The Influences of Pore Blockage by Natural Organic Matter and Pore Dimension Tuning on Pharmaceutical Adsorption onto GO-Fe3O4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GO-Fe3O4 Synthesis
2.3. NOM Fractionation
2.4. Characterization
2.5. Adsorption Experiment
2.6. Pharmaceutical Analysis
2.7. Adsorption Isotherm and Kinetics
3. Results and Discussion
3.1. Surface Characterization of GO-Fe3O4
3.2. Pore Blockage Effect of NOM
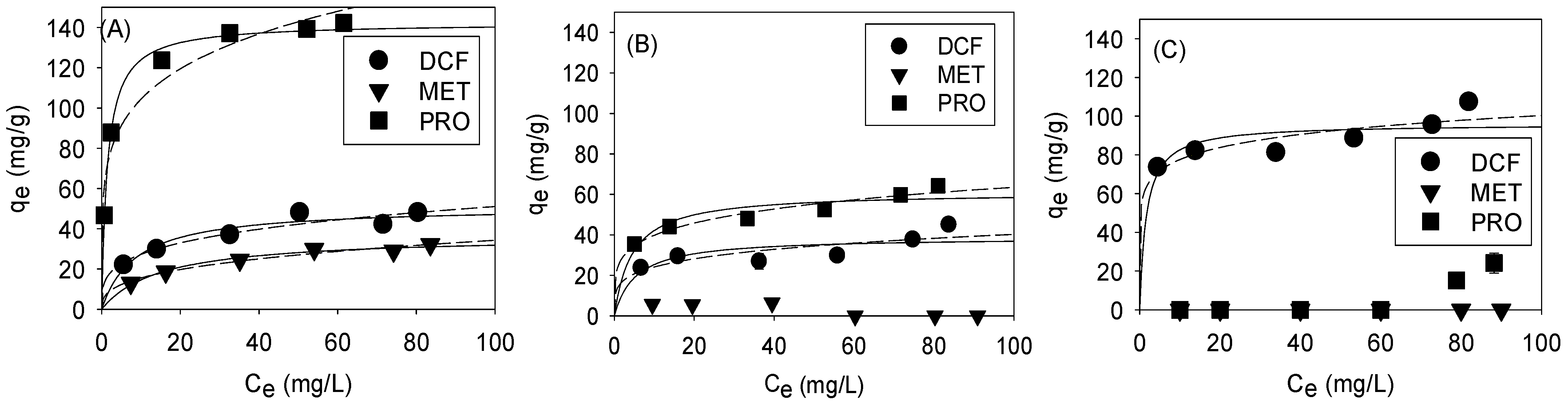
3.3. Selective Adsorption by Pore Size Tuning: Isotherm
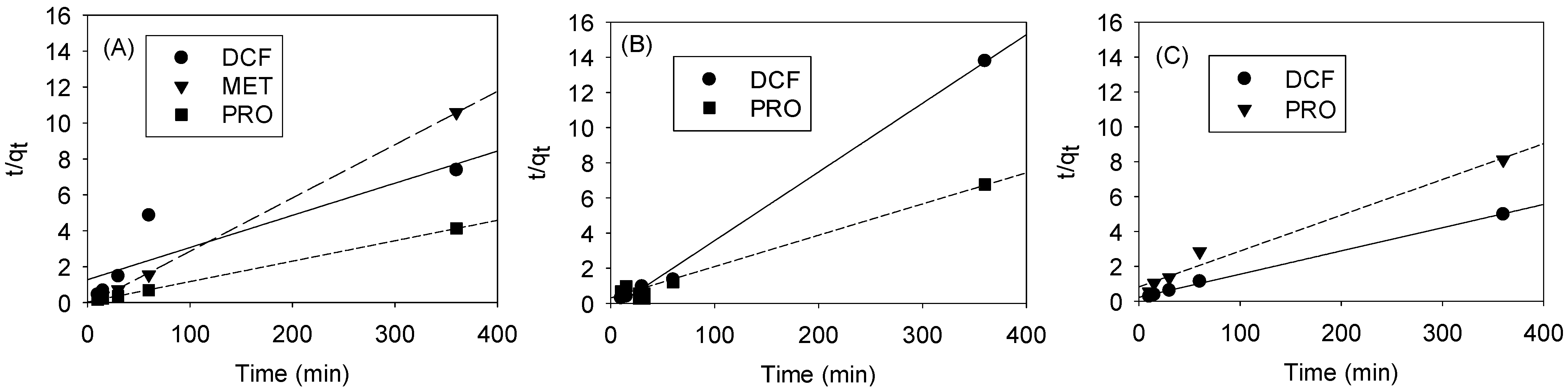
3.4. Selective Adsorption by Pore Size Tuning: Kinetics
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Su, C.C.; Cada, C.A.; Dalida, M.L.P.; Lu, M.C. Effect of UV light on acetaminophen degradation in the electro-Fenton process. Sep. Purif. Technol. 2013, 120, 43–51. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef]
- Roberts, J.; Kumar, A.; Du, J.; Hepplewhite, C.; Ellis, D.J.; Christy, A.G.; Beavis, S.G. Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Sci. Total Environ. 2016, 541, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-C.; Yang, R.-Y.; Chen, G.-F.; Chen, W.-H. The effect of metformin and drinking water quality variation on haloacetamide formation during chlor(am)ination of acetaminophen. J. Environ. Manag. 2023, 335, 117603. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, Y.H.; Hsu, T.H. The competitive effect of different chlorination disinfection methods and additional inorganic nitrogen on nitrosamine formation from aromatic and heterocyclic amine-containing pharmaceuticals. Chemosphere 2021, 267, 128922. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current research trends on emerging contaminants pharmaceutical and personal care products (PPCPs): A comprehensive review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef] [PubMed]
- Surana, D.; Gupta, J.; Sharma, S.; Kumar, S.; Ghosh, P. A review on advances in removal of endocrine disrupting compounds from aquatic matrices: Future perspectives on utilization of agri-waste based adsorbents. Sci. Total Environ. 2022, 826, 154129. [Google Scholar] [CrossRef]
- O’Connor, J.; Bolan, N.S.; Kumar, M.; Nitai, A.S.; Ahmed, M.B.; Bolan, S.S.; Vithanage, M.; Rinklebe, J.; Mukhopadhyay, R.; Srivastava, P.; et al. Distribution, transformation and remediation of poly- and per-fluoroalkyl substances (PFAS) in wastewater sources. Process Saf. Environ. Prot. 2022, 164, 91–108. [Google Scholar] [CrossRef]
- Ussery, E.; Bridges, K.N.; Pandelides, Z.; Kirkwood, A.E.; Bonetta, D.; Venables, B.J.; Guchardi, J.; Holdway, D. Effects of environmentally relevant metformin exposure on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2018, 205, 58–65. [Google Scholar] [CrossRef]
- Kong, L.; Kadokami, K.; Wang, S.; Duong, H.T.; Chau, H.T.C. Monitoring of 1300 organic micro-pollutants in surface waters from Tianjin, North China. Chemosphere 2015, 122, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yu, Y.; Men, Y. Emerging investigators series: Occurrence and fate of emerging organic contaminants in wastewater treatment plants with an enhanced nitrification step. Environ. Sci. Water Res. Technol. 2018, 4, 1412–1426. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Hlengwa, N.; Mahlambi, P. SPE-LC-PDA method development and application for the analysis of selected pharmaceuticals in river and wastewater samples from South Africa. Water SA 2020, 46, 514–522. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Di Cicco, M.; Di Censo, D.; Galante, A.; Boscaro, F.; Messana, G.; Galassi, D.M.P. Environmental risk assessment of propranolol in the groundwater bodies of Europe. Environ. Pollut. 2019, 255, 113189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.N.; Shen, Y.T.; Wang, H.X.; Wang, C.H.; Ye, X.X.; Xiang, Z. Determination of kaurenoic acid in rat plasma using UPLC-MS/MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 164, 27–31. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Z.; Zhang, L.; Yoshimura, C.; Ye, Z.; Yu, P.; Qian, Y.; Hatano, Y.; Wang, J.; Niu, J. Photodegradation of propranolol in surface waters: An important role of carbonate radical and enhancing toxicity phenomenon. Chemosphere 2022, 297, 134106. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, Z.J.; Ji, W.L.; Liu, D.Y.; Zhang, H.; Li, A.M.; Huo, Z.L.; Zhou, Q. An efficient resin for solid-phase extraction and determination by UPLCMS/MS of 44 pharmaceutical personal care products in environmental waters. Front. Environ. Sci. Eng. 2020, 14, 51. [Google Scholar] [CrossRef]
- Sood, A.K.; Lund, I.; Puri, Y.R.; Efstathiadis, H.; Haldar, P.; Dhar, N.K.; Lewis, J.; Dubey, M.; Zakar, E.; Wijewarnasuriya, P. Review of Graphene Technology and Its Applications for Electronic Devices; InTech: London, UK, 2015. [Google Scholar]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar]
- Li, C.M.; Chen, C.H.; Chen, W.H. Different influences of nanopore dimension and pH between chlorpheniramine adsorptions on graphene oxide-iron oxide suspension and particle. Chem. Eng. J. 2017, 307, 447–455. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, C.M.; Chen, C.H.; Chen, W.H. Removal of chlorpheniramine and variations of nitrosamine formation potentials in municipal wastewaters by adsorption onto the GO-Fe3O4. Environ. Sci. Pollut. Res. 2019, 26, 20701–20711. [Google Scholar] [CrossRef]
- Tishbi, P.; Mosayebi, M.; Salehi, Z.; Fatemi, S.; Faegh, E. Synthesizing magnetic graphene oxide nanomaterial (GO-Fe3O4) and kinetic modelling of methylene blue adsorption from water. Can. J. Chem. Eng. 2022, 100, 3321–3334. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, J.-R.; Lin, C.-H.; Huang, C.-P. Catalytic degradation of chlorpheniramine over GO-Fe3O4 in the presence of H2O2 in water: The synergistic effect of adsorption. Sci. Total Environ. 2020, 736, 139468. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, C.; Zhao, G.; Yang, X.; Li, J.; Wang, X. Removal of Cu(II) and Fulvic Acid by Graphene Oxide Nanosheets Decorated with Fe3O4 Nanoparticles. Acs Appl. Mater. Interfaces 2012, 4, 4991–5000. [Google Scholar] [CrossRef]
- Fan, L.L.; Luo, C.N.; Sun, M.; Qiu, H.M.; Li, X.J. Synthesis of magnetic beta-cyclodextrin-chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B-Biointerfaces 2013, 103, 601–607. [Google Scholar] [CrossRef]
- Liakos, E.V.; Gkika, D.A.; Mitropoulos, A.C.; Matis, K.A.; Kyzas, G.Z. On the combination of modern sorbents with cost analysis: A review. J. Mol. Struct. 2020, 1229, 129841. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
- Chen, W.H.; Wong, Y.T.; Huang, T.H.; Lin, J.G. Removals of pharmaceuticals in municipal wastewater using a staged anaerobic fluidized membrane bioreactor. Int. Biodeterior. Biodegrad. 2019, 140, 29–36. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.H.; LeBoeuf, E.J.; Pan, H.J.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, W.H. Influence of water, H2O2, H2SO4, and NaOH filtration on the surface characteristics of a graphene oxide-iron (GO-Fe) membrane. Sep. Purif. Technol. 2021, 262, 118317. [Google Scholar] [CrossRef]
- Li, Z.X.; Shakiba, S.; Deng, N.; Chen, J.W.; Louie, S.M.; Hu, Y.D. Natural Organic Matter (NOM) Imparts Molecular-Weight-Dependent Steric Stabilization or Electrostatic Destabilization to Ferrihydrite Nanoparticles. Environ. Sci. Technol. 2020, 54, 6761–6770. [Google Scholar] [CrossRef] [PubMed]
- Marais, S.S.; Ncube, E.J.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. Assessment of trihalomethane (THM) precursors using specific ultraviolet absorbance (SUVA) and molecular size distribution (MSD). J. Water Process Eng. 2019, 27, 143–151. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Pollard, A.J.; Losic, D. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon 2021, 179, 505–513. [Google Scholar] [CrossRef]
- Kar, K. Handbook of Fly Ash; Elsevier: Amsterdam, The Netherlands, 2022; p. 841. [Google Scholar]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pK(a) Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, R.Q.; Wang, J.P. A Mild Method for Preparation of Highly Selective Magnetic Biochar Microspheres. Int. J. Mol. Sci. 2020, 21, 3752. [Google Scholar] [CrossRef]
- Alizadeh, A.; Khodaei, M.M.; Beygzadeh, M.; Kordestani, D.; Feyzi, M. Biguanide-Functionalized Fe3O4/SiO2 Magnetic Nanoparticles: An Efficient Heterogeneous Organosuperbase Catalyst for Various Organic Transformations in Aqueous Media. Bull. Korean Chem. Soc. 2012, 33, 2546–2552. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, D.-D.; Li, L.; Li, W.-W.; Mu, Y.; Yu, H.-Q. Role of NOM molecular size on iodo-trihalomethane formation during chlorination and chloramination. Water Res. 2016, 102, 533–541. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Yang, H.; Li, H.; Li, A.; Cheng, R. Flocculation performance and mechanism of graphene oxide for removal of various contaminants from water. Water Res. 2013, 47, 3037–3046. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Snoeyink, V.L.; Mariñas, B.J.; Campos, C. Pore blockage effect of NOM on atrazine adsorption kinetics of PAC: The roles of PAC pore size distribution and NOM molecular weight. Water Res. 2003, 37, 4863–4872. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Goyne, K.W.; Chorover, J.; Komarneni, S.; Brantley, S.L. Mineral mesopore effects on nitrogenous organic matter adsorption. Org. Geochem. 2004, 35, 355–375. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Aniagor, C.O.; Oba, S.N.; Yap, P.-S.; Iwuchukwu, F.U.; Liu, T.; de Souza, E.C.; Ighalo, J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 104, 117–135. [Google Scholar] [CrossRef]
- Desai, D.; Wong, B.; Huang, Y.; Ye, Q.; Tang, D.; Guo, H.; Huang, M.; Timmins, P. Surfactant-mediated dissolution of metformin hydrochloride tablets: Wetting effects versus ion pairs diffusivity. J. Pharm. Sci. 2014, 103, 920–926. [Google Scholar] [CrossRef]
- Kumari, K.S.; Bandhakavi, S. Development and validation of stability-indicating RP-HPLC method for the simultaneous determination of ertugliflozin pidolate and metformin hydrochloride in bulk and tablets. Future J. Pharm. Sci. 2020, 6, 66. [Google Scholar] [CrossRef]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the Accuracy of Experimental and Predicted pKa Values of Basic and Acidic Compounds. Pharm. Res. 2014, 31, 1082–1095. [Google Scholar] [CrossRef]
- Chang, E.D.; Town, R.M.; Owen, S.F.; Hogstrand, C.; Bury, N.R. Effect of Water PH on the Uptake of Acidic (Ibuprofen) and Basic (Propranolol) Drugs in a Fish Gill Cell Culture Model. Environ. Sci. Technol. 2021, 55, 6848–6856. [Google Scholar] [CrossRef]
- Kong, F.X.; Liu, Q.; Dong, L.Q.; Zhang, T.; Wei, Y.B.; Chen, J.F.; Wang, Y.; Guo, C.M. Rejection of pharmaceuticals by graphene oxide membranes: Role of crosslinker and rejection mechanism. J. Membr. Sci. 2020, 612, 118338. [Google Scholar] [CrossRef]
- Nam, S.-W.; Jung, C.; Li, H.; Yu, M.; Flora, J.R.; Boateng, L.K.; Her, N.; Zoh, K.-D.; Yoon, Y. Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 2015, 136, 20–26. [Google Scholar] [CrossRef]
- Kimbi Yaah, V.B.; Zbair, M.; Botelho de Oliveira, S.; Ojala, S. Hydrochar-derived adsorbent for the removal of diclofenac from aqueous solution. Nanotechnol. Environ. Eng. 2021, 6, 3. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.M.; Chen, J.; Yu, C.G.; Huang, X.; Zhao, C.Z.; Duan, L.F.; Yang, Y.; Lu, W. Graphene nanosheets decorated with tunable magnetic nanoparticles and their efficiency of wastewater treatment. Mater. Res. Bull. 2015, 68, 234–239. [Google Scholar] [CrossRef]
- Yang, T.; Lin, H.; Zheng, X.; Loh, K.P.; Jia, B. Tailoring pores in graphene-based materials: From generation to applications. J. Mater. Chem. A 2017, 5, 16537–16558. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.-H.; Liu, Y.-G.; Zeng, G.-M.; Xiao, F.-Y.; Hu, X.-J.; Hu, X.; Wang, H.; Li, T.-T.; Zhou, L.; Tan, X.-F. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
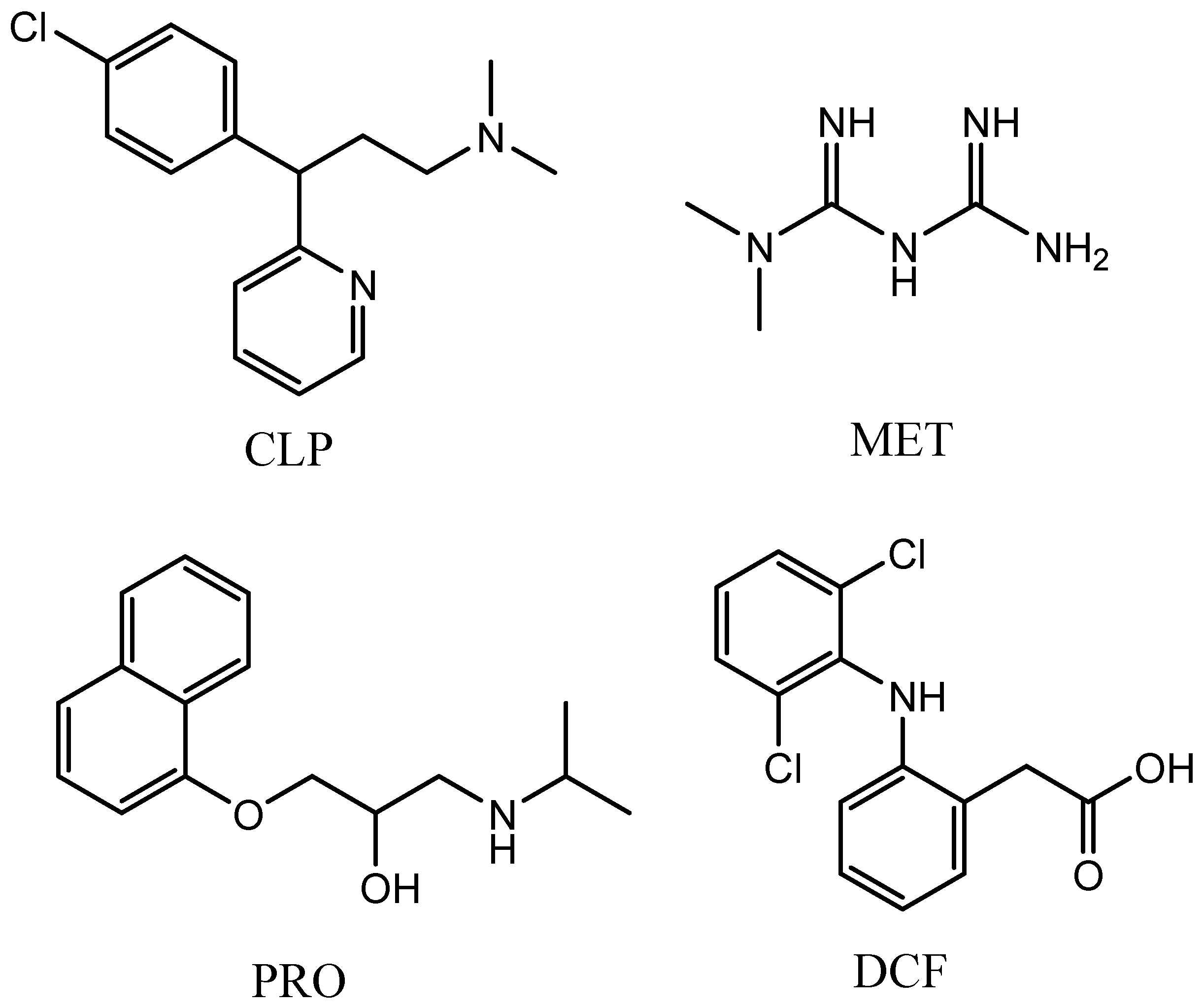
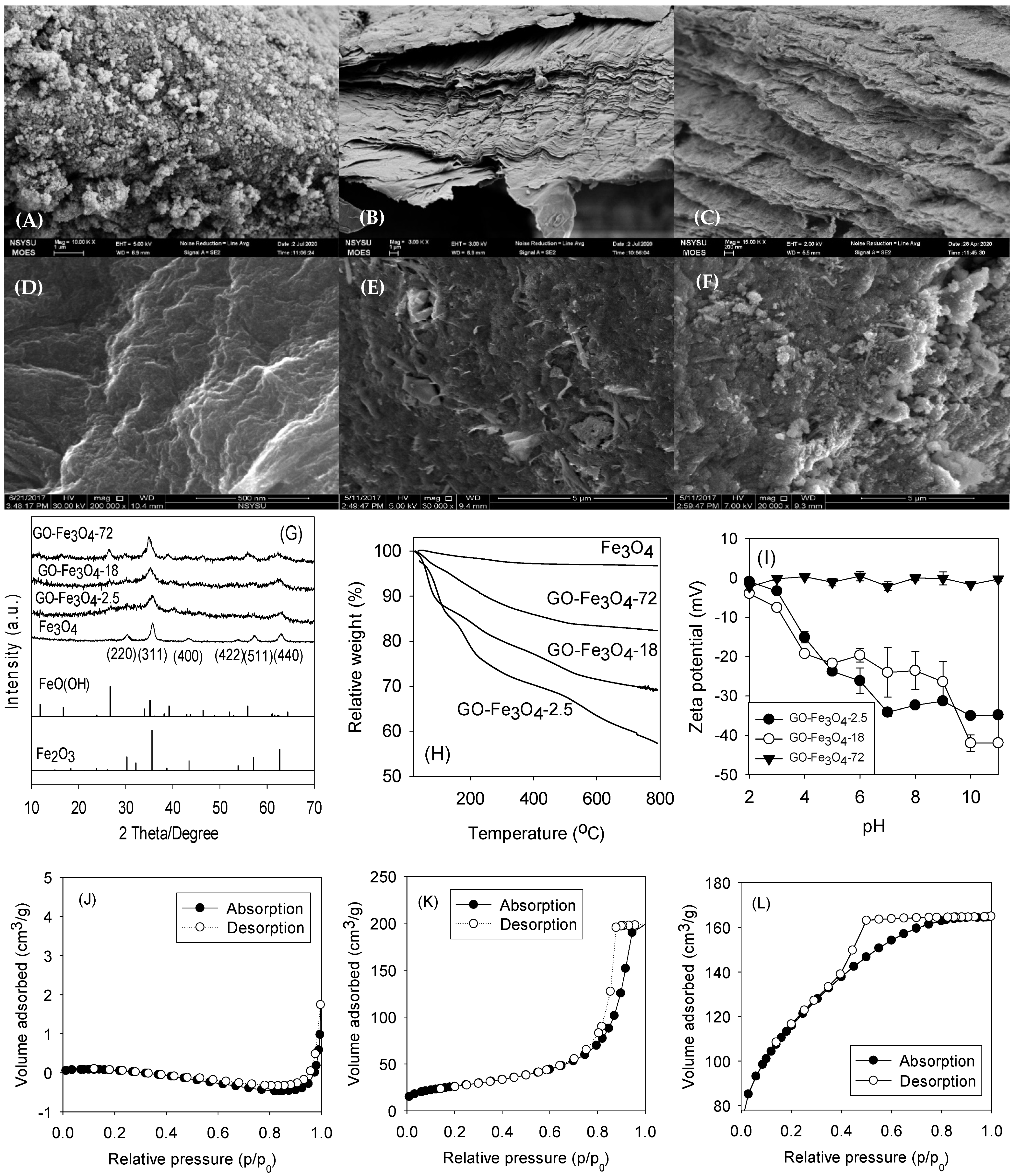
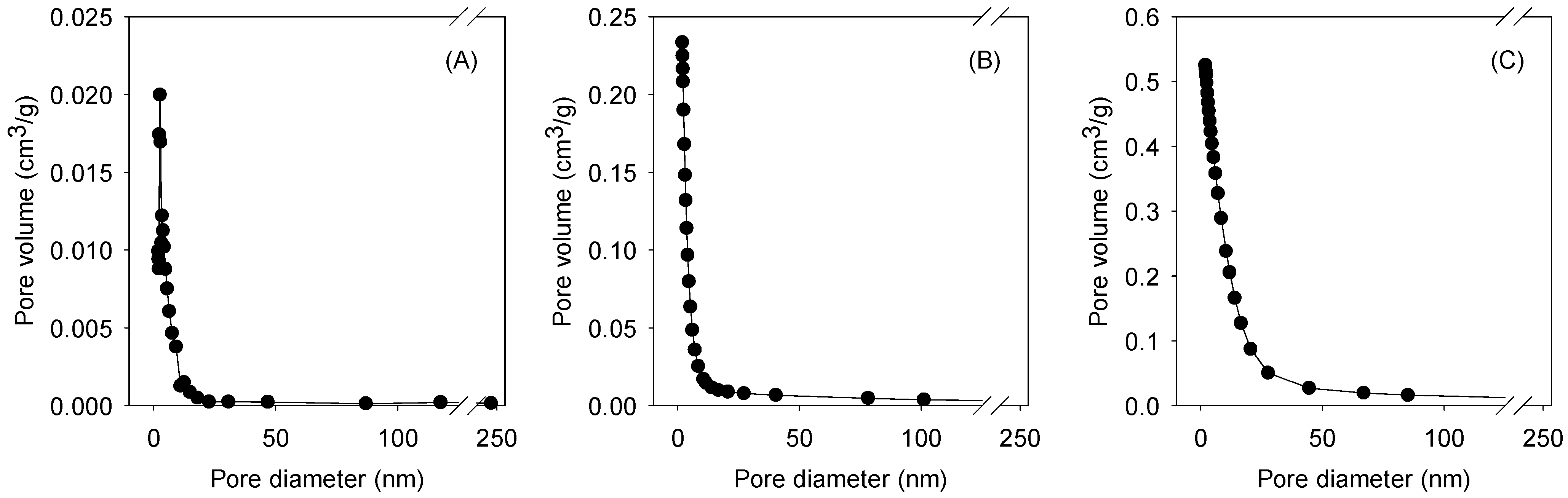

| Composite | Specific Surface Area (m2/g) | Pore Volume | |||||
|---|---|---|---|---|---|---|---|
| Total | Micro | External | Total (cm3/g) | <2 nm (%) 1 | 2–50 nm (%) 1 | >50 nm (%) 1 | |
| GO-Fe3O4-2.5 | 407 | 96 | 311 | 0.21 | 25 | 74 | 1 |
| GO-Fe3O4-18 | 331 | 31 | 300 | 0.30 | 22 | 77 | 1 |
| GO-Fe3O4-72 | 302 | 14 | 288 | 1.09 | 7 | 92 | 1 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | n | KF ((mg/g)/(mg/L)n) | R2 | ||
| GO-Fe3O4-2.5 | PRO | 144.93 | 0.54 | 0.99 | 0.23 | 59.99 | 0.92 |
| DCF | 51.28 | 0.11 | 0.98 | 0.28 | 13.95 | 0.94 | |
| MET | 36.76 | 0.07 | 0.99 | 0.36 | 6.55 | 0.98 | |
| GO-Fe3O4-18 | PRO | 66.67 | 0.12 | 0.98 | 0.19 | 25.69 | 0.95 |
| DCF | 45.05 | 0.07 | 0.89 | 0.19 | 16.14 | 0.65 | |
| GO-Fe3O4-72 | DCF | 106.38 | 0.18 | 0.98 | 0.10 | 61.62 | 0.77 |
| GO-Fe3O4-2.5 | GO-Fe3O4-18 | GO-Fe3O4-72 | |||||
|---|---|---|---|---|---|---|---|
| MET | PRO | DCF | PRO | DCF | PRO | DCF | |
| k (g/mg min) | 7.55 × 10−3 | 3.91 × 10−3 | 1.02×10−3 | 1.01 × 10−3 | 4.56 × 10−3 | 5.1 × 10−4 | 7.9 × 10−3 |
| qe (mg/g) | 33.67 | 87.72 | 51.02 | 56.18 | 25.58 | 48.78 | 75.19 |
| k × qe2 (g/mg-min) | 8.56 | 30.09 | 2.66 | 3.19 | 2.98 | 1.21 | 4.47 |
| R2 | 0.99 | 0.99 | 0.78 | 0.98 | 0.99 | 0.97 | 0.99 |
| GO-Fe3O4 | Adsorbed Concentration | ||||||
|---|---|---|---|---|---|---|---|
| Fe Content | SA | ZP | PV | MET | PRO | DCF | |
| Fe content | 1 | −0.67 | −0.94 | 0.95 | |||
| SA | −0.85 | 1 | 0.96 | 0.98 | −0.65 | ||
| ZP | 0.99 | −0.87 | 1 | −0.70 | −0.95 | 0.94 | |
| PV | 0.99 | −0.80 | 0.99 | 1 | −0.61 | −0.90 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.-C.; Lin, S.-J.; Huang, T.-C.; Chen, G.-F.; Peng, Y.-P.; Chen, W.-H. The Influences of Pore Blockage by Natural Organic Matter and Pore Dimension Tuning on Pharmaceutical Adsorption onto GO-Fe3O4. Nanomaterials 2023, 13, 2063. https://doi.org/10.3390/nano13142063
He M-C, Lin S-J, Huang T-C, Chen G-F, Peng Y-P, Chen W-H. The Influences of Pore Blockage by Natural Organic Matter and Pore Dimension Tuning on Pharmaceutical Adsorption onto GO-Fe3O4. Nanomaterials. 2023; 13(14):2063. https://doi.org/10.3390/nano13142063
Chicago/Turabian StyleHe, Ming-Cyuan, Sian-Jhang Lin, Tao-Cheng Huang, Guan-Fu Chen, Yen-Ping Peng, and Wei-Hsiang Chen. 2023. "The Influences of Pore Blockage by Natural Organic Matter and Pore Dimension Tuning on Pharmaceutical Adsorption onto GO-Fe3O4" Nanomaterials 13, no. 14: 2063. https://doi.org/10.3390/nano13142063





