, e0220779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, T.J.; Tran, P. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT—Food Sci. Technol. 2015, 63, 1001–1007. [Google Scholar] [CrossRef]
- Chaudhary, S.; Mehta, S.K. Selenium nanomaterials: Applications in electronics, catalysis and sensors. J. Nanosci. Nanotechnol. 2014, 14, 1658–1674. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [Green Version]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Zambonino, M.; Quizhpe, E.; Jaramillo, F.; Rahman, A.; Vispo, N.S.; Jeffryes, C.; Dahoumane, S. Green synthesis of selenium and tellurium nanoparticles: Current trends, biological properties and biomedical applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.-U. Biomedical potential of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic aspects. Int. J. Nanomed. 2021, 16, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdi, M.H.; Mahdavi, M.; Varastehmoradi, B.; Faramarzi, M.A.; Shahverdi, A.R. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: An in vivo study. Biol. Trace Elem. Res. 2012, 149, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Kumari, P.; Bontempi, E.; Yadav, S. Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal. Agric. Biotechnol. 2020, 24, 101518. [Google Scholar] [CrossRef]
- Yanchatuña Aguayo, O.P.; Mouheb, L.; Villota Revelo, K.; Vásquez-Ucho, P.A.; Pawar, P.P.; Rahman, A.; Jeffryes, C.; Terencio, T.; Dahoumane, S.A. Biogenic sulfur-based chalcogenide nanocrystals: Methods of fabrication, mechanistic aspects, and bio-applications. Molecules 2022, 27, 458. [Google Scholar] [PubMed]
- Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green nanotechnology: Advancement in phytoformulation research. Medicines 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wang, W.-H.; Yang, Y.; Zhang, H.-N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef]
- San Keskin, N.O.; Akbal Vural, O.; Abaci, S. Biosynthesis of noble selenium nanoparticles from Lysinibacillus sp. NOSK for antimicrobial, antibiofilm activity, and biocompatibility. Geomicrobiol. J. 2020, 37, 919–928. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Lens, P.N. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015, 33, 323–330. [Google Scholar] [CrossRef]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging nanopharmaceuticals and nanonutraceuticals in cancer management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef]
- Janjic, J.M.; Gorantla, V.S. Novel nanoimaging strategies for noninvasive graft monitoring in vascularized composite allotransplantation. Curr. Transplant. Rep. 2018, 5, 369–372. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. [Google Scholar] [CrossRef] [Green Version]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Sanmartin, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef]
- Sun, H.-J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P. Selenium in colorectal and differentiated thyroid cancer. Hormones 2020, 19, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-W.; Mo, H.-J.; Lau, A.T.Y.; Xu, Y.-M. Selenium species: Current status and potentials in cancer prevention and therapy. Int. J. Mol. Sci. 2019, 20, 75. [Google Scholar] [CrossRef] [Green Version]
- Hoefig, C.S.; Renko, K.; Köhrle, J.; Birringer, M.; Schomburg, L. Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J. Nutr. Biochem. 2011, 22, 945–955. [Google Scholar] [CrossRef]
- Cao, S.; A Durrani, F.; Tóth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Chen, J.; Wang, Y.; Wong, Y.-S.; Zhang, Y.; Zheng, W.; Cao, W.; Chen, T. Selenocystine potentiates cancer cell apoptosis induced by 5-fluorouracil by triggering reactive oxygen species-mediated DNA damage and inactivation of the ERK pathway. Free Radic. Biol. Med. 2013, 65, 305–316. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active aelenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, D.; Taylor, E.W.; Wang, Y.; Wan, X. Encapsulated nanoepigallocatechin-3-gallate and elemental selenium nanoparticles as paradigms for nanochemoprevention. Int. J. Nanomed. 2012, 7, 1711–1721. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Raju, V.; Gopal, V.B.; Gunasekaran, A.; Narayan, K.S.; Malairaj, S.; Lakshmikanthan, M.; Duraisamy, N.; Krishnan, K.; Perumal, P. A rapid synthesis and antibacterial property of selenium nanoparticles using egg white lysozyme as a stabilizing agent. SN Appl. Sci. 2019, 1, 1543. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Teng, Z.; Yuan, Y.; Zeng, Q.-Z.; Lou, Z.; Lee, S.-H.; Wang, Q. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int. J. Biol. Macromol. 2018, 107, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Zhang, J.; Wang, H.-Y.; Chen, H.-Y. Synthesis of selenium nanoparticles in the presence of polysaccharides. Mater. Lett. 2004, 58, 2590–2594. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-containing nanoparticles combine the NK Cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, T.; Li, J.; Mai, F.; Li, J.; Chen, Y.; Jing, Y.; Dong, X.; Lin, L.; He, J.; et al. Selenium nanoparticles as new strategy to potentiate gd T cell anti-tumor cytotoxicity through upregulation of tubulin-a acetylation. Biomaterials 2019, 222, 119397. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.; Mahmoud, E.; El Hakim, Y.A. Efficacy of dietary nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish Immunol. 2019, 94, 280–287. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I.; Aboel-Darag, M.A. The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 27, 9843–9852. [Google Scholar] [CrossRef]
- Zhu, M.L.; Wang, G.; Wang, H.; Guo, Y.M.; Song, P.; Xu, J.; Li, P.; Wang, S.; Yang, L. Amorphous nano-selenium quantum dots improve endothelial dysfunction in rats and prevent atherosclerosis in mice through Na+/H+ exchanger 1 inhibition. Vascul. Pharmacol. 2019, 115, 26–32. [Google Scholar] [CrossRef]
- Shi, L.-G.; Yang, R.-J.; Yue, W.-B.; Xun, W.-J.; Zhang, C.-X.; Ren, Y.-S.; Shi, L.; Lei, F.-L. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim. Reprod. Sci. 2010, 118, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Selvaraj, K. Biogenic Synthesis of Selenium Nanoparticles and Their Effect on As(III)-Induced Toxicity on Human Lymphocytes. Biol. Trace Elem. Res. 2014, 157, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hashem, K.; Hassnin, K.M.A.; AbdEl-Kawi, S.H. The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int. J. Nanomed. 2013, 8, 1713–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabelsi, H.; Azzouz, I.; Ferchichi, S.; Tebourbi, O.; Sakly, M.; Abdelmelek, H. Nanotoxicological evaluation of oxidative responses in rat nephrocytes induced by cadmium. Int. J. Nanomed. 2013, 8, 3447–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheiha, A.M.; Abdelnour, S.A.; El-Hack, M.E.A.; Khafaga, A.F.; Metwally, K.A.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; El-Saadony, M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals 2020, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.A.; Sarin, L.; Hurt, R.H.; Webster, T.J. Differential effects of nanoselenium doping on healthy and cancerous osteoblasts in coculture on titanium. Int. J. Nanomed. 2010, 5, 351–358. [Google Scholar]
- Bartůněk, V.; Vokatá, B.; Kolářová, K.; Ulbrich, P. Preparation of amorphous nano-selenium-PEG composite network with selective antimicrobial activity. Mater. Lett. 2019, 238, 51–53. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, M.; El-Batal, A.I. Gentamicin-assisted mycogenic selenium nanoparticles synthesized under gamma irradiation for robust reluctance of resistant urinary tract infection-causing pathogens. Biol. Trace Elem. Res. 2020, 195, 323–342. [Google Scholar] [CrossRef]
- Yazhiniprabha, M.; Vaseeharan, B. In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109763. [Google Scholar] [CrossRef]
- Yu, B.; You, P.; Song, M.; Zhou, Y.; Yu, F.; Zheng, W. A facile and fast synthetic approach to create selenium nanoparticles with diverse shapes and their antioxidation ability. New J. Chem. 2016, 40, 1118–1123. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Salavati-Niasari, M.; Hosseinpour-Mashkani, S.M. Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J. Alloys Compd. 2014, 617, 627–632. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Mosallam, F.M.; Ghorab, M.; Hanora, A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; Pal, K.; Fathy, R.M.; Elkodous, M.A.; et al. Factorial design-optimized and gamma irradiation-assisted fabrication of selenium nanoparticles by chitosan and Pleurotus ostreatus fermented fenugreek for a vigorous in vitro effect against carcinoma cells. Int. J. Biol. Macromol. 2020, 156, 1584–1599. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Guisbiers, G.; Mendoza, J.; Mimun, L.C.; A Vincent, B.; Lopez-Ribot, J.L.; Nash, K.L. Synergistic antifungal effect of chitosan-stabilized selenium nanoparticles synthesized by pulsed laser ablation in liquids against Candida albicans biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menazea, A.; Ismail, A.; Awwad, N.S.; Ibrahium, H.A. Physical characterization and antibacterial activity of PVA/Chitosan matrix doped by selenium nanoparticles prepared via one-pot laser ablation route. J. Mater. Res. Technol. 2020, 9, 9598–9606. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.J.; Nash, K.L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef] [Green Version]
- Luesakul, U.; Komenek, S.; Puthong, S.; Muangsin, N. Shape-controlled synthesis of cubic-like selenium nanoparticles via the self-assembly method. Carbohydr. Polym. 2016, 153, 435–444. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Mousavi-Kamazani, M.; Salavati-Niasari, M.; Hosseinpour-Mashkani, S.M. A simple sonochemical approach for synthesis of selenium nanostructures and investigation of its light harvesting application. Ultrason. Sonochem. 2015, 23, 246–256. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Kumar, A.; Sevonkaev, I.; Goia, D.V. Synthesis of selenium particles with various morphologies. J. Colloid Interface Sci. 2014, 416, 119–123. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Zhou, R.; Webster, T.J. Green synthesized BSA-coated selenium nanoparticles inhibit bacterial growth while promoting mammalian cell growth. Int. J. Nanomed. 2020, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ramos, J.F. Cytotoxicity of selenium nanoparticles in rat dermal fibroblasts. Int. J. Nanomed. 2012, 7, 3907–3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. The synthesis and characterisation of highly stable and reproducible selenium nanoparticles. Inorg. Nano-Metal Chem. 2017, 47, 1568–1576. [Google Scholar] [CrossRef]
- Ye, X.; Chen, L.; Liu, L.; Bai, Y. Electrochemical synthesis of selenium nanoparticles and formation of sea urchin-like selenium nanoparticles by electrostatic assembly. Mater. Lett. 2017, 196, 381–384. [Google Scholar] [CrossRef]
- Langi, B.; Shah, C.; Singh, K.; Chaskar, A.; Kumar, M.; Bajaj, P.N. Ionic liquid-induced synthesis of selenium nanoparticles. Mater. Res. Bull. 2010, 45, 668–671. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, Y.; Zeng, H.; Wang, Q.; Tian, F.; Song, J.; Cheng, W.-H. Encapsulation of selenium in chitosan nanoparticles improves selenium availability and protects cells from selenium-induced DNA damage response. J. Nutr. Biochem. 2011, 22, 1137–1142. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, B.; Cheng, W.-H.; Wang, Q. Preparation, characterization and evaluation of selenite-loaded chitosan/TPP nanoparticles with or without zein coating. Carbohydr. Polym. 2010, 82, 942–951. [Google Scholar] [CrossRef]
- Cui, D.; Yan, C.; Miao, J.; Zhang, X.; Chen, J.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater. Sci. Eng. C 2018, 90, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Modrzejewska-Sikorska, A.; Konował, E.; Klapiszewski, Ł.; Nowaczyk, G.; Jurga, S.; Jesionowski, T.; Milczarek, G. Lignosulfonate-stabilized selenium nanoparticles and their deposition on spherical silica. Int. J. Biol. Macromol. 2017, 103, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Medina Cruz, D.; Mi, G.; Webster, T.J. Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginos. J. Biomed. Mater. Res. A 2018, 106, 1400–1412. [Google Scholar] [CrossRef]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Cecconi, D.; Monti, F.; Micaroni, M.; Turner, R.J.; Butler, C.S.; Vallini, G. Selenite biotransformation and detoxification by Stenotrophomonas maltophilia SeITE02: Novel clues on the route to bacterial biogenesis of selenium nanoparticles. J. Hazard. Mater. 2017, 324, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J. Bacillus cereus, selenite-reducing bacterium from contaminated lake of an industrial area: A renewable nanofactory for the synthesis of selenium nanoparticles. Bioresour. Bioprocess. 2018, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Khoei, N.S.; Lampis, S.; Zonaro, E.; Yrjälä, K.; Bernardi, P.; Vallini, G. Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnol. 2017, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.A.; Mamchenkova, P.V.; Dyatlova, Y.A.; Tugarova, A.V. FTIR spectroscopic studies of selenite reduction by cells of the rhizobacterium Azospirillum brasilense Sp7 and the formation of selenium nanoparticles. J. Mol. Struct. 2017, 1140, 106–112. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Gonzalez-Gil, G.; Saikaly, P.; van Hullebusch, E.D.; Lens, P.N.L. Effects of selenium oxyanions on the white-rot fungus Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2015, 99, 2405–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasteen, T.G.; Bentley, R. Biomethylation of selenium and tellurium: Microorganisms and plants. Chem. Rev. 2003, 103, 1–26. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Mu, J.; Ho, C.-T.; Su, J.; Zhang, Y.; Lin, X.; Chen, Z.; Li, B.; Xie, Y. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporus umbellatus polysaccharide. Int. J. Biol. Macromol. 2020, 152, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Vetchinkina, E.; Loshchinina, E.; Kursky, V.; Nikitina, V. Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J. Microbiol. 2013, 51, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Nikitina, V. Shape and size diversity of gold, silver, selenium, and silica nanoparticles prepared by green synthesis using fungi and bacteria. Ind. Eng. Chem. Res. 2019, 58, 17207–17218. [Google Scholar] [CrossRef]
- Liang, X.; Perez, M.A.M.-J.; Nwoko, K.C.; Egbers, P.; Feldmann, J.; Csetenyi, L.; Gadd, G.M. Fungal formation of selenium and tellurium nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 7241–7259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Yu, Z.; Lin, Z.; Lei, Z.; Ning, Z.; Regenstein, J.M.; Yang, J.; Ren, J. Biofunctionalization of selenium nanoparticle with Dictyophora Indusiata polysaccharide and its antiproliferative activity through death-receptor and mitochondria-mediated apoptotic pathways. Sci. Rep. 2015, 5, 18629. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Joshi, S.; De Britto, S.; Jogaiah, S.; Ito, S.-I. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Faramarzi, S.; Anzabi, Y.; Jafarizadeh-Malmiri, H. Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae—Fabrication and characterization. Arch. Microbiol. 2020, 202, 1203–1209. [Google Scholar] [CrossRef]
- Bartosiak, M.; Giersz, J.; Jankowski, K. Analytical monitoring of selenium nanoparticles green synthesis using photochemical vapor generation coupled with MIP-OES and UV–Vis spectrophotometry. Microchem. J. 2019, 145, 1169–1175. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Gao, P. Expulsion of selenium/protein nanoparticles through vesicle-like structures by Saccharomyces cerevisiae under microaerophilic environment. World J. Microbiol. Biotechnol. 2012, 28, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Elahian, F.; Reiisi, S.; Shahidi, A.; Mirzaei, S.A. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered Pichia pastoris. Nanomedicine 2017, 13, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M. Biosynthesis of selenium nanoparticles using yeast Nematospora coryli and examination of their anti-candida and anti-oxidant activities. IET Nanobiotechnol. 2019, 13, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Hassanien, R.; Abed-Elmageed, A.A.I.; Husein, D.Z. Eco-friendly approach to synthesize selenium nanoparticles: Photocatalytic degradation of sunset yellow azo dye and anticancer activity. ChemistrySelect 2019, 4, 9018–9026. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef] [Green Version]
- Fardsadegh, B.; Vaghari, H.; Mohammad-Jafari, R.; Najian, Y.; Jafarizadeh-Malmiri, H. Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract. Green Process. Synth. 2019, 8, 191–198. [Google Scholar] [CrossRef]
- Liang, T.; Qiu, X.; Ye, X.; Liu, Y.; Li, Z.; Tian, B.; Yan, D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech 2020, 10, 23. [Google Scholar] [CrossRef]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium sanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Alam, H.; Khatoon, N.; Raza, M.; Ghosh, P.C.; Sardar, M. Synthesis and characterization of nano selenium using plant biomolecules and their potential applications. Bionanoscience 2018, 9, 96–104. [Google Scholar] [CrossRef]
- Anu, K.; Devanesan, S.; Prasanth, R.; AlSalhi, M.S.; Ajithkumar, S.; Singaravelu, G. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J. King Saud Univ. Sci. 2020, 32, 2520–2526. [Google Scholar] [CrossRef]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf. B Biointerfaces 2013, 103, 261–266. [Google Scholar] [CrossRef]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling antimicrobial and insecticidal activities of biosynthesized selenium nanoparticles using prickly pear peel waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef]
- Ghaderi, R.S.; Adibian, F.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Jamehdar, S.A.; Meshkat, Z.; Soleimanpour, S.; Daroudi, M. Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity. Mater. Technol. 2021, 37, 1289–1297. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green biosynthesis of selenium nanoparticles using orange peel waste: Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Li, H.; Wang, Y.; Zhao, K. Nano-selenium and Macleaya cordata extracts improved immune functions of intrauterine growth retardation piglets under maternal oxidation stress. Biol. Trace Elem. Res. 2022, 200, 3975–3982. [Google Scholar] [CrossRef]
- Fan, D.; Li, L.; Li, Z.; Zhang, Y.; Ma, X.; Wu, L.; Zhang, H.; Guo, F. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci. Technol. Adv. Mater. 2020, 21, 505–514. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539–553. [Google Scholar] [CrossRef]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of inorganic nanoparticles: A fresh look at the control of shape, size and composition. Bioengineering 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259. [Google Scholar] [CrossRef] [PubMed]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef] [Green Version]
- Nayak, V.; Singh, K.R.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Gates, B.; Yin, Y.; Xia, Y. A solution-phase approach to the synthesis of uniform nanowires of crystalline selenium with lateral dimensions in the range of 10−30 nm. J. Am. Chem. Soc. 2000, 122, 12582–12583. [Google Scholar] [CrossRef]
- Johnson, J.A.; Saboungi, M.-L.; Thiyagarajan, P.; Csencsits, R.; Meisel, D. Selenium nanoparticles: A small-angle neutron scattering study. J. Phys. Chem. B 1999, 103, 59–63. [Google Scholar] [CrossRef]
- Xiong, S.; Xi, B.; Wang, W.; Wang, C.; Fei, L.; Zhou, H.; Qian, Y. The Fabrication and characterization of single-crystalline selenium nanoneedles. Cryst. Growth Des. 2006, 6, 1711–1716. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, P.; Sun, S.; Bao, D.; Wang, Y.; Li, X.; Wu, T.; Chen, Y.; Yang, P. Amorphous, crystalline and crystalline/amorphous selenium nanowires and their different (de)lithiation mechanisms. Chem. Mater. 2015, 27, 6730–6736. [Google Scholar] [CrossRef]
- Di, Y.; Zeng, Q.; Huang, C.; Tang, D.; Sun, K.; Yan, C.; Wang, Y.; Ke, S.; Jiang, L.; Hao, X.; et al. Thermal-evaporated selenium as a hole-transporting material for planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2018, 185, 130–135. [Google Scholar] [CrossRef]
- Cui, Y.; Abouimrane, A.; Lu, J.; Bolin, T.; Ren, Y.; Weng, W.; Sun, C.; Maroni, V.A.; Heald, S.M.; Amine, K. (De)lithiation mechanism of Li/SeSx (x = 0–7) batteries determined by in situ synchrotron X-ray diffraction and X-ray absorption spectroscopy. J. Am. Chem. Soc. 2013, 135, 8047–8056. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, X.; Feng, J.; Xiong, S. Selenium in nitrogen-doped microporous carbon spheres for high-performance lithium–selenium batteries. J. Mater. Chem. A 2015, 3, 4539–4546. [Google Scholar] [CrossRef]
- Kubota, M.; Kato, T.; Suzuki, S.; Maruyama, H.; Shidara, K.; Tanioka, K.; Sameshima, K.; Makishima, T.; Tsuji, K.; Hirai, T.; et al. Ultrahigh-sensitivity new super-HARP camera. IEEE Trans. Broadcast. 1996, 42, 251–258. [Google Scholar] [CrossRef]
- Yamazaki, J.; Maruyama, H.; Andoh, F.; Tanioka, K.; Araki, S.; Tsuji, K.; Kawamura, T. A solid-state imager joined to an avalanche multiplier film with micro-bump electrodes. In Proceedings of the International Electron Devices Meeting, Washington, DC, USA, 10–13 December 1995; pp. 175–178. [Google Scholar] [CrossRef]
- Imura, S.; Watabe, T.; Miyakawa, K.; Hagiwara, K.; Ohtake, H.; Kubota, M. Effects of grain refinement on surface enhancement of thin-film chlorine-doped crystalline selenium. J. Mater. Sci. Mater. Electron. 2017, 28, 7064–7069. [Google Scholar] [CrossRef]
- Takiguchi, Y.; Maruyama, H.; Kosugi, M.; Andoh, F.; Kato, T.; Tanioka, K.; Yamazaki, J.; Tsuji, K.; Kawamura, T. A CMOS imager hybridized to an avalanche multiplied film. IEEE Trans. Electron Devices 1997, 44, 1783–1788. [Google Scholar] [CrossRef]
- Liu, P.; Ma, Y.; Cai, W.; Wang, Z.; Wang, J.; Qi, L.; Chen, D. Photoconductivity of single-crystalline selenium nanotubes. Nanotechnology 2007, 18, 205704. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef]
- Hageman, S.P.; van der Weijden, R.D.; Stams, A.J.; Buisman, C.J. Bio-production of selenium nanoparticles with diverse physical properties for recovery from water. Int. J. Miner. Process. 2017, 169, 7–15. [Google Scholar] [CrossRef]
- Jiang, F.; Cai, W.; Tan, G. Facile synthesis and optical properties of small selenium nanocrystals and nanorods. Nanoscale Res. Lett. 2017, 12, 401. [Google Scholar] [CrossRef] [Green Version]
- Frey, J.B.; Belev, G.; Tousignant, O.; Mani, H.; Laperriere, L.; Kasap, S.O. Dark current in multilayer stabilized amorphous selenium based photoconductive x-ray detectors. J. Appl. Phys. 2012, 112, 14502. [Google Scholar] [CrossRef]
- Kasap, S.; Frey, J.B.; Belev, G.; Tousignant, O.; Mani, H.; Laperriere, L.; Reznik, A.; Rowlands, J.A. Amorphous selenium and its alloys from early xeroradiography to high resolution X-ray image detectors and ultrasensitive imaging tubes. Phys. Status solidi (B) 2009, 246, 1794–1805. [Google Scholar] [CrossRef]
- Kannan, H.; Stavro, J.; Mukherjee, A.; Léveillé, S.; Kisslinger, K.; Guan, L.; Zhao, W.; Sahu, A.; Goldan, A.H. Ultralow dark currents in avalanche amorphous selenium photodetectors using solution-processed quantum dot blocking layer. ACS Photon. 2020, 7, 1367–1374. [Google Scholar] [CrossRef]
- Imura, S.; Mineo, K.; Miyakawa, K.; Nanba, M.; Ohtake, H.; Kubota, M. Low-dark-current photodiodes comprising highly (100)-oriented hexagonal selenium with crystallinity-enhanced tellurium nucleation layers. IEEE Sens. J. 2018, 18, 3108–3113. [Google Scholar] [CrossRef]
- Wronski, M.M.; Rowlands, J.A. Direct-conversion flat-panel imager with avalanche gain: Feasibility investigation for HARP-AMFPI. Med. Phys. 2008, 35, 5207–5218. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, D.; Reznik, A.; Lui, B.J.M.; Hunt, D.C.; Rowlands, J.A.; Ohkawa, Y.; Tanioka, K. Indirect flat-panel detector with avalanche gain: Fundamental feasibility investigation for SHARP-AMFPI (scintillator HARP active matrix flat panel imager). Med. Phys. 2005, 32, 2954–2966. [Google Scholar] [CrossRef]
- Okano, K.; Saito, I.; Mine, T.; Suzuki, Y.; Yamada, T.; Rupesinghe, N.; Amaratunga, G.; Milne, W.; Zahn, D. Characterizations of a-Se based photodetectors using X-ray photoelectron spectroscopy and Raman spectroscopy. J. Non-Cryst. Solids 2007, 353, 308–312. [Google Scholar] [CrossRef]
- Oonuki, K.; Suzuki, Y.; Yamaguchi, H.; Okano, K.; Okamura, Y. Diode structure amorphous selenium photodetector with nitrogen (N)-doped diamond cold cathode. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2003, 21, 1586. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamaguchi, H.; Oonuki, K.; Okamura, Y.; Okano, K. Amorphous selenium photodetector driven by diamond cold cathode. IEEE Electron Device Lett. 2003, 24, 16–18. [Google Scholar] [CrossRef]
- Hadar, I.; Hu, X.; Luo, Z.-Z.; Dravid, V.P.; Kanatzidis, M.G. Nonlinear band gap tunability in selenium–tellurium alloys and its utilization in solar cells. ACS Energy Lett. 2019, 4, 2137–2143. [Google Scholar] [CrossRef]
- Platzer-Björkman, C.; Zabierowski, P.; Pettersson, J.; Törndahl, T.; Edoff, M. Improved fill factor and open circuit voltage by crystalline selenium at the Cu(In,Ga)Se2/buffer layer interface in thin film solar cells. Prog. Photovolt. Res. Appl. 2010, 18, 249–256. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, R.; Tamboli, R.A.; Kanhere, D.G. Ab initio investigation of structural and electronic properties of selenium and tellurium clusters. Eur. Phys. J. B 2019, 92, 51. [Google Scholar] [CrossRef]
- Wang, L.; Kafshgari, M.H.; Meunier, M. Optical properties and applications of plasmonic-metal nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Jara, N.; Milán, N.; Rahman, A.; Mouheb, L.; Boffito, D.; Jeffryes, C.; Dahoumane, S. Photochemical synthesis of gold and silver nanoparticles—A review. Molecules 2021, 26, 4585. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Wang, J. Optical properties of biomass-derived nanomaterials for sensing, catalytic, biomedical and environmental applications. TrAC Trends Anal. Chem. 2020, 124, 115800. [Google Scholar] [CrossRef]
- Lesnichaya, M.; Shendrik, R.; Sukhov, B. Relation between excitation dependent luminescence and particle size distributions for the selenium nanoparticles in κ-carrageenan shell. J. Lumin. 2019, 211, 305–313. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Arora, A. Optical properties of selenium nanoparticles dispersed in polymer. Solid State Commun. 1999, 110, 75–80. [Google Scholar] [CrossRef]
- Singh, S.C.; Mishra, S.K.; Srivastava, R.K.; Gopal, R. Optical properties of selenium quantum dots produced with laser irradiation of water suspended Se nanoparticles. J. Phys. Chem. C 2010, 114, 17374–17384. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.-S.; Lee, S.-S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lampis, S.; Vallini, G.; Turner, R.J. Selenium and tellurium nanomaterials. Phys. Sci. Rev. 2018, 3, 20170100. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef]
- Colmenares, L.; Jusys, Z.; Behm, R.J. Activity, selectivity, and methanol tolerance of Se-modified Ru/C cathode catalysts. J. Phys. Chem. C 2007, 111, 1273–1283. [Google Scholar] [CrossRef]
- Zehl, G.; Bogdanoff, P.; Dorbandt, I.; Fiechter, S.; Wippermann, K.; Hartnig, C. Carbon supported Ru–Se as methanol tolerant catalysts for DMFC cathodes. Part I: Preparation and characterization of catalysts. J. Appl. Electrochem. 2007, 37, 1475–1484. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Mehta, S.K. Surface functionalized selenium nanoparticles for biomedical applications. J. Biomed. Nanotechnol. 2014, 10, 3004–3042. [Google Scholar] [CrossRef]
- Dumore, N.S.; Mukhopadhyay, M. Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struct. 2019, 1205, 127637. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, M.; Wang, M.; Feng, C.; Wang, Z.-S. Metal selenides as efficient counter electrodes for dye-sensitized solar cells. Accounts Chem. Res. 2017, 50, 895–904. [Google Scholar] [CrossRef]
- Verjulio, R.W.; Santander, J.; Ma, J.; Alonso-Vante, N. Selective CoSe2/C cathode catalyst for passive air-breathing alkaline anion exchange membrane μ-direct methanol fuel cell (AEM-μDMFC). Int. J. Hydrogen Energy 2016, 41, 19595–19600. [Google Scholar] [CrossRef]
- Gurkan, Y.Y.; Kasapbasi, E.; Cinar, Z. Enhanced solar photocatalytic activity of TiO2 by selenium(IV) ion-doping: Characterization and DFT modeling of the surface. Chem. Eng. J. 2013, 214, 34–44. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Heyne, B.; Turner, R.J. Tunable photoluminescence properties of selenium nanoparticles: Biogenic versus chemogenic synthesis. Nanophotonics 2020, 9, 3615–3628. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Biosynthesis of SnO2 nanoparticles using bacterium Erwinia herbicola and their photocatalytic activity for degradation of dyes. Ind. Eng. Chem. Res. 2014, 53, 13971–13979. [Google Scholar] [CrossRef]
- Kumari, C.; Sharma, P.; Katyal, S.; Tanwar, M.; Bamola, P.; Sharma, H.; Kumar, R.; Chhoker, S. Photocatalytic activity of GeSbSeEr quaternary chalcogenide for efficient methylene blue degradation in visible light. Results Surf. Interfaces 2022, 9, 100088. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.; Almahri, A.; Ahmed, M.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97. [Google Scholar] [CrossRef]
- Altaf, S.; Ajaz, H.; Imran, M.; Ul-Hamid, A.; Naz, M.; Aqeel, M.; Shahzadi, A.; Shahbaz, A.; Ikram, M. Synthesis and characterization of binary selenides of transition metals to investigate its photocatalytic, antimicrobial and anticancer efficacy. Appl. Nanosci. 2020, 10, 2113–2127. [Google Scholar] [CrossRef]
- Talukdar, S.; Dutta, R.K. A mechanistic approach for superoxide radicals and singlet oxygen mediated enhanced photocatalytic dye degradation by selenium doped ZnS nanoparticles. RSC Adv. 2016, 6, 928–936. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Ayeshamariam, A.; Manikandan, E.; Kennedy, J.; Ladchumananandasivam, R.; Gomes, U.U.; Jayachandran, M.; Maaza, M. Solution processing of CuSe quantum dots: Photocatalytic activity under RhB for UV and visible-light solar irradiation. Mater. Sci. Eng. B 2016, 210, 1–9. [Google Scholar] [CrossRef]
- Karthikeyan, N.; Sivaranjani, T.; Dhanavel, S.; Gupta, V.; Narayanan, V.; Stephen, A. Visible light degradation of textile effluent by electrodeposited multiphase CuInSe2 semiconductor photocatalysts. J. Mol. Liq. 2017, 227, 194–201. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Feng, Z.; Lan, F.; Teng, C.; Li, D.; Tang, J. A new HgSe photocatalyst for degradation of organic dyes under visible light irradiation. Mater. Lett. 2015, 161, 591–594. [Google Scholar] [CrossRef]
- Ameri, A.; Shakibaie, M.; Pournamdari, M.; Ameri, A.; Foroutanfar, A.; Doostmohammadi, M.; Forootanfar, H. Degradation of diclofenac sodium using UV/biogenic selenium nanoparticles/H2O2: Optimization of process parameters. J. Photochem. Photobiol. A Chem. 2020, 392, 112382. [Google Scholar] [CrossRef]
- Chiou, Y.-D.; Hsu, Y.-J. Room-temperature synthesis of single-crystalline Se nanorods with remarkable photocatalytic properties. Appl. Catal. B Environ. 2011, 105, 211–219. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Hameed, P.; Rao, R.P.; Shrivastava, N.; Mittal, J.; Mohapatra, S. Biosynthesis of highly stable fluorescent selenium nanoparticles and the evaluation of their photocatalytic degradation of dye. Bionanoscience 2020, 10, 389–396. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, X.; Ma, L.; Yang, C.; Zhou, Z.; Wang, Y.; Wang, S. Visible-light-responsive t-Se nanorod photocatalysts: Synthesis, properties, and mechanism. RSC Adv. 2015, 5, 45165–45171. [Google Scholar] [CrossRef]
- Che, L.; Dong, Y.; Wu, M.; Zhao, Y.; Liu, L.; Zhou, H. Characterization of selenite reduction by Lysinibacillus sp. ZYM-1 and photocatalytic performance of biogenic selenium nanospheres. ACS Sustain. Chem. Eng. 2017, 5, 2535–2543. [Google Scholar] [CrossRef]
- Song, L.; Chen, C.; Zhang, S. Preparation and photocatalytic activity of visible light-sensitive selenium-doped bismuth sulfide. Powder Technol. 2011, 207, 170–174. [Google Scholar] [CrossRef]
- Sharma, A.; Dutta, R.K. Se-doped CuO NPs/H2O2/UV as a highly efficient and sustainable photo-fenton catalytic system for enhanced degradation of 4-bromophenol. J. Clean. Prod. 2018, 185, 464–475. [Google Scholar] [CrossRef]
- Huang, L.; Tong, X.; Li, Y.; Teng, J.; Bai, Y. Preparation of a novel supported selenium nanoparticles adsorbent and its application for copper removal from aqueous solution. J. Chem. Eng. Data 2014, 60, 151–160. [Google Scholar] [CrossRef]
- Bai, Y.; Rong, F.; Wang, H.; Zhou, Y.; Xie, X.; Teng, J. Removal of copper from aqueous solutions by adsorption on elemental selenium nanoparticles. J. Chem. Eng. Data 2011, 56, 2563–2568. [Google Scholar] [CrossRef]
- Jain, R.; Jordan, N.; Schild, D.; van Hullebusch, E.D.; Weiss, S.; Franzen, C.; Farges, F.; Hübner, R.; Lens, P.N. Adsorption of zinc by biogenic elemental selenium nanoparticles. Chem. Eng. J. 2015, 260, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Song, C.; Sun, X.; Tan, L.; Wang, Y.; Wang, S. Adsorption of Cd(II) from aqueous solution by biogenic selenium nanoparticles. RSC Adv. 2016, 6, 15201–15209. [Google Scholar] [CrossRef]
- Inukai, J.; Cao, D.; Wieckowski, A.; Chang, K.-C.; Menzel, A.; Komanicky, V.; You, H. In situ synchrotron X-ray spectroscopy of ruthenium nanoparticles modified with selenium for an oxygen reduction reaction. J. Phys. Chem. C 2007, 111, 16889–16894. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium-fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An antioxidant with a critical role in anti-aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food sources of selenium and its relationship with chronic diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Kohrle, J.; Jakob, F.; Contempre, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Gereben, B.; McAninch, E.A.; Ribeiro, M.O.; Bianco, A.C. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat. Rev. Endocrinol. 2015, 11, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Orozco, A.; Valverde, R.C.; Olvera, A.; Garcia, G.C. Iodothyronine deiodinases: A functional and evolutionary perspective. J. Endocrinol. 2012, 215, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Lee, K.H. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Qin, S.; Huang, B.; Ma, J.; Wang, X.; Zhang, J.; Li, L.; Chen, F. Effects of selenium-chitosan on blood selenium concentration, antioxidation status, and cellular and humoral immunity in mice. Biol. Trace Elem. Res. 2015, 165, 145–152. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Cole-Ezea, P.; Swan, D.; Shanley, D.; Hesketh, J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free Radic. Biol. Med. 2012, 53, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Aitken, J.B.; Finney, L.; Vogt, S.; Witting, P.K.; Harris, H.H. Selenium metabolism in cancer cells: The combined application of XAS and XFM techniques to the problem of selenium speciation in biological systems. Nutrients 2013, 5, 1734–1756. [Google Scholar] [CrossRef] [Green Version]
- Yeo, J.E.; Kang, S.K. Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim. Biophys. Acta 2007, 1772, 1199–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganther, H.E. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenesis 1999, 20, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Tobe, R.; Mihara, H. Delivery of selenium to selenophosphate synthetase for selenoprotein biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2433–2440. [Google Scholar] [CrossRef]
- Forootanfar, H.; Adeli-Sardou, M.; Nikkhoo, M.; Mehrbani, M.; Amirheidari, B.; Shahverdi, A.R.; Shakibaie, M. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014, 28, 75–79. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of nano-Se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Torres, S.K.; Campos, V.L.; León, C.G.; Rodríguez-Llamazares, S.M.; Rojas, S.M.; González, M.; Smith, C.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Rao, S.; Lin, Y.; Du, Y.; He, L.; Huang, G.; Chen, B.; Chen, T. Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J. Mater. Chem. B 2019, 7, 2648–2656. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, N.-H.; Patel, K.D.; Jun, S.-K.; Park, J.-H.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. A study on myogenesis by regulation of reactive oxygen species and cytotoxic activity by selenium nanoparticles. Antioxidants 2021, 10, 1727. [Google Scholar] [CrossRef]
- Varlamova, E.; Goltyaev, M.; Mal’Tseva, V.; Turovsky, E.; Sarimov, R.; Simakin, A.; Gudkov, S. Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Sen, T.; Bhattacharya, S. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumor-bearing mice. Mol. Cell Biochem. 2017, 424, 13–33. [Google Scholar] [CrossRef]
- Luesakul, U.; Puthong, S.; Neamati, N.; Muangsin, N. pH-responsive selenium nanoparticles stabilized by folate-chitosan delivering doxorubicin for overcoming drug-resistant cancer cells. Carbohydr. Polym. 2018, 181, 841–850. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Liu, W.; Fan, C.; Zheng, W.; Wong, Y.-S.; Chen, T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [Google Scholar] [CrossRef]
- Pi, J.; Jiang, J.; Cai, H.; Yang, F.; Jin, H.; Yang, P.; Cai, J.; Chen, Z.W. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv. 2017, 24, 1549–1564. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zheng, S.; Li, X.; Zhang, Y.; Wong, Y.-S. PEG-nanolized ultrasmall selenium nanoparticles overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int. J. Nanomed. 2012, 7, 3939–3949. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Yu, Y.; Zhang, X.; Wang, Y.; Chen, H.; Zhao, Y.; Wang, F.; Rong, G.; Wang, W.; Kang, X.; et al. Breaking the intracellular redox balance with diselenium nanoparticles for maximizing chemotherapy efficacy on patient-derived xenograft models. ACS Nano 2020, 14, 16984–16996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, T.; Liu, H.; Han, Y.; Zheng, Q.; Xu, Q.; Bao, B.; Xing, W.; Li, Z. Boost therapy of hepatocellular carcinoma by amplifying vicious cycle between mitochondrial oxidative stress and endoplasmic reticulum stress via biodegradable ultrasmall nanoparticles and old drug. Nano Today 2022, 46, 101601. [Google Scholar] [CrossRef]
- Qiao, L.; Yan, S.; Dou, X.; Song, X.; Chang, J.; Pi, S.; Zhang, X.; Xu, C. Biogenic selenium nanoparticles alleviate intestinal epithelial barrier damage through regulating endoplasmic reticulum stress-mediated mitophagy. Oxidative Med. Cell Longev. 2022, 2022, 3982613. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan nanoparticles induced the antitumor effect in hepatocellular carcinoma cells by regulating ROS-mediated mitochondrial damage and endoplasmic reticulum stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.J.; Qin, Y.; Zhao, Z.H.; Zhang, Y.; Yang, J.H.; Zhai, D.H.; Cui, F.; Luo, C.; Lu, M.X.; Liu, P.P.; et al. Lentinan-functionalized selenium nanoparticles target tumor cell mitochondria via TLR4/TRAF3/MFN1 pathway. Theranostics 2020, 10, 9083–9099. [Google Scholar] [CrossRef]
- Jiang, W.; Fu, Y.; Yang, F.; Yang, Y.; Liu, T.; Zheng, W.; Zeng, L.; Chen, T. Gracilaria lemaneiformis Polysaccharide as sntegrin-targeting surface decorator of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl. Mater. Interfaces 2014, 6, 13738–13748. [Google Scholar] [CrossRef]
- Guo, M.; Li, Y.; Lin, Z.; Zhao, M.; Xiao, M.; Wang, C.; Xu, T.; Xia, Y.; Zhu, B. Surface decoration of selenium nanoparticles with curcumin induced HepG2 cell apoptosis through ROS mediated p53 and AKT signaling pathways. RSC Adv. 2017, 7, 52456–52464. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Liu, T.; Chen, T. Overcoming blood-brain barrier by HER2-targeted nanosystem to suppress glioblastoma cell migration, invasion and tumor growth. J. Mater. Chem. B 2018, 6, 568–579. [Google Scholar] [CrossRef]
- Xia, Y.; Zhong, J.; Zhao, M.; Tang, Y.; Han, N.; Hua, L.; Xu, T.; Wang, C.; Zhu, B. Galactose-modified selenium nanoparticles for targeted delivery of doxorubicin to hepatocellular carcinoma. Drug Deliv. 2019, 26, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Yu, Z.; Luo, T.; Zhou, B.; Song, Q.; Li, Z.; Yu, X.; Jiang, S.; Zhou, Y.; Dong, W.; et al. Chitosan-coated selenium nanoparticles attenuate PRRSV replication and ROS/JNK-mediated apoptosis in vitro. Int. J. Nanomed. 2022, 17, 3043–3054. [Google Scholar] [CrossRef]
- Al-Noshokaty, T.M.; Mesbah, N.M.; Abo-Elmatty, D.M.; Abulsoud, A.I.; Abdel-Hamed, A.R. Selenium nanoparticles overcomes sorafenib resistance in thioacetamide induced hepatocellular carcinoma in rats by modulation of mTOR, NF-κB pathways and LncRNA-AF085935/GPC3 axis. Life Sci. 2022, 303, 120675. [Google Scholar] [CrossRef]
- Vundela, S.R.; Kalagatur, N.K.; Nagaraj, A.; Kadirvelu, K.; Chandranayaka, S.; Kondapalli, K.; Hashem, A.; Abd_Allah, E.F.; Poda, S. Multi-biofunctional properties of phytofabricated selenium nanoparticles from Carica papaya fruit extract: Antioxidant, antimicrobial, antimycotoxin, anticancer, and biocompatibility. Front. Microbiol. 2021, 12, 769891. [Google Scholar] [CrossRef]
- Pi, J.; Yang, F.; Jin, H.; Huang, X.; Liu, R.; Yang, P.; Cai, J. Selenium nanoparticles induced membrane bio-mechanical property changes in MCF-7 cells by disturbing membrane molecules and F-actin. Bioorg. Med. Chem. Lett. 2013, 23, 6296–6303. [Google Scholar] [CrossRef]
- Zeebaree, S.Y.S.; Zebari, O.I.H. Diagnosis of the multiple effect of selenium nanoparticles decorated by Asteriscus graveolens components in inhibiting HepG2 cell proliferation. Sustain. Chem. Pharm. 2020, 15, 1002101. [Google Scholar] [CrossRef]
- Juan, C.; de la Lastra, J.P.; Plou, F.; Pérez-Lebeña, E. The Chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trend Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Carrier, L.; Rowan, B.G. Methylseleninic acid synergizes with tamoxifen to induce caspase-mediated apoptosis in breast cancer cells. Mol. Cancer Ther. 2008, 7, 3056–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhang, J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef]
- Kong, Q.; Beel, J.; Lillehei, K. A threshold concept for cancer therapy. Med. Hypotheses 2000, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Dong, R.; Teng, J.; Yang, L.; Liu, T.; Wu, X.; He, Y.; Wang, Z.; Pu, H.; Wang, Y. N-Acetyl-l-cysteine enhances the effect of selenium nanoparticles on cancer cytotoxicity by increasing the production of selenium-induced reactive oxygen species. ACS Omega 2020, 5, 11710–11720. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, K.; Tan, Y.; Wu, S.; Zhang, J. Efficacy and safety of selenium nanoparticles administered intraperitoneally for the prevention of growth of cancer cells in the peritoneal cavity. Free Radic. Biol. Med. 2014, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 43. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, P.; Zhao, G.; Sun, K.; Li, D.; Wan, X.; Zhang, J. Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem. Toxicol. 2015, 85, 71–77. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Pasaro, E.; Méndez, J.; Laffon, B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: A review. Arch. Toxicol. 2010, 84, 337–351. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Ge, X.; Wang, C.; Zeng, H.; Li, D.; Dong, L. The inhibitory effect of a novel organoselenium compound BBSKE on the tongue cancer Tca8113 in vitro and in vivo. Oral Oncol. 2008, 44, 963–969. [Google Scholar] [CrossRef]
- Shiah, H.-S.; Lee, W.-S.; Juang, S.-H.; Hong, P.-C.; Lung, C.-C.; Chang, C.-J.; Chou, K.-M.; Chang, J.-Y. Mitochondria-mediated and p53-associated apoptosis induced in human cancer cells by a novel selenophene derivative, D-501036. Biochem. Pharmacol. 2007, 73, 610–619. [Google Scholar] [CrossRef]
- Shang, D.; Li, Y.; Wang, C.; Wang, X.; Yu, Z.; Fu, X. A novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol. Rep. 2010, 25, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Shang, D.; Zhang, J.; Wen, L.; Li, Y.; Cui, Q. Preparation, characterization, and antiproliferative activities of the Se-containing polysaccharide SeGLP-2B-1 from Se-enriched Ganoderma lucidum. J. Agric. Food Chem. 2009, 57, 7737–7742. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Clavijo, C.D.F.; Medina, E.; Sinche, F.; Vispo, N.S.; Dahoumane, S.A.; Alexis, F. Biomedical science to tackle the COVID-19 pandemic: Current status and future perspectives. Molecules 2020, 25, 4620. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Lopez-Ribot, J.L. Nanotechnology as an alternative to reduce the spread of COVID-19. Challenges 2020, 11, 15. [Google Scholar] [CrossRef]
- Rai, P.K.; Usmani, Z.; Thakur, V.K.; Gupta, V.K.; Mishra, Y.K. Tackling COVID-19 pandemic through nanocoatings: Confront and exactitude. Curr. Res. Green Sustain. Chem. 2020, 3, 100011. [Google Scholar] [CrossRef]
- Rao, L.; Xia, S.; Xu, W.; Tian, R.; Yu, G.; Gu, C.; Pan, P.; Meng, Q.-F.; Cai, X.; Qu, D.; et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2020, 117, 27141–27147. [Google Scholar] [CrossRef]
- Tharayil, A.; Rajakumari, R.; Kumar, A.; Choudhary, M.D.; Palit, P.; Thomas, S. New insights into application of nanoparticles in the diagnosis and screening of novel coronavirus (SARS-CoV-2). Emergent Mater. 2021, 4, 101–117. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Soufi, G.J.; Iravani, S.; Varma, R.S. Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials 2020, 10, 1072. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Gao, X.-Y.; Zhang, L.-D.; Bao, Y.-P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef]
- Jia, X.; Li, N.; Chen, J. A subchronic toxicity study of elemental nano-Se in Sprague-Dawley rats. Life Sci. 2005, 76, 1989–2003. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Xiong, L.; Zhang, H.; Song, J.; Xia, M. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed. Sci. Technol. 2012, 177, 204–210. [Google Scholar] [CrossRef]
- Benko, I.; Nagy, G.; Tanczos, B.; Ungvari, E.; Sztrik, A.; Eszenyi, P.; Prokisch, J.; Banfalvi, G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ. Toxicol. Chem. 2013, 31, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2012, 51, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, L.; Jiang, W.; Fu, Y.; Zheng, W.; Chen, T. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 947–958. [Google Scholar] [CrossRef]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Soflaei, S.; Dalimi, A.; Abdoli, A.; Kamali, M.; Nasiri, V.; Shakibaie, M.; Tat, M. Anti-leishmanial activities of selenium nanoparticles and selenium dioxide on Leishmania infantum. Comp. Clin. Pathol. 2012, 23, 15–20. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Shen, X. Effects of nano-selenium poisoning on immune function in the Wumeng semi-fine wool sSheep. Biol. Trace Elem. Res. 2021, 199, 2919–2924. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int. J. Nanomed. 2018, 13, 2005–2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, H.; Chen, D.; Zhao, M.; Lin, Z.; Guo, M.; Xu, T.; Chen, Y.; Hua, L.; Lin, T.; et al. The inhibition of H1N1 influenza virus-induced apoptosis by surface decoration of selenium nanoparticles with b-thujaplicin through reactive oxygen species-mediated AKT and p53 signaling pathways. ACS Omega 2020, 5, 30633–30642. [Google Scholar] [CrossRef]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Ma, J.; Mu, Y.; Wang, Y.; Yang, S.; Wu, Y.; Wu, F.; Zhou, Y. Selenium (Se) plays a key role in the biological effects of some viruses: Implications for COVID-19. Environ. Res. 2021, 196, 110984. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA virus interactions: Potential implications for SARS-CoV-2 infection (COVID-19). Front. Nutr. 2020, 7, 164. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a South Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Manzanares, W.; Moreira, E.; Hardy, G. Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): Does selenium have a place? Nutrition 2021, 81, 110989. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef]
- Peng, T.; Sui, Z.; Huang, Z.; Xie, J.; Wen, K.; Zhang, Y.; Huang, W.; Mi, W.; Peng, K.; Dai, X.; et al. Point-of-care test system for detection of immunoglobulin-G and -M against nucleocapsid protein and spike glycoprotein of SARS-CoV-2. Sens. Actuator B Chem. 2021, 331, 129415. [Google Scholar] [CrossRef] [PubMed]
- Bayin, Q.; Huang, L.; Ren, C.; Fu, Y.; Ma, X.; Guo, J. Anti-SARS-CoV-2 IgG and IgM detection with a GMR based LFIA system. Talanta 2021, 227, 122207. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-H.; Opadele, A.E.; Onodera, Y.; Nam, J.-M. Targeting integrins in cancer nanomedicine: Applications in cancer diagnosis and therapy. Cancers 2019, 11, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, S.; Tiwary, S.K.; Sonker, M.; Joshi, A.; Gupta, V.; Kumar, Y.; Shreyash, N.; Biswas, S. Recent advances in nanoparticle-based cancer treatment: A review. ACS Appl. Nano Mater. 2021, 4, 6441–6470. [Google Scholar] [CrossRef]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef]
- Xie, Q.; Deng, W.; Yuan, X.; Wang, H.; Ma, Z.; Wu, B.; Zhang, X. Selenium-functionalized liposomes for systemic delivery of doxorubicin with enhanced pharmacokinetics and anticancer effect. Eur. J. Pharm. Biopharm. 2018, 122, 87–95. [Google Scholar] [CrossRef]
- Treuel, L.; Eslahian, K.A.; Docter, D.; Lang, T.; Zellner, R.; Nienhaus, K.; Nienhaus, G.U.; Stauber, R.H.; Maskos, M. Physicochemical characterization of nanoparticles and their behavior in the biological environment. Phys. Chem. Chem. Phys. 2014, 16, 15053–15067. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Merging worlds of nanomaterials and biological environment: Factors governing protein corona formation on nanoparticles and its biological consequences. Nanoscale Res. Lett. 2015, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Gharbavi, M.; Johari, B.; Mousazadeh, N.; Rahimi, B.; Leilan, M.P.; Eslami, S.S.; Sharafi, A. Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Mol. Biol. Rep. 2020, 47, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.; Daniels, A.; Habib, S.; Singh, M. Poly-L-lysine–lactobionic acid-capped selenium nanoparticles for liver-targeted gene delivery. Int. J. Mol. Sci. 2022, 23, 1492. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Singh, M. Polymerized selenium nanoparticles for folate-receptor-targeted delivery of anti-luc-siRNA: Potential for gene silencing. Biomedicines 2020, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korany, M.; Marzook, F.; Mahmoud, B.; Ahmed, S.A.; Ayoub, S.M.; Sakr, T.M. Exhibiting the diagnostic face of selenium nanoparticles as a radio-platform for tumor imaging. Bioorg. Chem. 2020, 100, 103910. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yu, Q.; Deng, G.; Wang, Q.; Ma, X.; Wang, Q.; Lu, J. Selenium nanocomposites as multifunctional nanoplatform for imaging guiding synergistic chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2018, 166, 161–169. [Google Scholar] [CrossRef]
- Yu, C.; Wo, F.; Shao, Y.; Dai, X.; Chu, M. Bovine serum albumin nanospheres synchronously encapsulating “gold selenium/gold” nanoparticles and photosensitizer for high-efficiency cancer phototherapy. Appl. Biochem. Biotechnol. 2013, 169, 1566–1578. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of nanoparticles and biosystems: Microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Kumar Yadav, S. Cellular interactions of therapeutically delivred nanoparticules. Expert Opin. Drug Deliv. 2011, 8, 141–151. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Jiang, J.; Gao, M.; Wang, W.; Zheng, H.; Xu, S.; Li, R. Molecular mechanisms, characterization methods, and utilities of nanoparticle biotransformation in nanosafety assessments. Small 2020, 16, e1907663. [Google Scholar] [CrossRef]
- Chiappi, M.; Conesa, J.J.; Pereiro, E.; Sorzano, C.O.S.; Rodríguez, M.J.; Henzler, K.; Schneider, G.; Chichón, F.J.; Carrascosa, J.L. Cryo-soft X-ray tomography as a quantitative three-dimensional tool to model nanoparticle:cell interaction. J. Nanobiotechnol. 2016, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Mitchell, A.J.; Malysheva, A.; Voelcker, N.H.; Lombi, E. Methodologies and approaches for the analysis of cell–nanoparticle interactions. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1486. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Toy, R.; Bauer, L.; Hoimes, C.; Ghaghada, K.B.; Karathanasis, E. Targeted nanotechnology for cancer imaging. Adv. Drug Deliv. Rev. 2014, 76, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, C.C.; Payne, C.K. Nanoparticle–cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Gräfe, C.; Weidner, A.; Lühe, M.V.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle–cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Runa, S.; Hussey, M.; Payne, C.K. Nanoparticle–cell interactions: Relevance for public health. J. Phys. Chem. B 2017, 122, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Ivask, A.; Mitchell, A.J.; Hope, C.M.; Barry, S.C.; Lombi, E.; Voelcker, N.H. Single cell level quantification of nanoparticle–cell interactions using mass cytometry. Anal. Chem. 2017, 89, 8228–8232. [Google Scholar] [CrossRef]
- Rasmussen, L.; Shi, H.; Liu, W.; Shannon, K.B. Quantification of silver nanoparticle interactions with yeast Saccharomyces cerevisiae studied using single-cell ICP-MS. Anal. Bioanal. Chem. 2022, 414, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.J.; Ha, M.K.; Yoon, T.H. Quantitative estimation of cell-associated silver nanoparticles using the normalized side scattering intensities of flow cytometry. Nanomaterials 2021, 11, 3079. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska, B.; Fonseca, M.P.; Gorshkov, V.; Skytte, L.; Rasmussen, K.L.; Schrøder, H.D.; Kjeldsen, F. The cytotoxicity of metal nanoparticles depends on their synergistic interactions. Part. Part. Syst. Charact. 2020, 37, 2000135. [Google Scholar] [CrossRef]
- Khanehzar, A.; Fraire, J.C.; Xi, M.; Feizpour, A.; Xu, F.; Wu, L.; Coronado, E.A.; Reinhard, B.M. Nanoparticle–cell interactions induced apoptosis: A case study with nanoconjugated epidermal growth factor. Nanoscale 2018, 10, 6712–6723. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, X.; Li, Q.; Yin, M.; Song, H.; Hu, J.; Wang, L.; Fan, C.; Chen, N. Unraveling cell-type-specific targeted delivery of membrane-camouflaged nanoparticles with plasmonic imaging. Nano Lett. 2020, 20, 5228–5235. [Google Scholar] [CrossRef]
- Mutalik, S.P.; Pandey, A.; Mutalik, S. Nanoarchitectronics: A versatile tool for deciphering nanoparticle interaction with cellular proteins, nucleic acids and phospholipids at biological interfaces. Int. J. Biol. Macromol. 2020, 151, 136–158. [Google Scholar] [CrossRef]
- Nash, J.A.; Kwansa, A.L.; Peerless, J.S.; Kim, H.S.; Yingling, Y.G. Advances in molecular modeling of nanoparticle–nucleic acid interfaces. Bioconjug. Chem. 2017, 28, 3–10. [Google Scholar] [CrossRef]
- Samanta, A.; Medintz, I.L. Nanoparticles and DNA—A powerful and growing functional combination in bionanotechnology. Nanoscale 2016, 8, 9037–9095. [Google Scholar] [CrossRef] [Green Version]
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef]
- Fusco, L.; Avitabile, E.; Armuzza, V.; Orecchioni, M.; Istif, A.; Bedognetti, D.; Da Ros, T.; Delogu, L.G. Impact of the surface functionalization on nanodiamond biocompatibility: A comprehensive view on human blood immune cells. Carbon 2020, 160, 390–404. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Wang, T. A new view for nanoparticle assemblies: From crystalline to binary cooperative complementarity. Chem. Soc. Rev. 2017, 46, 1483–1509. [Google Scholar] [CrossRef] [PubMed]
- Dyawanapelly, S.; Mehrotra, P.; Ghosh, G.; Jagtap, D.D.; Dandekar, P.; Jain, R. How the surface functionalized nanoparticles affect conformation and activity of proteins: Exploring through protein-nanoparticle interactions. Bioorg. Chem. 2019, 82, 17–25. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Lartigue, L.; Javed, Y.; Luciani, N.; Pellegrino, T.; Wilhelm, C.; Alloyeau, D.; Gazeau, F. Biotransformations of magnetic nanoparticles in the body. Nano Today 2016, 11, 280–284. [Google Scholar] [CrossRef]
- Ruiz, A.; Hernández, Y.; Cabal, C.; González, E.; Veintemillas-Verdaguer, S.; Martínez, E.; Morales, M.P. Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale 2013, 5, 11400–11408. [Google Scholar] [CrossRef] [Green Version]
- Vlasova, I.I.; Kapralov, A.A.; Michael, Z.P.; Burkert, S.C.; Shurin, M.R.; Star, A.; Shvedova, A.A.; Kagan, V.E. Enzymatic oxidative biodegradation of nanoparticles: Mechanisms, significance and applications. Toxicol. Appl. Pharmacol. 2016, 299, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 2019, 31, e1805730. [Google Scholar] [CrossRef]
- de Dios, A.S.; Diaz-Garcia, M.E. Multifunctional nanoparticles: Analytical prospects. Anal. Chim. Acta 2010, 666, 1–22. [Google Scholar] [CrossRef]
- Murar, M.; Albertazzi, L.; Pujals, S. Advanced optical imaging-guided nanotheranostics towards personalized cancer drug delivery. Nanomaterials 2022, 12, 399. [Google Scholar] [CrossRef]
- Russell, P.; Hagemeyer, C.E.; Esser, L.; Voelcker, N.H. Theranostic nanoparticles for the management of thrombosis. Theranostics 2022, 12, 2773–2800. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.-L.; Hsieh, P.C.-H. Advances in biomimetic nanoparticles for targeted cancer therapy and diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, Q.; Liu, Y.; Bhavsar, D.; Yang, L.; Ren, X.; Sun, D.; Zheng, W.; Liu, J.; Chen, L.M. Multifunctional selenium nanoparticles: Chiral selectivity of delivering MDR-siRNA for reversal of multidrug resistance and real-time biofluorescence imaging. Nanomedicine 2015, 11, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Cao, C.; Liu, Y.; Yu, Q.; Zheng, C.; Sun, D.; Ren, X.; Liu, J. Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomater. 2015, 11, 368–380. [Google Scholar] [CrossRef]
- Zhu, B.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Xia, H.; Wang, H.; Li, Y. Multifunctional selenium nanoparticles as carriers of HSP70 siRNA to induce apoptosis of HepG2 cells. Int. J. Nanomed. 2016, 11, 3065–3076. [Google Scholar] [CrossRef] [Green Version]
- Siemer, S.; Westmeier, D.; Barz, M.; Eckrich, J.; Wünsch, D.; Seckert, C.; Thyssen, C.; Schilling, O.; Hasenberg, M.; Pang, C.; et al. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials 2019, 192, 551–559. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Xu, X.; Tang, W.; Xiong, L.; Sun, Q. Formation of protein corona on nanoparticles with digestive enzymes in simulated gastrointestinal fluids. J. Agric. Food Chem. 2019, 67, 2296–2306. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, J.; Zhang, T.; Zhang, T.-X.; Zhang, C.-L.; Mu, H. Digestive enzyme corona formed in the gastrointestinal tract and its impact on epithelial cell uptake of nanoparticles. Biomacromolecules 2019, 20, 1789–1797. [Google Scholar] [CrossRef]
- Obst, K.; Yealland, G.; Balzus, B.; Miceli, E.; Dimde, M.; Weise, C.; Eravci, M.; Bodmeier, R.; Haag, R.; Calderón, M.; et al. Protein corona formation on colloidal polymeric nanoparticles and polymeric nanogels: Impact on cellular uptake, toxicity, immunogenicity, and drug release properties. Biomacromolecules 2017, 18, 1762–1771. [Google Scholar] [CrossRef]
- Gupta, M.N.; Roy, I. How corona formation impacts nanomaterials as drug carriers. Mol. Pharm. 2020, 17, 725–737. [Google Scholar] [CrossRef]
- Pyrgiotakis, G.; Blattmann, C.O.; Demokritou, P. Real-time nanoparticle–cell interactions in physiological media by atomic force microscopy. ACS Sustain. Chem. Eng. 2014, 2, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Tabatabaei, M.; Farokhzad, O.C.; Mahmoudi, M. Personalized protein corona on nanoparticles and its clinical implications. Biomater. Sci. 2017, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Chauhan, P.; Alex, S.A.; Chaudhary, S.; Ethiraj, K.; Chandrasekaran, N.; Mukherjee, A. Comprehensive study on biocorona formation on functionalized selenium nanoparticle and its biological implications. J. Mol. Liq. 2018, 268, 335–342. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chauhan, P.; Kumar, S.; Chaudhary, S.; Chandrasekaran, N.; Mukherjee, A.; Ethiraj, K. Utilizing corona on functionalized selenium nanoparticles for loading and release of doxorubicin payload. J. Mol. Liq. 2019, 296, 111864. [Google Scholar] [CrossRef]
- Borowska, M.; Pawlik, E.; Jankowski, K. Investigation of interaction between biogenic selenium nanoparticles and human serum albumin using microwave plasma optical emission spectrometry operating in a single-particle mode. Monatsh. Chem. 2020, 151, 1283–1290. [Google Scholar] [CrossRef]
- Swierczewska, M.; Han, H.; Kim, K.; Park, J.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreani, T.; Fangueiro, J.F.; Severino, P.; de Souza, A.L.R.; Martins-Gomes, C.; Fernandes, P.M.V.; Calpena, A.C.; Gremião, M.P.; Souto, E.B.; Silva, A.M. The Influence of polysaccharide coating on the physicochemical parameters and cytotoxicity of silica nanoparticles for hydrophilic biomolecules delivery. Nanomaterials 2019, 9, 1081. [Google Scholar] [CrossRef] [Green Version]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef]
- Salatin, S.; Khosroushahi, A.Y. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.; ElSohly, H.; ElSohly, M.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shen, B.; Nie, S.; Duan, Z.; Chen, K. A combination of selenium and polysaccharides: Promising therapeutic potential. Carbohydr. Polym. 2019, 206, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010, 316, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [Green Version]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery, J.C.; Hoffmann, P.R. Selenium, selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Labunskyy, V.; Lee, B.C.; Handy, D.; Loscalzo, J.; Hatfield, D.L.; Gladyshev, V.N. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid. Redox Signal. 2011, 14, 2327–2336. [Google Scholar] [CrossRef]
- Shchedrina, V.A.; Zhang, Y.; Labunskyy, V.; Hatfield, D.L.; Gladyshev, V.N. Structure–function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antioxid. Redox Signal. 2010, 12, 839–849. [Google Scholar] [CrossRef]
- Arbogast, S.; Ferreiro, A. Selenoproteins and protection against oxidative stress: Selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The impact of selenium deficiency on cardiovascular function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; Boukhzar, L.; Granieri, M.C.; Alsharif, I.; Mazza, R.; Lefranc, B.; Tota, B.; Leprince, J.; Cerra, M.C.; Anouar, Y.; et al. A selenoprotein T-derived peptide protects the heart against ischaemia/reperfusion injury through inhibition of apoptosis and oxidative stress. Acta Physiol. 2018, 223, e13067. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2011, 8, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Valea, A.; Georgescu, C.E. Selenoproteins in human body: Focus on thyroid pathophysiology. Hormones 2018, 17, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Vassalle, C. Selenium and selenoproteins at the intersection of type 2 diabetes and thyroid pathophysiology. Antioxidants 2022, 11, 1188. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.-S.; Kim, J.-H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Gong, T.; Torres, D.; Berry, M.J.; Pitts, M.W. Hypothalamic redox balance and leptin signaling—Emerging role of selenoproteins. Free Radic. Biol. Med. 2018, 127, 172–181. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.-J.; Meng, Q.; Han, H.; et al. Role of selenium and selenoproteins in male reproductive function: A review of past and present evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Sahakian, D.C.; De Morais, S.M.F.; Xu, J.J.; Polzer, R.J.; Winter, S.M. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr. Top. Med. Chem. 2003, 3, 1125–1154. [Google Scholar] [CrossRef]
- Kazi, M.; Alhajri, A.; AlShehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing oral bioavailability of apigenin using a bioactive self-nanoemulsifying drug delivery system (Bio-SNEDDS): In vitro, in vivo and stability evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Al-Kassas, R.; Bansal, M.; Shaw, J. Nanosizing techniques for improving bioavailability of drugs. J. Control. Release 2017, 260, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.H.; Han, D.-G.; Cho, H.-J.; Yoon, I.-S.; Jung, I.H. Recent advances in physiologically based pharmacokinetic and pharmacodynamic models for anticancer nanomedicines. Arch. Pharmacal Res. 2020, 43, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiio, T.M.; Park, S. Physical properties of nanoparticles do matter. J. Pharm. Investig. 2020, 51, 35–51. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Kadam, R.S.; Bourne, D.W.A.; Kompella, U.B. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: Contribution of reduced clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Liu, W.; Liu, F.; Nasr, K.; Misra, P.; Bawendi, M.G.; Frangioni, J.V. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010, 5, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef]
- Li, Y.; Kröger, M.; Liu, W.K. Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.; Muthana, M. Designer nanocarriers for navigating the systemic delivery of oncolytic viruses. Nanomedicine 2020, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Obrezanova, O.; Martinsson, A.; Whitehead, T.; Mahmoud, S.; Bender, A.; Miljković, F.; Grabowski, P.; Irwin, B.; Oprisiu, I.; Conduit, G.; et al. Prediction of in vivo pharmacokinetic parameters and time–exposure curves in rats using machine learning from the chemical structure. Mol. Pharm. 2022, 19, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Hadrup, N.; Hansen, M.; Pereira, S.A.; Gammelgaard, B.; Møller, L.H.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Absorption, distribution, metabolism and excretion of selenium following oral administration of elemental selenium nanoparticles or selenite in rats. Metallomics 2014, 6, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Jia, X. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity. Regul. Toxicol. Pharmacol. 2014, 70, 720–727. [Google Scholar] [CrossRef]
- Zheng, S.; Xing, H.; Zhang, Q.; Xue, H.; Zhu, F.; Xu, S. Pharmacokinetics of sodium selenite administered orally in blood and tissues of selenium-deficient ducklings. Biol. Trace Elem. Res. 2019, 190, 509–516. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Shen, S.; Zhang, Y. Comparison of bioavailability, pharmacokinetics, and biotransformation of selenium-enriched yeast and sodium selenite in rats using plasma selenium and selenomethionine. Biol. Trace Elem. Res. 2020, 196, 512–516. [Google Scholar] [CrossRef]
- Xing, H.; Zheng, S.; Zhang, Z.; Zhu, F.; Xue, H.; Xu, S. Pharmacokinetics of selenium in healthy piglets after different routes of administration: Application of pharmacokinetic data to the risk assessment of selenium. Biol. Trace Elem. Res. 2019, 191, 403–411. [Google Scholar] [CrossRef]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [Green Version]
- Manzanares, W.; Biestro, A.; Galusso, F.; Torre, M.H.; Mañáy, N.; Facchin, G.; Hardy, G. High-dose selenium for critically ill patients with systemic inflammation: Pharmacokinetics and pharmacodynamics of selenious acid: A pilot study. Nutrition 2010, 26, 634–640. [Google Scholar] [CrossRef]
- Evans, S.O.; Jacobson, G.M.; Goodman, H.J.B.; Bird, S.; Jameson, M.B. Comparative safety and pharmacokinetic evaluation of three oral selenium compounds in cancer patients. Biol. Trace Elem. Res. 2019, 189, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium nanoparticles for biomedical applications: From development and characterization to therapeutics. Adv. Health Mater. 2021, 10, e2100598. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yuan, Q.; Gao, L.; Cai, P.; Zhu, H.; Liu, R.; Wang, Y.; Wei, Y.; Huang, G.; Liang, J.; et al. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials 2014, 35, 8854–8866. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; Rao, L.; Zhuang, M.; Luo, T.; Wang, Y.; Ma, Y. Chitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic BAY 55-9837 for type 2 diabetes mellitus. Int. J. Nanomed. 2014, 9, 4819–4828. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Xie, Q.; Wang, H.; Ma, Z.; Wu, B.; Zhang, X. Selenium nanoparticles as versatile carriers for oral delivery of insulin: Insight into the synergic antidiabetic effect and mechanism. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1965–1974. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Y.; Li, X.; Lai, H.; Huang, Y.; He, L.; Zheng, W.; Chen, T. RGD peptide-conjugated selenium nanoparticles: Antiangiogenesis by suppressing VEGF-VEGFR2-ERK/AKT pathway. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1627–1639. [Google Scholar] [CrossRef]
- Rojekar, S.; Pai, R.; Abadi, L.F.; Mahajan, K.; Prajapati, M.K.; Kulkarni, S.; Vavia, P. Dual loaded nanostructured lipid carrier of nano-selenium and Etravirine as a potential anti-HIV therapy. Int. J. Pharm. 2021, 607, 120986. [Google Scholar] [CrossRef]
- Xiao, J.; Yan, M.; Zhou, K.; Chen, H.; Xu, Z.; Gan, Y.; Hong, B.; Tian, G.; Qian, J.; Zhang, G.; et al. A nanoselenium-coating biomimetic cytomembrane nanoplatform for mitochondrial targeted chemotherapy- and chemodynamic therapy through manganese and doxorubicin codelivery. J. Nanobiotechnol. 2021, 19, 227. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, E.; Medina-Cruz, D.; Vernet-Crua, A.; Chen, J.; Cholula-Diaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanomedicine: The path to the next generation of nanomaterials for diagnosing brain tumors and therapeutics? Expert Opin. Drug Deliv. 2021, 18, 715–736. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondarenko, O.; Mortimer, M.; Kahru, A.; Feliu, N.; Javed, I.; Kakinen, A.; Lin, S.; Xia, T.; Song, Y.; Davis, T.P.; et al. Nanotoxicology and nanomedicine: The Yin and Yang of nano-bio interactions for the new decade. Nano Today 2021, 39, 101184. [Google Scholar] [CrossRef]
- Madani, M.; Hosny, S.; Alshangiti, D.M.; Nady, N.; Alkhursani, S.A.; Alkhaldi, H.; Al-Gahtany, S.A.; Ghobashy, M.M.; Gaber, G.A. Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes. Nanotechnol. Rev. 2022, 11, 731–759. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. Nanoimpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Alvarez, F.J.; Agathos, S.N.; Jeffryes, C. Microalgae: An outstanding tool in nanotechnology. Bionatura 2016, 1, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Lin, J.; Jaramillo, F.E.; Bazylinski, D.A.; Jeffryes, C.; Dahoumane, S.A. In vivo biosynthesis of inorganic nanomaterials using eukaryotes—A review. Molecules 2020, 25, 3246. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef]
- Varma, R.S. Greener approach to nanomaterials and their sustainable applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green nanotechnology-driven drug delivery assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangirian, H.; Lemraski, E.G.; Rafiee-Moghaddam, R.; Webster, T.J. A review of using green chemistry methods for biomaterials in tissue engineering. Int. J. Nanomed. 2018, 13, 5953–5969. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [Green Version]
- Sakr, T.M.; Korany, M.; Katti, K.V. Selenium nanomaterials in biomedicine—An overview of new opportunities in nanomedicine of selenium. J. Drug Deliv. Sci. Technol. 2018, 46, 223–233. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Harandi, M.F.; Shakibaie, M.; Aflatoonian, M.R.; ZiaAli, N.; Makki, M.S.; Jahanbakhsh, S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 2014, 12, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2021, 12, 467–480. [Google Scholar] [CrossRef]
- Sonkusre, P.; Nanduri, R. Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, J.; Thakur, N.S.; Aili, P.K.; Ghanghoriya, A.; Mittal, A.K.; Banerjee, U.C. Bioinspired nanotheranostic agents: Synthesis, surface functionalization, and antioxidant potential. ACS Biomater. Sci. Eng. 2015, 1, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Harsij, M.; Kanani, H.G.; Adineh, H. Effects of antioxidant supplementation (nano-selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquaculture 2020, 521, 734942. [Google Scholar] [CrossRef]
- Rathore, S.S.; Murthy, H.S.; Mamun, M.A.-A.; Nasren, S.; Rakesh, K.; Kumar, B.T.N.; Abhiman, P.B.; Khandagale, A.S. Nano-selenium supplementation to ameliorate nutrition physiology, immune response, antioxidant system and disease resistance against Aeromonas hydrophila in monosex Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2021, 199, 3073–3088. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Azeez, L.; Lateef, A.; Adebisi, S.A. Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl. Nanosci. 2017, 7, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Interplay between selenium, selenoproteins and HIV-1 replication in human CD4 T-lymphocytes. Int. J. Mol. Sci. 2022, 23, 1394. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, H.; Xia, W. Construction of Polygonatum sibiricum polysaccharide functionalized selenium nanoparticles for the enhancement of stability and antioxidant activity. Antioxidants 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Ponnampalam, E.N.; Celi, P.; Hopkins, D.L.; Leury, B.J.; Dunshea, F.R. High dietary vitamin E and selenium improves feed intake and weight gain of finisher lambs and maintains redox homeostasis under hot conditions. Small Rumin. Res. 2016, 137, 17–23. [Google Scholar] [CrossRef]
- Adeyemi, J.A.; Ogunwole, G.A.; Bamidele, O.S.; Adedire, C.O. Effects of pre-treatment with waterborne selenium on redox homeostasis and humoral innate immune parameters in African catfish, Clarias gariepinus (Burchell, 1822), experimentally challenged with Serratia marcescens. Fish Physiol. Biochem. 2022, 48, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, M.; Qiu, Q.; Wang, Y.; Shen, X.; Zhao, K. Nano-selenium and Macleaya cordata extracts improved immune function and reduced oxidative damage of Sows and IUGR piglets after heat stress of Sows in late gestation. Biol. Trace Elem. Res. 2022, 200, 5081–5090. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Y.; Wang, Y.; Wu, Q.; Cheng, B.; Li, Y.; Wang, Z.; Chai, X.; Ren, A.; Li, G. Role of endoplasmic reticulum stress in nano-selenium alleviating prehierarchical follicular atresia induced by mercury in laying hens. Biol. Trace Elem. Res. 2022, 200, 5205–5217. [Google Scholar] [CrossRef]
- Mellinas, C.; Jiménez, A.; Garrigós, M.D.C. Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 2019, 24, 4048. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Qiu, W.-Y.; Sun, L.; Ding, Z.-C.; Yan, J.-K. Preparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide–protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Chen, W.; Yue, L.; Jiang, Q.; Xia, W. Effect of chitosan with different molecular weight on the stability, antioxidant and anticancer activities of well-dispersed selenium nanoparticles. IET Nanobiotechnol. 2019, 13, 30–35. [Google Scholar] [CrossRef]
- Kokila, K.; Elavarasan, N.; Sujatha, V. Diospyros montana leaf extract-mediated synthesis of selenium nanoparticles and their biological applications. New J. Chem. 2017, 41, 7481–7490. [Google Scholar] [CrossRef]
- El-Zayat, M.M.; Eraqi, M.M.; Alrefai, H.; El-Khateeb, A.Y.; Ibrahim, M.A.; Aljohani, H.M.; Aljohani, M.M.; Elshaer, M.M. The antimicrobial, antioxidant, and anticancer activity of greenly synthesized selenium and zinc composite nanoparticles using Ephedra aphylla extract. Biomolecules 2021, 11, 470. [Google Scholar] [CrossRef]
- Liu, X.; Mao, Y.; Huang, S.; Li, W.; Zhang, W.; An, J.; Jin, Y.; Guan, J.; Wu, L.; Zhou, P. Selenium nanoparticles derived from Proteus mirabilis YC801 alleviate oxidative stress and inflammatory response to promote nerve repair in rats with spinal cord injury. Regen. Biomater. 2022, 9, rbac042. [Google Scholar] [CrossRef] [PubMed]
- Akçay, F.A.; Avcı, A. Effects of process conditions and yeast extract on the synthesis of selenium nanoparticles by a novel indigenous isolate Bacillus sp. EKT1 and characterization of nanoparticles. Arch. Microbiol. 2020, 202, 2233–2243. [Google Scholar] [CrossRef]
- Qi, Y.; Yi, P.; He, T.; Song, X.; Liu, Y.; Li, Q.; Zheng, J.; Song, R.; Liu, C.; Zhang, Z.; et al. Quercetin-loaded selenium nanoparticles inhibit amyloid-β aggregation and exhibit antioxidant activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125058. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, X.; Yan, S.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020, 11, 3020–3031. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, Y.; Qiao, L.; Ma, L.; Cheng, Y.; Roman, A. Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front. Microbiol. 2018, 9, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Yan, S.; Guo, Y.; Dou, X.; Zhang, B.; Roman, A. Biosynthesis of polysaccharides-capped selenium nanoparticles using Lactococcus lactis NZ9000 and their antioxidant and anti-inflammatory activities. Front. Microbiol. 2019, 10, 1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Muehlmann, L.A.; Deng, S.-E.; Wang, X.; Li, W.; Zhang, W. Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1463–1470. [Google Scholar] [CrossRef]
- Menon, S.; Shrudhi Devi, K.S.; Agarwal, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, M.M.G.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J. Clust. Sci. 2020, 32, 351–361. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Xu, J.-F.; Pi, J. The Advancing of selenium nanoparticles against infectious diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.M.D.S.; Dibo, M.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, I.M.; Kobayashi, R.K.T.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Curr. Res. Green Sustain. Chem. 2022, 5, 100303. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cittrarasu, V.; Kaliannan, D.; Dharman, K.; Maluventhen, V.; Easwaran, M.; Liu, W.C.; Balasubramanian, B.; Arumugam, M. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 2021, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf. B Biointerfaces 2015, 136, 1051–1057. [Google Scholar] [CrossRef]
- Chudobova, D.; Cihalova, K.; Dostalova, S.; Ruttkay-Nedecky, B.; Rodrigo, M.A.; Tmejova, K.; Kopel, P.; Nejdl, L.; Kudr, J.; Gumulec, J.; et al. Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. FEMS Microbiol. Lett. 2014, 351, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Alghuthaymi, M.A.; Diab, A.M.; Elzahy, A.F.; Mazrou, K.E.; Tayel, A.A.; Moussa, S.H. Green biosynthesized selenium nanoparticles by cinnamon extract and their antimicrobial activity and application as edible coatings with nano-chitosan. J. Food Qual. 2021, 2021, 6670709. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Rajeshkumar, S.; Rosy, P.J.; Shanmugam, V.K. Investigating the antimicrobial activities of the biosynthesized selenium nanoparticles and its statistical analysis. Bionanoscience 2020, 10, 122–135. [Google Scholar] [CrossRef]
- Kheradmand, E.; Rafii, F.; Yazdi, M.H.; Sepahi, A.A.; Shahverdi, A.R.; Oveisi, M.R. The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans. DARU J. Pharm. Sci. 2014, 22, 48. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Shakibaie, M.; Ezzatkhah, F.; Gabal, E.; Badparva, E.; Jahanbakhsh, S.; Mahmoudvand, H. Prophylactic effects of biogenic selenium nanoparticles on acute toxoplasmosis: An in vivo study. Ann. Med. Surg. 2020, 54, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, N.; Soflaei, S.; Shakibaie, M.; Yazdi, M.H.; Ghaffarifar, F.; Dalimi, A.; Shahverdi, A.R. Efficacy of biogenic selenium nanoparticles against Leishmania major: In vitro and in vivo studies. J. Trace Elem. Med. Biol. 2013, 27, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, H.; Barabadi, H.; Saravanan, M. Emerging selenium nanoparticles to combat cancer: A systematic review. J. Clust. Sci. 2019, 31, 301–309. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Chen, Y.; Zhao, G.; Sun, H.; Che, H.; Leng, X. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr. Polym. 2020, 231, 115689. [Google Scholar] [CrossRef]
- Ullah, A.; Mu, J.; Wang, F.; Chan, M.W.H.; Yin, X.; Liao, Y.; Mirani, Z.A.; Sebt-E-Hassan, S.; Aslam, S.; Naveed, M.; et al. Biogenic selenium nanoparticles and their anticancer effects pertaining to probiotic bacteria—A Review. Antioxidants 2022, 11, 1916. [Google Scholar] [CrossRef]
- Sholkamy, E.; Ahmad, M.; Yaser, M.M.; Ali, A.; Mehanni, M. Anticancer activity of biostabilized selenium nanorods synthesized by Streptomyces bikiniensis strain Ess_amA-1. Int. J. Nanomed. 2015, 10, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): Biophysical characterization and cytotoxicity on Vero cells. J. Clust. Sci. 2016, 28, 551–563. [Google Scholar] [CrossRef]
- Cruz, L.Y.; Wang, D.; Liu, J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J. Photochem. Photobiol. B Biol. 2019, 191, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: An in vitro and in vivo study. Front. Oncol. 2020, 9, 1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamurthy, C.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef]
- Srivastava, P.; Kowshik, M. Anti-neoplastic selenium nanoparticles from Idiomarina sp. PR58-8. Enzym. Microb. Technol. 2016, 95, 192–200. [Google Scholar] [CrossRef]
- A Wadhwani, S.; Gorain, M.; Banerjee, P.; Shedbalkar, U.U.; Singh, R.; Kundu, G.C.; A Chopade, B. Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: Optimization, characterization and its anticancer activity in breast cancer cells. Int. J. Nanomed. 2017, 12, 6841–6855. [Google Scholar] [CrossRef] [Green Version]
- Shakibaie, M.; Khorramizadeh, M.; Faramarzi, M.A.; Sabzevari, O.; Shahverdi, A.R. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol. Appl. Biochem. 2010, 56, 7–15. [Google Scholar] [CrossRef]
- Cui, D.; Liang, T.; Sun, L.; Meng, L.; Yang, C.; Wang, L.; Liang, T.; Li, Q. Green synthesis of selenium nanoparticles with extract of hawthorn fruit induced HepG2 cells apoptosis. Pharm. Biol. 2018, 56, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Rajasekar, S.; Kuppusamy, S. Eco-friendly formulation of selenium nanoparticles and its functional characterization against breast cancer and normal cells. J. Clust. Sci. 2020, 32, 907–915. [Google Scholar] [CrossRef]
- Krishnan, V.; Loganathan, C.; Thayumanavan, P. Green synthesized selenium nanoparticle as carrier and potent delivering agent of s-allyl glutathione: Anticancer effect against hepatocarcinoma cell line (HepG2) through induction of cell cycle arrest and apoptosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101207. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.-S.; Zheng, W.; Bai, Y.; Huang, L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf. B Biointerfaces 2008, 67, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Nonsuwan, P.; Puthong, S.; Palaga, T.; Muangsin, N. Novel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivatives. Carbohydr. Polym. 2018, 184, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liao, W.; Zhang, R.; Dong, C.; Yu, Z. Novel walnut peptide–selenium hybrids with enhanced anticancer synergism: Facile synthesis and mechanistic investigation of anticancer activity. Int. J. Nanomed. 2016, 11, 1305–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Spyridopoulou, K.; Aindelis, G.; Pappa, A.; Chlichlia, K. Anticancer activity of biogenic selenium nanoparticles: Apoptotic and immunogenic cell death markers in colon cancer cells. Cancers 2021, 13, 5335. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist. Updat. 2022, 63, 100844. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Vekariya, K.K.; Kaur, J.; Tikoo, K. Alleviating anastrozole induced bone toxicity by selenium nanoparticles in SD rats. Toxicol. Appl. Pharmacol. 2013, 268, 212–220. [Google Scholar] [CrossRef]
- Shirsat, S.; Kadam, A.; Mane, R.S.; Jadhav, V.V.; Zate, M.K.; Naushad, M.; Kim, K.H. Protective role of biogenic selenium nanoparticles in immunological and oxidative stress generated by enrofloxacin in broiler chicken. Dalton Trans. 2016, 45, 8845–8853. [Google Scholar] [CrossRef]
- Dahdouh, F.; Bendjeffal, H.; Nouacer, Z.; Moumene, W.; Zeminour, M.E.-H.; Naous, M.; Djebar, H. Selenium nanoparticles attenuate gentamycin-induced nephrotoxicity and hematotoxicity in female Swiss albino mice. Bionanoscience 2019, 9, 356–364. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Rezvanfar, M.A.; Shahverdi, A.R.; Ahmadi, A.; Baeeri, M.; Mohammadirad, A.; Abdollahi, M. Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium nano-particles. Toxicol. Appl. Pharmacol. 2013, 266, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wong, Y.-S.; Chen, T.; Zhang, H.; Liu, C.; Zheng, W. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 2011, 32, 9068–9076. [Google Scholar] [CrossRef]
- Saif-Elnasr, M.; Abdel-Aziz, N.; El-Batal, A.I. Ameliorative effect of selenium nanoparticles and fish oil on cisplatin and gamma irradiation-induced nephrotoxicity in male albino rats. Drug Chem. Toxicol. 2019, 42, 94–103. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zheng, W.; Fan, C.; Zhang, Y.; Chen, T. Functionalized selenium nanoparticles with nephroprotective activity, the important roles of ROS-mediated signaling pathways. J. Mater. Chem. B 2013, 1, 6365–6372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Kulkarni, A.; Khurana, A.; Kaur, J.; Tikoo, K. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem. Interact. 2014, 223, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, S.; Kojouri, G.A.; Mohebbi, A. Nanoparticles of selenium as species with stronger physiological effects in sheep in comparison with sodium selenite. Biol. Trace Elem. Res. 2012, 146, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Sadek, K.M.; Lebda, M.A.; Abouzed, T.K.; Nasr, S.M.; Shoukry, M. Neuro- and nephrotoxicity of subchronic cadmium chloride exposure and the potential chemoprotective effects of selenium nanoparticles. Metab. Brain Dis. 2017, 32, 1659–1673. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Talukder, M.; Ge, J.; Lv, M.-W.; Bi, S.-S.; Li, J.-L. Selenium sources differ in their potential to alleviate the cadmium-induced testicular dysfunction. Environ. Pollut. 2020, 267, 115610. [Google Scholar] [CrossRef]
- Qamar, N.; John, P.; Bhatti, A. Toxicological and anti-rheumatic potential of Trachyspermum ammi derived biogenic selenium nanoparticles in arthritic balb/c mice. Int. J. Nanomed. 2020, 15, 3497–3509. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-X.; Zhang, B.; Lin, Y.; Ma, D.-S.; Yan, H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. J. Pharmacol. Exp. Ther. 2019, 25, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Fu, Z.; Ji, P.; Guo, L.; O Al-Ghamdy, A.; Alkandiri, A.; A Habotta, O.; Moneim, A.E.A.; Kassab, R.B. Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int. J. Nanomed. 2020, 15, 6339–6353. [Google Scholar] [CrossRef] [PubMed]
- A Alkhudhayri, A.; A Dkhil, M.; Al-Quraishy, S. Nanoselenium prevents eimeriosis-induced inflammation and regulates mucin gene expression in mice jejunum. Int. J. Nanomed. 2018, 13, 1993–2003. [Google Scholar] [CrossRef] [Green Version]
- El-Ghazaly, M.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 101–110. [Google Scholar] [CrossRef]
- Ebokaiwe, A.P.; Okori, S.; Nwankwo, J.O.; Ejike, C.E.C.C.; Osawe, S.O. Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 591–602. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-kB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.Y.; Lin, P.Y.; Chuang, E.Y.; Shih, C.M.; Cheng, T.M.; Lin, T.Y.; Sung, H.W.; Mi, F.L. H2O2-depleting and O2-generating selenium nanoparticles for fluorescence imaging and photodynamic treatment of proinflammatory-activated macrophages. ACS Appl. Mater. Interfaces 2017, 9, 5158–5172. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yuan, Y.; Yue, T. Immunomodulatory of selenium nano-particles decorated by sulfated Ganoderma lucidum polysaccharides. Food Chem. Toxicol. 2014, 68, 183–189. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Zeng, C.; Gong, Q.-Y.; Yang, H.-B.; Li, X.-X.; Lei, G.-H.; Yang, T.-B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokmen, B.B.; Basaraner, H.; Yanardag, R. Combined effects of treatment with vitamin C, vitamin E and selenium on the skin of diabetic rats. Hum. Exp. Toxicol. 2013, 32, 379–384. [Google Scholar] [CrossRef]
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic. Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, K.; Lei, X.G. Selenium and diabetes—Evidence from animal studies. Free Radic. Biol. Med. 2013, 65, 1548–1556. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Xiao, Y.; Yu, Y.; Liu, Y.; Feng, W.; Qiu, G.; Wang, H.; Liu, B.; Wang, J.; Zhou, L.; et al. Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: The Dongfeng-Tongji Cohort. Environ. Pollut. 2018, 237, 917–925. [Google Scholar] [CrossRef]
- Galan-Chilet, I.; Grau-Perez, M.; De Marco, G.; Guallar, E.; Martin-Escudero, J.C.; Dominguez-Lucas, A.; Gonzalez-Manzano, I.; Lopez-Izquierdo, R.; Briongos-Figuero, L.S.; Redon, J.; et al. A gene-environment interaction analysis of plasma selenium with prevalent and incident diabetes: The Hortega study. Redox Biol. 2017, 12, 798–805. [Google Scholar] [CrossRef]
- Zhou, C.; Na, L.; Shan, R.; Cheng, Y.; Li, Y.; Wu, X.; Sun, C. Dietary vitamin C intake reduces the risk of type 2 diabetes in Chinese adults: HOMA-IR and T-AOC as potential mediators. PLoS ONE 2016, 11, e0163571. [Google Scholar] [CrossRef] [Green Version]
- Bahmani, F.; Kia, M.; Soleimani, A.; Mohammadi, A.A.; Asemi, Z. The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1222–1228. [Google Scholar] [CrossRef]
- Oztürk, Z.; Gurpinar, T.; Vural, K.; Boyacıoglu, S.; Korkmaz, M.; Var, A. Effects of selenium on endothelial dysfunction and metabolic profile in low dose streptozotocin induced diabetic rats fed a high fat diet. Biotech. Histochem. 2015, 90, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-J.; Wang, D.-H.; Li, Y.-W.; Han, L.; Xiao, X.; Ma, M.; Wan, D.C.-C.; Hong, A.; Ma, Y. A novel selective VPAC2 agonist peptide-conjugated chitosan modified selenium nanoparticles with enhanced anti-type 2 diabetes synergy effects. Int. J. Nanomed. 2017, 12, 2143–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.H.; El-Maksoud, M.D.A.; Moneim, A.E.A.; Aglan, H.A. Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol. Trace Elem. Res. 2017, 177, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hakim, Y.M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-stabilized selenium nanoparticles and metformin synergistically rescue testicular oxidative damage and steroidogenesis-related genes dysregulation in high-fat diet/streptozotocin-induced diabetic rats. Antioxidants 2021, 10, 17. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Khater, S.I.; Arisha, A.H.; Metwally, M.M.; Mostafa-Hedeab, G.; El-Shetry, E.S. Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 2020, 768, 145288. [Google Scholar] [CrossRef]
- Golmohammadi, R.; Najar-Peerayeh, S.; Moghadam, T.T.; Hosseini, S.M.J. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: An in vivo study on rat diabetic Staphylococcus aureus wound infection model. Sci. Rep. 2020, 10, 2854. [Google Scholar] [CrossRef] [Green Version]
- Shakeri-Zadeh, A.; Zareyi, H.; Sheervalilou, R.; Laurent, S.; Ghaznavi, H.; Samadian, H. Gold nanoparticle-mediated bubbles in cancer nanotechnology. J. Control. Release 2021, 330, 49–60. [Google Scholar] [CrossRef]
- Bromma, K.; Chithrani, D.B. Advances in gold nanoparticle-based combined cancer therapy. Nanomaterials 2020, 10, 1671. [Google Scholar] [CrossRef]
- Kerry, R.G.; Mahapatra, G.P.; Maurya, G.K.; Patra, S.; Mahari, S.; Das, G.; Patra, J.K.; Sahoo, S. Molecular prospect of type-2 diabetes: Nanotechnology based diagnostics and therapeutic intervention. Rev. Endocr. Metab. Disord. 2021, 22, 421–451. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [Green Version]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural polysaccharide-based nanodrug delivery systems for treatment of diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xu, L.; Xu, W.; Yang, M.; Zhang, Y. Application of nanotechnology in the diagnosis and treatment of acute pancreatitis. Nanoscale Adv. 2022, 4, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Ma, Y.; Yan, X.; Gong, L.; Zhang, M.; Zhang, B. Nanoparticle-based therapeutics of inflammatory bowel diseases: A narrative review of the current state and prospects. J. Bio-X Res. 2020, 3, 157–173. [Google Scholar] [CrossRef]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for diagnosis and treatment of infectious diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, X.; Zeng, Y.; Lin, D.; Wu, J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021, 14, 2067–2089. [Google Scholar] [CrossRef]
- Tobin, E.; Brenner, S. Nanotechnology fundamentals applied to clinical infectious diseases and public health. Open Forum Infect. Dis. 2021, 8, ofab583. [Google Scholar] [CrossRef]
- Homayoonnia, S.; Lee, Y.; Andalib, D.; Rahman, S.; Shin, J.; Kim, K.; Kim, S. Micro/nanotechnology-inspired rapid diagnosis of respiratory infectious diseases. Biomed. Eng. Lett. 2021, 11, 335–365. [Google Scholar] [CrossRef]
- Pehlivan, S.B. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm. Res. 2013, 30, 2499–2511. [Google Scholar] [CrossRef]
- Re, F.; Gregori, M.; Masserini, M. Nanotechnology for neurodegenerative disorders. Maturitas 2012, 73, 45–51. [Google Scholar] [CrossRef]
- Tapeinos, C. Graphene-based nanotechnology in neurodegenerative disorders. Adv. NanoBiomed Res. 2021, 1, 2000059. [Google Scholar] [CrossRef]
- De Andrade, V.; Nikitin, V.; Wojcik, M.; Deriy, A.; Bean, S.; Shu, D.; Mooney, T.; Peterson, K.; Kc, P.; Li, K.; et al. Fast X-ray Nanotomography with sub-10 nm resolution as a powerful imaging tool for nanotechnology and energy storage applications. Adv. Mater. 2021, 33, 2008653. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, Y.-Y.; Liu, X.; Zhang, X.; Ferrari, M.; Qin, L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014, 32, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Bayford, R.H.; Rademacher, T.; Roitt, I.; Wang, S.X. Emerging applications of nanotechnology for diagnosis and therapy of disease: A review. Physiol. Meas. 2017, 38, R183–R203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Central Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Kolay, A.; Maity, D.; Ghosal, P.; Deepa, M. Selenium nanoparticle-decorated silicon nanowires with enhanced liquid-junction photoelectrochemical solar cell performance. J. Phys. Chem. C 2019, 123, 8614–8622. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Yang, T.; Duncan, T.V. Challenges and potential solutions for nanosensors intended for use with foods. Nat. Nanotechnol. 2021, 16, 251–265. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Lei, J.; Huo, D.; Huang, Q.; Tan, J.; Li, Y.; Hou, C.; Tian, F. A portable and automatic dual-readout detector integrated with 3D-printed microfluidic nanosensors for rapid carbamate pesticides detection. Sens. Actuators B Chem. 2021, 346, 130454. [Google Scholar] [CrossRef]
- Sargazi, S.; Fatima, I.; Kiani, M.H.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Díez-Pascual, A.M.; Behzadmehr, R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: A comprehensive review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Steffens, C.; Ballen, S.C.; Scapin, E.; da Silva, D.M.; Steffens, J.; Jacques, R.A. Advances of nanobiosensors and its application in atrazine detection in water: A review. Sens. Actuators Rep. 2022, 4, 100096. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; Rodrigues, P.D.A.; Joshi, N.; Ferrari, R.G.; Conte-Junior, C.A. Nucleic acid-based nanobiosensor (NAB) used for Salmonella detection in foods: A Systematic Review. Nanomaterials 2022, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Farouq MA, H.; Al Qaraghuli, M.M.; Kubiak-Ossowska, K.; Ferro, V.A.; Mulheran, P.A. Biomolecular interactions with nanoparticles: Applications for COVID-2019. Curr. Opin. Colloid Interface Sci. 2021, 54, 101461. [Google Scholar] [CrossRef]
- Yu, L.; Banerjee, I.A.; Gao, X.; Nuraje, N.; Matsui, H. Fabrication and application of enzyme-incorporated peptide nanotubes. Bioconjug. Chem. 2005, 16, 1484–1487. [Google Scholar] [CrossRef]
- Rouse, I.; Lobaskin, V. A hard-sphere model of protein corona formation on spherical and cylindrical nanoparticles. Biophys. J. 2021, 120, 4457–4471. [Google Scholar] [CrossRef]
- Malik, P.; Katyal, V.; Malik, V.; Asatkar, A.; Inwati, G.; Mukherjee, T.K. Nanobiosensors: Concepts and variations. ISRN Nanomater. 2013, 2013, 327435. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Kaur, N.; Bhardwaj, P.; Singh, G.; Arya, S.K. Applicative insights on nascent role of biochar production, tailoring and immobilization in enzyme industry—A review. Process Biochem. 2021, 107, 153–163. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of enzymes by polymeric materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Rafiee, F.; Rezaee, M. Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Gan, J.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review. Int. J. Biol. Macromol. 2021, 167, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Sosa-Hernández, J.E.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Enzyme (single and multiple) and nanozyme biosensors: Recent developments and their novel applications in the water-food-health nexus. Biosensors 2021, 11, 410. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kiyota, Y.; Miyazaki, M. Techniques for preparation of cross-linked enzyme aggregates and their applications in bioconversions. Catalysts 2018, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, challenges and opportunities of enzyme immobilization on porous silicon for biosensing applications. J. Environ. Chem. Eng. 2020, 8, 104266. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Karthik, V.; Kumar, P.S.; Vo, D.-V.N.; Selvakumar, P.; Gokulakrishnan, M.; Keerthana, P.; Audilakshmi, V.; Jeyanthi, J. Enzyme-loaded nanoparticles for the degradation of wastewater contaminants: A review. Environ. Chem. Lett. 2021, 19, 2331–2350. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.-S.; Danquah, M.K. Potential and challenges of enzyme incorporated nanotechnology in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Panda, A.K. Recent advances in nano-engineered approaches used for enzyme immobilization with enhanced activity. J. Mol. Liq. 2021, 338, 116602. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Bin Emran, T.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.; Abbasi, B. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalaized silver nanoparticles. Sci. Rep. 2020, 10, 19615. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Biosynthetic conversion of Ag+ to highly Stable Ag0 nanoparticles by wild type and cell wall deficient strains of Chlamydomonas reinhardtii. Molecules 2018, 24, 98. [Google Scholar] [CrossRef] [Green Version]
- Bulgarini, A.; Lampis, S.; Turner, R.J.; Vallini, G. Biomolecular composition of capping layer and stability of biogenic selenium nanoparticles synthesized by five bacterial species. Microb. Biotechnol. 2020, 14, 198–212. [Google Scholar] [CrossRef]
- Torres-Chavolla, E.; Ranasinghe, R.J.; Alocilja, E.C. Characterization and functionalization of biogenic gold nanoparticles for biosensing enhancement. IEEE Trans. Nanotechnol. 2010, 9, 533–538. [Google Scholar] [CrossRef]
- Pumera, M.; Sánchez, S.; Ichinose, I.; Tang, J. Electrochemical nanobiosensors. Sens. Actuators B Chem. 2007, 123, 1195–1205. [Google Scholar] [CrossRef]
- Lopez-Marzo, A.M.; Hoyos-de-la-Torre, R.; Baldrich, E. NaNO3/NaCl oxidant and polyethylene glycol (PEG) capped gold nanoparticles (AuNPs) as a novel green route for AuNPs detection in electrochemical biosensors. Anal. Chem. 2018, 90, 4010–4018. [Google Scholar] [CrossRef]
- Ma, E.; Chen, P.; Wilkins, H.M.; Wang, T.; Swerdlow, R.H.; Chen, Q. Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic. Biol. Med. 2017, 113, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Eno, C.O.; Zhao, G.; Venkatanarayan, A.; Wang, B.; Flores, E.R.; Li, C. Noxa couples lysosomal membrane permeabilization and apoptosis during oxidative stress. Free Radic. Biol. Med. 2013, 65, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiser, T.; Ishigaki, M.; van Leer, C.; Matthay, M.A.; Broaddus, V.C. H2O2 inhibits alveolar epithelial wound repair in vitro by induction of apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L448–L453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, J.; M Hofferber, E.; A Stapleton, J.; Iverson, N.M. Hydrogen peroxide sensors for biomedical applications. Chemosensors 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Sawant, V.J.; Sawant, V.J. Biogenic capped selenium nano rods as naked eye and selective hydrogen peroxide spectrometric sensor. Sens. Bio-Sens. Res. 2020, 27, 100314. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B Biointerfaces 2010, 80, 94–102. [Google Scholar] [CrossRef]
- Prasad, K.S.; Vaghasiya, J.V.; Soni, S.; Patel, J.; Patel, R.; Kumari, M.; Jasmani, F.; Selvaraj, K. Microbial selenium nanoparticles (SeNPs) and their application as a sensitive hydrogen peroxide biosensor. Appl. Biochem. Biotechnol. 2015, 177, 1386–1393. [Google Scholar] [CrossRef]
- Dwivedi, S.; Al-Khedhairy, A.; Ahamed, M.; Musarrat, J. Biomimetic synthesis of selenium nanospheres by bacterial strain JS-11 and its role as a biosensor for nanotoxicity assessment: A novel Se-bioassay. PLoS ONE 2013, 8, e57404. [Google Scholar] [CrossRef]
- Cao, H.; Xiao, J.; Liu, H. Enhanced oxidase-like activity of selenium nanoparticles stabilized by chitosan and application in a facile colorimetric assay for mercury (II). Biochem. Eng. J. 2019, 152, 107384. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Zhang, X.; Baeyens, J.; Su, H. Self-assembled selenium nanoparticles and their application in the rapid diagnostic detection of small cell lung cancer biomarkers. Soft Matter 2018, 14, 481–489. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Y.; Yu, Q.; Qin, X.; Yang, L.; Zhou, Y.; Chen, L.; Liu, J. Inhibition of tumor growth and vasculature and fluorescence imaging using functionalized ruthenium-thiol protected selenium nanoparticles. Biomaterials 2014, 35, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, W.; Zhang, Z.; Lin, X.; Lin, H.; Peng, L.; Chen, T. Highly uniform synthesis of selenium nanoparticles with EGFR targeting and tumor microenvironment-responsive ability for simultaneous diagnosis and therapy of nasopharyngeal carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 11177–11193. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, Y.; Cao, C.; Le, F.; Qin, X.; Sun, D.; Liu, J. The use of pH-sensitive functional selenium nanoparticles shows enhanced in vivo VEGF-siRNA silencing and fluorescence imaging. Nanoscale 2014, 6, 9279–9292. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Tran, P.A.; Norello, R.; Simpson, D.A.; O’Connor, A.J.; Tomljenovic-Hanic, S. Intrinsic fluorescence of selenium nanoparticles for cellular imaging applications. Nanoscale 2016, 8, 3376–3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, W.; Hui, D.; Lau, D. Nanoengineering in biomedicine: Current development and future perspectives. Nanotechnol. Rev. 2020, 9, 700–715. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Thorat, N.D.; Chen, G. New advances in pre-clinical diagnostic imaging perspectives of functionalized upconversion nanoparticle-based nanomedicine. Coord. Chem. Rev. 2021, 440, 213971. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wu, J.-Y.; Liu, J.; Xu, W.; Qiu, X.; Huang, S.; Hu, X.-B.; Xiang, D.-X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021, 19, 242. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Guidolin, K.; Zheng, G.; Karpus, A.; Majoral, J.-P. Clinical diagonal translation of nanoparticles: Case studies in dendrimer nanomedicine. J. Control. Release 2021, 337, 356–370. [Google Scholar] [CrossRef]
- Zhu, G.H.; Gray, A.B.; Patra, H.K. Nanomedicine: Controlling nanoparticle clearance for translational success. Trends Pharmacol. Sci. 2022, 43, 709–711. [Google Scholar] [CrossRef]
- Murday, J.S.; Siegel, R.W.; Stein, J.; Wright, J.F. Translational nanomedicine: Status assessment and opportunities. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 251–273. [Google Scholar] [CrossRef]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine-Challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Van Der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Nishiyama, N.; Kataoka, K. Supramolecular nanodevices: From design validation to theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirshafiee, V.; Jiang, W.; Sun, B.; Wang, X.; Xia, T. Facilitating translational nanomedicine via predictive safety assessment. Mol. Ther. 2017, 25, 1522–1530. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Xu, H. Selenium-containing nanomaterials for cancer treatment. Cell Rep. Phys. Sci. 2020, 1, 100111. [Google Scholar] [CrossRef]
- Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent advancements of nanomedicine towards antiangiogenic therapy in cancer. Int. J. Mol. Sci. 2020, 21, 455. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the nanoparticle–protein corona on colloidal stability and protein structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J.; et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: Where are we now, and where we are going. J. Neurooncol. 2020, 147, 261–278. [Google Scholar] [CrossRef]
- Omidi, Y.; Kianinejad, N.; Kwon, Y.; Omidian, H. Drug delivery and targeting to brain tumors: Considerations for crossing the blood-brain barrier. Expert Rev. Clin. Pharmacol. 2021, 14, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Jiang, C. Evolution of blood–brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, Y.; Zhu, X.; Hu, X.; Chen, T. Efficient overcoming of blood–brain barrier by functionalized selenium nanoparticles to treat glioma. Adv. Ther. 2018, 1, 1800074. [Google Scholar] [CrossRef]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Sharifi, E.; Bakhshi, N.; Ramzanpoor, M. Biogenic silver nanoparticles using Oxalis corniculata characterization and their clinical implications. J. Drug Deliv. Sci. Technol. 2019, 54, 101263. [Google Scholar] [CrossRef]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostruct. Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef] [Green Version]
- Hosny, M.; Fawzy, M. Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv. Powder Technol. 2021, 32, 2891–2904. [Google Scholar] [CrossRef]
- Marouzi, S.; Sabouri, Z.; Darroudi, M. Greener synthesis and medical applications of metal oxide nanoparticles. Ceram. Int. 2021, 47, 19632–19650. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Divya, M.; Vaseeharan, B.; Ranjan, S.; Kalaiselvi, V.; Dasgupta, N.; Chen, J.; Durán-Lara, E.F. Biogenic preparation and characterization of ZnO nanoparticles from natural polysaccharide Azadirachta indica L. (neem gum) and its clinical implications. J. Cluster Sci. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Fierascu, I.; Fierascu, I.C.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C. Phytosynthesized metallic nanoparticles-between nanomedicine and toxicology. A brief review of 2019’s findings. Materials 2020, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Raza, A.; Islam, N.U.; Ayaz, M.; Saravanan, M.; Ali, M.; Ahmad, I.; Shahid, M.; Shinwari, Z.K. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Schomburg, L.; Buentzel, J.; Kisters, K.; Muecke, R. Selenium in oncology: From chemistry to clinics. Molecules 2009, 14, 3975–3988. [Google Scholar] [CrossRef]
- Gabizon, A.A.; De Rosales, R.T.M.; La-Beck, N.M. Translational considerations in nanomedicine: The oncology perspective. Adv. Drug Deliv. Rev. 2020, 158, 140–157. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Current trends of nnobiosensors for point-of-care diagnostics. J. Anal. Method Chem. 2019, 2019, 2179718. [Google Scholar] [CrossRef] [PubMed]
- Bellah, M.; Christensen, S.M.; Iqbal, S. Nanostructures for medical diagnostics. J. Nanomater. 2012, 2012, 486301. [Google Scholar] [CrossRef]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]












| Selenium Compound | Type |
|---|---|
| Selenoamino acids | Selenomethionine (SeMet) |
| Methyl selenocysteine (MeSeCys) | |
| Selenocysteine (SeCys) | |
| Selenocystamine | |
| Se-heterocyclic compounds | 1,3-Selenazolin-4-one derivatives |
| 2-Phenyl-1,2-benzisoselenazol-3(2H)-one (ebselen) | |
| 2,5-Bis(5-hydroxymethyl-2-selenienyl)-3-hydroxymethyl-N-methylpyrrole (D-501036) | |
| 1,2-[Bis(1,2-benzisoselenazolone-3-(2H)-ketone)] ethane (BBSKE) | |
| 2-Cyclohexylselenazolidine-4-(R)-carboxylic acid (ChSCA) | |
| 2-Buthylselenazolidine-4-(R)-carboxylic acid (BSCA) | |
| Selenocyanates | Isatin analogs |
| Diphenylmethylselenocyanate | |
| 1-4-Phenylenebis(methylene) selenocyanate (p-XSC) | |
| Temozolomide (TMZ)-Se | |
| 5-Phenylcarbamoylpentyl selenocyanide (SelSA-2) | |
| Selenides | Methylimidoselenocarbamates |
| 5-Phenylselenyl-methyl-2′-deoxyuridine (PhSe-T) | |
| 5-Methylselenyl-methyl-2′-deoxyuridine (MeSe-T) | |
| β-Selenium amine derivatives | |
| Se,Se’-1,4-phenylenebis(1,2-ethanediyl) bisisoselenourea (PBISe) | |
| Di-selenides | Bis(4-aminophenyl) diselenide |
| Bis(5-phenylcarbamoylpentyl) diselenide (SelSA-1) | |
| Diselenodipropio nic acid (DSePA) | |
| 2-Selenium-bridged β-cyclodextrin (2-SeCD) | |
| Se(IV) compounds | Sodium selenite (Na2SeO3) |
| Selenous acid (H2SeO3) | |
| Methyl selenic acid (MeSeA) | |
| Selenium dioxide (SeO2) |
| Selenoproteins | Abbreviation | Localization | Function | Ref. |
|---|---|---|---|---|
| Glutathione peroxidase | GPX | Protection against oxidative stress. Catalytic reduction of H2O2 | [75,246] | |
| Cytosolic GPX1 | GPX1 | Cytoplasm, ubiquitous | Antioxidative defense | |
| Extracellular GPX | GPX3 | Plasma and thyroid follicle | Anti-inflammatory activity | |
| Phospholipid GPX | GPX4 | Mitochondrial membrane | Reduction of the phospholipid hydroperoxides. Membrane antioxidant | |
| Thioredoxin reductase | TrxR | Oxidoreductase activity with NADPH as the cofactor | [244] | |
| Cytosolic TrxR | TrxR1 | Mainly cytosolic, ubiquitous | Main antioxidant “weapon” at the cellular level. Inhibition of apoptosis and redox state of transcription factors | |
| Mitochondrial TrxR | TrxR2 | Mitochondrial, ubiquitous | Regulation of cell proliferation | |
| Mitochondrial TrxR | TrxR3 | Mainly mitochondrial, ubiquitous | Regulation of apoptosis and signaling pathway | |
| Iodothyronine deiodinase | DIO | Catalytic conversion of T4 and T3 | [247] | |
| Type I DIO | DIO1 | Liver, lung, eyes, kidney, thyroid, pituitary, CNS * | Conversion of T4 into T3, T4 into rT3, and of T3 into T2 | |
| Type II DIO | DIO2 | Local (intracellular) production of T3 from T4 and T2 from rT3 | ||
| Type III DIO | DIO3 | Placenta, fetus, liver, gravid uterus, fetal and neonatal brain, skin | Production of T2 from T4 and rT3 from T4 | |
| Selenoprotein P | SePP | Thyroid and blood | Selenium transport and storage, endothelial antioxidant | [248] |
| Biological System Used for Synthesis | Shape and Size of SeNPs | Antioxidant Measurement Technique | Antioxidant Activity | IC50 (μg/mL) | EC50 (μg/mL) | Ref. |
|---|---|---|---|---|---|---|
| Bacillus sp. MSh-1. | Spherical; 80–220 nm | DPPH and reducing power assays | RSA of 23.1 ± 3.4% Dose-dependent reducing power within a 0–200 μg·mL−1 concentration range | 41.5 ± 0.9 | N/A | [260] |
| Saccharomyces cerevisiae | Spherical; 50 nm | DPPH | RSA increase of 21.7–48.5% in a dose-dependent manner | N/A | N/A | [150] |
| Bacillus sp. EKT1 | Spherical; 31–335 nm | DPPH | RSA of up to 56.5 ± 5.0% at 400 μg·mL−1 | 322.8 | N/A | [493] |
| Quercetin and gallic acid | Bimetallic Ag-Se NPs capped by flavonoids and phenolics; 30–35 nm | ABTS, DPPH and MTT assays | 59–62% T-AOC | 30–66 | N/A | [495] |
| Aqueous chitosan microspheres | Spherical; 36–95 nm | H2O2 levels; measurement of GSH, TBARS (MDA equivalent), GSH-Px, SOD and CAT | Increase in both intracorporeal Se retention and levels of GSH-Px, SOD and CAT; reduced levels of TBARS | N/A | N/A | [129] |
| Emblica officinalis extract | Spherical; 15–40 nm | DPPH and ABTS radical scavenging assays | Dose-dependent RSA, linear relationship with NP concentration | 127.28 ± 3.73 | DPPH: 15.67 ± 1.41 mg·mL−1 ABTS: 18.84 ± 1.02 mg·mL−1 | [155] |
| Lactobacillus casei ATCC 393 | 50–80 nm | Cellular methods: T-AOC MDA T-SOD GSH-Px levels TrxR | Increased T-AOC, T-SOD, TrxR and GSH-Px Reduced MDA levels in serum and jejunum | N/A | N/A | [496] |
| Pantoea agglomerans | Spherical; 30–300 nm | Production of ROS using HUVEC fluorescence determined using a microplate reader at 485-nm excitation and 583-nm emission | Decrease in fluorescence resulting from the oxidation of the intracellular probe dichlorofluorescein (DCF) | N/A | N/A | [262] |
| L. casei ATCC 393 | Spherical; capped with proteins and polysaccharides; 50–80 nm | H2O2 levels | Increased GPX activity, reduced MDA | N/A | N/A | [497] |
| H2O2-induced oxidative damage model of human colon mucosal epithelial cells | Alleviated increase in ROS, reduced ATP and MMP Improved levels of Nrf2, HO-1, and NQO-1 proteins | N/A | N/A | [498] | ||
| L. lactis NZ9000 | Spherical; 38–152 nm | H2O2 levels measurement; MDA T-SOD GPX | Alleviated IPEC-J2 cell oxidative damage caused by H2O2 Inhibition of intracellular ROS production | N/A | N/A | [499] |
| Cordyceps sinensis EPS conjugation | Amorphous and monoclinic; 80–125 nm. | ABTS and superoxide anion radical (O2) scavenging assays | Smaller SeNPs present high O2 scavenging ability. Se/P ratios (1:3, 1:1 and 4:3) had a higher ABTS•+ scavenging ability, and could reach 88.89%, 85.53% and 69.88%, respectively, at 0.2 mg·mL−1. | N/A | N/A | [148] |
| Ephedra aphylla extract | Spherical and tetragonal; 13.95–26.26 nm | DPPH assay | Lower activity of SeNPs a than the plant extract | 0.213 and 0.296 mg·mL−1 | N/A | [491] |
| Green tea extract and Lycium barbarum polysaccharides | Spherical and triangular; 83–160 nm | DPPH and ABTS assays | Strong, concentration-dependent DPPH-scavenging activity at 5–25 µM High antioxidant activity with low EC50 Dose-dependent inhibition of ABTS free radicals | N/A | 22 µM | [500] |
| Theobroma cacao L. bean shell extract | Spherical; 1–3 nm | ABTS and FRAP assays | ABTS: 28.6 ± 0.1 mg TE/g FRAP: 12.4 ± 0.2 mg TE/g | N/A | N/A | [487] |
| Chitosan | Spherical; 102–104 nm | DPPH, ABTS and superoxide anion radical (O2) scavenging assays | DPPH: 83.06% at 0.5 mM ABTS: 74.33, 80.23 and 81.99% at 2 mM Superoxide: 25.20, 27.54, 31.44% at 1 mM | DPPH: 0.296, 0.306, 0.325, 0.370 mM ABTS: 1.314, 1.249, 1.143 and 1.101 mM | N/A | [489] |
| Streptomyces minutiscleroticus | Spherical; 100–250 nm | DPPH; Reducing power assay; T-AOC: Phosphomolybdenum method | All measurements increase in a dose-dependent manner T-AOC was more or less equal to the standard ascorbic acid | N/A | N/A | [316] |
| Withania somnifera | Spherical; 45–90 nm | DPPH | RSA increase in a dose-dependent manner in the range of 20–100 mg·mL−1 * | 14.81 μg·mg−1 | N/A | [160] |
| Diospyros montana leaf extract | Spherical; 4–16 nm | DPPH and FRAP assays | DPPH: color change from purple to pale yellow. RSA of 61.12% at 200 μg·mL−1 FRAP: color change from yellow to shades of green and blue | 0.225 | 0.435 | [490] |
| Corbicula fluminea | Spherical; 40–70 nm | DPPH, TEAC and FRAP of plasma assays. | DPPH RSA: 70, 77, 83, 79, and 53% at 1.5 mg·mL−1 *. Increase in a dose-dependent manner TEAC: highest RSA at 226 μmol Trolox/g sample FRAP: highest RSA at 150 μmol Fe2+/g sample | 1.5 mg·mL−1 * | N/A | [488] |
| Murraya koenigii | Spherical; 50–150 nm | DPPH and Superoxide anion (O2) scavenging assay | Concentration-dependent RSA increase | 25 and 50 | N/A | [109] |
| Ginger plant (Zingiber officinale) extract | Spherical; 100–150 nm | DPPH | Dose-dependent RSA increase (disappearance of the purple color) SeNPs are free radical inhibitors or scavengers acting possibly as primary antioxidants | 125 | N/A | [501] |
| Biological System/Green Method | Shape and Size (nm) | Concentration/Dosage | Assay/Pathway | Cell Line * | Key Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Streptomyces bikiniensis | Nanorods; 17 nm | 10, 25, 50, and 100 μg·mL−1 | MTT dye reduction assay | Hep-G2 (M) MCF-7 (M) | ID50%: 75.96 and 61.86 μg·mL−1, respectively. Loss of cell-to-cell contact, cell shrinkage, and formation of apoptotic bodies. Higher reduction of cell viability in Hep-G2 (42.3–86.9%) than in MCF-7 (37.5–69.1%). | [521] |
| Cassia auriculata leaf extract | 10–20 nm | 0.5 to 150 μg·mL −1 | MTT assay | HL-60 (M) Vero cell line (NM) | Antileukemia activity in a dose-dependent manner with a CC50: 7.01 μg·mL−1 (HL-60) and 109.13 μg·mL−1 (Vero cells) | [162] |
| Garlic (Allium sativum) clove extract | Spherical; 40–100 nm | 15, 30, 60 and 90 μg·mL−1 | MTT assay | Vero cell line (NM) | CC50 of 31.8 ± 0.6 μg·mL−1 | [522] |
| Chitosan (CTS-SeNPs) and Pleurotus ostreatus fermented fenugreek (SeNPs-AEFFP) | Spherical; CTS-SeNPs: 45 nm SeNPs-AEFFP: 11.8 nm | CTS-SeNPs: 1.187–38 μg·mL−1 SeNPs-AEFFP: 0.594–19 μg·mL−1 NPs exposed to γ-ray doses of 60 and 15 kGy, respectively, against EaC and CaCo-2. | Trypan blue (0.5%) assay | EaC (M) CaCo-2 (M) | For EaC: CTS-SeNPs: IC50 = 23.12% SeNPs-AEFFP: IC50 = 7.21% For CaCo-2: CTS-SeNPs: IC50 = 25.32% SeNPs-AEFFP: IC50 = 8.57% SeNPs exhibit a concentration-dependent repression against EaC and CaCo-2 and a selective cytotoxic effect. | [112] |
| E. coli | Spherical, elliptical and nanorods; 60 nm | 20, 60, and 100 μg·mL−1 | MTT and addition of DMSO. Caspase 3-involved apoptosis pathway. | A549 (M) IMR-90 (NM) | Cell viability of ∼70%, ∼45% and ∼25%, high ROS generation and elevated caspase-3 activity. | [523] |
| B. licheniformis JS2 | Spherical; 110 nm | 1, 2, 4, 6, 50, or 200 μg Se/mL for 24 h | Colorimetric XTT assay, activation of caspases 3 and 7, DMSO treatment and hemolysis assays | LNCaP-FGC (M) | Overexpression of TNF and IRF1, reducing the expression of androgen receptors. SeNPs decrease the cell viability regardless of apoptosis and necrosis. SeNPs induce cell death through neither apoptosis nor necrosis. | [524] |
| S. minutiscleroticus M10A62 | Spherical; 10–250 nm | 50–100 μg·mL−1 | MTT | Hep-G2 (M) HeLa (NM) | 50 μg·mL−1 concentration of SeNPs was required for 99.5% HepG2 growth inhibition and 100 μg·mL−1 for 100% growth inhibition of HeLa. | [316] |
| B. licheniformis JS2 | 110 nm | Minimum of 2 μg Se/mL | Real-time qPCR analysis; confocal microscopy; treatment with cytochalasin D | PC-3 (M) | ROS mediated necroptosis of PC-3 cells independent of RIP3 and MLKL and regulated by a RIP1 kinase. Increased expression of necroptosis associated to TNF and IRF1 | [295] |
| Fenugreek seed extract | Amorphous; 50–150 nm | 25, 50, 75, and 100 μg·mL−1 for 24 h | MTT assay | MCF-7 (M) | SeNPs augment the cytotoxicity of doxorubicin and induce MCF 7 cell death through apoptosis. | [525] |
| Idiomarina sp. PR58-8 | Spherical; 150–350 nm | 5–100 μg·mL−1 for 24 h | MTT assay, ROS assay, apoptotic index assay, Western blot assay. | HaCaT (NM) HeLa cells (M) | Caspase-dependent apoptosis in HeLa cell lines: decrease in expression of pro-caspase 3. SeNPs exhibited dose-dependent cytotoxicity with only 3% viability at 100 μg·mL−1. | [526] |
| Acinetobacter sp. SW30 | Nanospheres and crystalline nanorods of 78 nm. Polygonal-shaped SeNPs of 79 nm. | 0–100 μg·mL−1 | MTT assay | 4T1 (M) MCF-7 (M) NIH/3T3 (NM) HEK293 (NM) | Antiproliferative activity. | [527] |
| Bacillus sp. MSh-1 | Spherical; 80–220 nm | 10, 20, 50 and 100 μg·mL−1 | MTT assay and gelatin zymography | HT-1080 (NM) | SeNP dose of 100 μg·mL−1 decreases the viability of the cell line to 50%, whereas a lower dose (10 μg·mL−1) shows a low level of cytotoxicity with a viability of more than 80%. | [528] |
| Asteriscus graveolens extract | Spherical; 20 nm | 25–125 mg·mL–1 ‡ for 24 h | MTT assay, flow cytometry analysis, measurement of ROS (conversion of DCFH-DA to DCFH); measurement of MMP and lipid peroxidation | HepG2 (M) | Cell viability (IC50): 51.8% at 3.98 μg·mL−1. SeNPs inhibit the growth of HepG2 cells mainly by induction of apoptosis. They also significantly and rapidly increase the ROS level. | [285] |
| Moringa oleifera extract | Spherical; 23–35 nm Polygonal; 25–45 nm | N/A | MTT assay | CaCo-2 (M) HepG2 (M) MCF-7 (M) | IC50: 50.3% at 392.57 μg·mL−1 | [156] |
| Penicillium corylophilum | Spherical; 29.1–48.9 nm | 1000, 500, 250, 125, 62.5 and 31.25 ppm incubated in 5% CO2 at 37 °C for 24 h | MTT assay | WI-38 (NM) CaCo-2 (M) | IC50: 171.8 ppm (Wi 38) and 104.3 ppm (CaCo-2) | [502] |
| Hawthorn fruit extract | 113 nm | 0, 5, 10 and 20 μg·mL−1 for 24 h | MTT assay; flow cytometric analysis; ROS detection; MMP measurement; Western blot assay | HepG2 (M) | IC50: 19.22 ± 5.3 μg·mL−1 | [529] |
| L. casei 393 | Spherical; 50–80 nm | 4, 8, and 16 μg·mL−1 for 12 h | RT-PCR; mRNA expression levels of Bax, caspase 3, p53 and bcl-2. | HepG2 (M) NCM460 (NM) | Endocytosis of SeNPs induces cell death by reducing the viability of HepG2, increasing mRNA levels of caspase 3, Bax, and p53, and reducing mRNA expression of bcl-2. | [497] |
| Carica papaya latex | Spherical; 70 nm | 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 μg·mL−1 for 48 h | MTT assay | HBL100 (NM) MDA-MB-231 (M) | IC50 (HBL100): 50 μg·mL−1 IC50 (MDA-MB-231): 34 μg·mL−1 | [530] |
| Spermacoce hispida aqueous leaf extract (Sh-SeNPs) + S-allyl glutathione conjugation (SAG-Sh-SeNPs) | Sh-SeNPs: aggregation SAG-Sh-SeNPs: spherical; 50 nm | HepG2: 1.88, 3.75, 7.50, 15.00 and 30.00 μg·mL−1 for 24 h Vero cells: 3.7–60.0 μg·mL−1 for 48 h | MTT assay; determination of intracellular ROS production and MMP; cell cycle analysis by flow cytometry; DNA fragmentation assay; apoptosis determination by acridine orange/ethidium bromide staining | HepG2 (M) Vero cells (NM) | IC50 (Sh-SeNPs): 30.0 μg·mL−1 IC50 (SAG-Sh-SeNPs): 18.7 μg·mL−1 SAG-Sh-SeNPs induce cell cycle arrest at sub-G1 phase and further lead to apoptosis. The NPs increase ROS levels, disrupt MMP, initiate DNA fragmentation and decrease the endogenous levels of antioxidants, such as GSH, superoxide dismutase, catalase and GSH peroxidase. | [531] |
| Undaria pinnatifida polysaccharides | Spherical; 44–92 nm (average of 59 nm) | N/A | MTT assay; flow cytometry analysis; Annexin-V-FLUOS staining assay; measurement of ROS levels; MMP evaluation | A375 (M) CNE2 (M) Hep G2 (M) MCF-7 (M) | IC50 values ranging from 3.0 to 14.1 μM. Apoptosis with the involvement of oxidative stress and mitochondrial dysfunction. | [532] |
| Ceropegia bulbosa tuber’s aqueous extracts | Spherical; 55.9 nm | 0, 5, 10.0, 15.0, 20.0, 25.0, 30.0, 35.0, 40.0, 45.0, 50.0 μg·mL−1 | MTT assay | MDA-MB-231 (M) HBL-100 (M) | IC50 (MDA-MB-231): 34 μg·mL−1 for 48 h. IC50 (HBL-100): more than 50 μg·mL−1 for 48 h. | [506] |
| Diospyros montana leaf extract | 4–16 nm | 50, 150, 250, 350 μg·mL−1 | MTT assay | MCF-7 (M) | IC50: 80.83 μg·mL−1, SeNPs enhance the cytotoxicity. | [490] |
| Chitosan decoration | Spherical; 50 nm | 10, 50, 100 μM | WST-1 assay | HepG2 (M) | SeNPs decrease the cell viability to 76.63, 63.31 and 56.34% and inhibit the growth of HepG2 cells in a time- and dose-dependent manner. | [489] |
| Ephedra aphylla extract | Spherical and tetragonal; 13.95–26.26 nm | N/A | MTT assay | HepG-2 (M) MCF-7 (M) HCT-116 (M) HeLa (M) PC3 (M) HEp2 (M) | IC50 (HePG-2): 7.56 ± 0.60 μg·mL−1 IC50 (MCF-7): 15.65 ± 1.40 μg·mL−1 IC50 (HCT-116): 10.02 ± 0.90 μg·mL−1 IC50 (HeLa): 9.23 ± 0.80 μg·mL−1 IC50 (PC3): 18.63 ± 1.50 μg·mL−1 IC50 (HEp2): 12.10 ± 1.20 μg·mL−1 | [491] |
| Lentinan (LNT, denatured β-glucan) | Spherical; 28 nm | N/A | MTT assay | HeLa (M) | IC50 of three complexes Se/s-LNT-1, Se/s-LNT-2, Se/s-LNT-3 were estimated to be 85, 37, and 19 μM. SeNPs with small, uniform size greatly enhanced the antitumor activity and bioavailability. | [533] |
| Quercetin and gallic acid | Bimetallic Ag-Se NPs of 30–35 nm and capped by flavonoids and phenolics. | 50, 100, 250 and 500 μg·mL−1 | MTT assay | DL (M) | The viability of DL cells was 20% at 50 μg·mL−1 of Ag-SeNPs, while at 100 μg·mL−1, it was reduced to 15%. The Ag-SeNPs showed strong anticancer activity at a lower concentration. | [495] |
| Cationic pullulan (CP) | Spherical and microflowers; 50 nm | N/A | MTT assay; Annexin-V-FITC and propidium iodide (PI) staining | L929 (M) KB (M) | IC50: 0.060 μM. The early- and late-stage apoptotic rates of KB cells treated with doxorubicin only reached 0.52% and 4.64%, respectively, and the highest induction of 55.8% was arrested in the necrosis rate. | [534] |
| Walnut peptides | Spherical; 89.22 nm | 200 μL·mL−1 | MTT assay; POM, flow cytometry; MMP assay; nuclear morphology analysis by Hoechst 33258; measurement of ROS production; DNA fragmentation assay; caspase activity assay; Western blot assay | HL-7702 (L02) (M) MCF-7 (M) SGC-7901 (M) A549 (M) PC3 (M) HeLa (M) | MCF-7 cells were the most sensitive to SeNPs. The apoptosis-inducing activity was proved by the accumulation of S-phase cell arrest, nuclear condensation, and DNA breakage. The intrinsic signal pathway was through the activation of FADD and caspases 3, 8, and 9, in combination with the MMP depletion and ROS generation. | [535] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambonino, M.C.; Quizhpe, E.M.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine. Nanomaterials 2023, 13, 424. https://doi.org/10.3390/nano13030424
Zambonino MC, Quizhpe EM, Mouheb L, Rahman A, Agathos SN, Dahoumane SA. Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine. Nanomaterials. 2023; 13(3):424. https://doi.org/10.3390/nano13030424
Chicago/Turabian StyleZambonino, Marjorie C., Ernesto Mateo Quizhpe, Lynda Mouheb, Ashiqur Rahman, Spiros N. Agathos, and Si Amar Dahoumane. 2023. "Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine" Nanomaterials 13, no. 3: 424. https://doi.org/10.3390/nano13030424