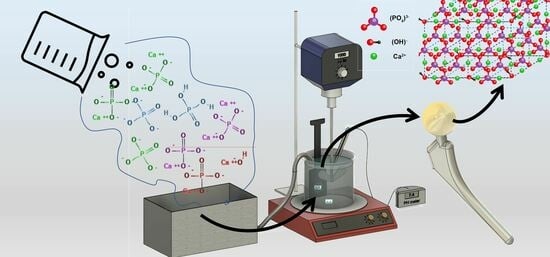
Development of Hydroxyapatite Coatings for Orthopaedic Implants from Colloidal Solutions: Part 1—Effect of Solution Concentration and Deposition Kinetics
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Kinetics of Process Solutions
3.2. Analysis of HA Films Formed with Various Process Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Szczęsny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A Review on Biomaterials for Orthopaedic Surgery and Traumatology: From Past to Present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial Adhesion on Orthopedic Implants. Adv. Colloid. Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Walter, N.; Stich, T.; Docheva, D.; Alt, V.; Rupp, M. Evolution of Implants and Advancements for Osseointegration: A Narrative Review. Injury 2022, 53, S69–S73. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving Biocompatibility for next Generation of Metallic Implants. Progress. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef]
- Kothari, A.; Razdan, R.A.; Jain, R.; Patel, V.; Parihar, N.; Pandey, D. Role of Hydroxyapatite (HA) Coatings in Implants: A Review. Univ. J. Dent. Sci. 2022, 8, 116–119. [Google Scholar] [CrossRef]
- Horváth, T.; Hanák, L.; Hegyi, P.; Butt, E.; Solymár, M.; Szűcs, Á.; Varga, O.; Thien, B.Q.; Szakács, Z.; Csonka, E.; et al. Hydroxyapatite-Coated Implants Provide Better Fixation in Total Knee Arthroplasty. A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2020, 15, e0232378. [Google Scholar] [CrossRef]
- Jiang, J.; Han, G.; Zheng, X.; Chen, G.; Zhu, P. Characterization and Biocompatibility Study of Hydroxyapatite Coating on the Surface of Titanium Alloy. Surf. Coat. Technol. 2019, 375, 645–651. [Google Scholar] [CrossRef]
- Zaman, S.U.; Irfan, M.; Irfan, M.; Zaman, M.K.U.; Muhammad, N. Overview of Hydroxyapatite; Composition, Structure, Synthesis Methods and Its Biomedical Uses. Biomed. Lett. 2020, 6, 84–99. [Google Scholar]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery—Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.U.; Salman, S.; Ihsan, A.; Elsaman, T. Synthesis, Characterization, Functionalization and Bio-Applications of Hydroxyapatite Nanomaterials: An Overview. Int. J. Nanomed. 2022, 17, 1903–1925. [Google Scholar] [CrossRef] [PubMed]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A Review on the Synthesis and Properties of Hydroxyapatite for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhao, J.; Esmeryan, K.D.; Lu, X.; Li, Z.; Wang, K.; Ren, F.; Wang, Q.; Wang, M.; Qian, B. Cicada-Inspired Fluoridated Hydroxyapatite Nanostructured Surfaces Synthesized by Electrochemical Additive Manufacturing. Mater. Des. 2020, 193, 108790. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]
- Gil, J.; Manero, J.M.; Ruperez, E.; Velasco-Ortega, E.; Jiménez-Guerra, A.; Ortiz-García, I.; Monsalve-Guil, L. Mineralization of Titanium Surfaces: Biomimetic Implants. Materials 2021, 14, 2879. [Google Scholar] [CrossRef]
- Riau, A.K.; Venkatraman, S.S.; Mehta, J.S. Biomimetic vs. Direct Approach to Deposit Hydroxyapatite on the Surface of Low Melting Point Polymers for Tissue Engineering. Nanomaterials 2020, 10, 2162. [Google Scholar] [CrossRef]
- Huang, C.; Bhagia, S.; Hao, N.; Meng, X.; Liang, L.; Yong, Q.; Ragauskas, A.J. Biomimetic Composite Scaffold from an in Situ Hydroxyapatite Coating on Cellulose Nanocrystals. RSC Adv. 2019, 9, 5786–5793. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, W.; Xiong, Z.; Hu, Y.; Xiao, J. Effects of Biomimetic Hydroxyapatite Coatings on Osteoimmunomodulation. Biomater. Adv. 2022, 134, 112640. [Google Scholar] [CrossRef]
- Murphy, B.; Baez, J.; Morris, M.A. Characterising Hydroxyapatite Deposited from Solution onto Novel Substrates: Growth Mechanism and Physical Properties. Nanomaterials 2023, 13, 2483. [Google Scholar] [CrossRef]
- György, S.; Károly, Z.; Fazekas, P.; Németh, P.; Bódis, E.; Menyhárd, A.; Kótai, L.; Klébert, S. Effect of the Reaction Temperature on the Morphology of Nanosized HAp. J. Therm. Anal. Calorim. 2019, 138, 145–151. [Google Scholar] [CrossRef]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and Characterization of Nano-Hydroxyapatite Added with Magnesium Obtained by Wet Chemical Precipitation. Progress. Nat. Sci. Mater. Int. 2021, 31, 575–582. [Google Scholar] [CrossRef]
- Mobarak, M.B.; Hossain, M.S.; Yeasmin, Z.; Mahmud, M.; Rahman, M.M.; Sultana, S.; Masum, S.M.; Ahmed, S. Probing the Photocatalytic Competency of Hydroxyapatite Synthesized by Solid State and Wet Chemical Precipitation Method. J. Mol. Struct. 2022, 1252, 132142. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Alipal, J.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis of Eggshell Derived Hydroxyapatite via Chemical Precipitation and Calcination Method. Mater. Today Proc. 2021, 42, 172–177. [Google Scholar] [CrossRef]
- Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Zubieta-Otero, L.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Synthesis and Characterization of Bioinspired Nano-Hydroxyapatite by Wet Chemical Precipitation. Ceram. Int. 2021, 47, 32775–32785. [Google Scholar] [CrossRef]
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-Situ Raman Spectroscopy of Amorphous Calcium Phosphate to Crystalline Hydroxyapatite Transformation. MethodsX 2018, 5, 1241–1250. [Google Scholar] [CrossRef]
- Patty, D.J.; Nugraheni, A.D.; Dewi Ana, I.; Yusuf, Y. Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering. Bioengineering 2022, 9, 784. [Google Scholar] [CrossRef]
- Beig, B.; Liaqat, U.; Niazi, M.F.K.; Douna, I.; Zahoor, M.; Niazi, M.B.K. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A Review on Hydroxyapatite Coatings for the Biomedical Applications: Experimental and Theoretical Perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Y.; Li, S.; Pang, Y.; Sun, Z. Dual Function of Poly(Acrylic Acid) on Controlling Amorphous Mediated Hydroxyapatite Crystallization. J. Cryst. Growth 2021, 557, 125991. [Google Scholar] [CrossRef]
- Posner, A.S. Crystal Chemistry of Bone Mineral. Physiol. Rev. 1969, 49, 760–792. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the Hydroxyapatite Structure. Acta Cryst. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Harper, R.A.; Posner, A.S. Measurement of Non-Crystalline Calcium Phosphate in Bone Mineral. Proc. Soc. Exp. Biol. Med. 1966, 122, 137–142. [Google Scholar] [CrossRef]
- Jin, B.; Shao, C.; Wang, Y.; Mu, Z.; Liu, Z.; Tang, R. Anisotropic Epitaxial Behavior in the Amorphous Phase-Mediated Hydroxyapatite Crystallization Process: A New Understanding of Orientation Control. J. Phys. Chem. Lett. 2019, 10, 7611–7616. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Sawczyk, M.; Liu, C.; Yuan, Y.; Song, B.; Deivanayagam, R.; Nie, A.; Hu, X.; Dravid, V.P.; Lu, J.; et al. Revealing Nanoscale Mineralization Pathways of Hydroxyapatite Using in Situ Liquid Cell Transmission Electron Microscopy. Sci. Adv. 2020, 6, eaaz7524. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, L.; Iafisco, M. Amorphous Calcium Phosphate, the Lack of Order Is an Abundance of Possibilities. Biomater. Biosyst. 2022, 5, 100037. [Google Scholar] [CrossRef]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier Transform Raman Spectroscopy of Synthetic and Biological Calcium Phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef]
- Evcin, A.; Buyukleblebici, B. Ti6Al4V Coating with B2O3 and Al2O3 Containing Hydroxyapatite by HVOF Technique. Sci. Iran. 2019, 26, 1980–1989. [Google Scholar] [CrossRef]
- Robertson, S.F.; Bandyopadhyay, A.; Bose, S. Titania Nanotube Interface to Increase Adhesion Strength of Hydroxyapatite Sol-Gel Coatings on Ti-6Al-4V for Orthopedic Applications. Surf. Coat. Technol. 2019, 372, 140–147. [Google Scholar] [CrossRef]
- Lu, M.; Chen, H.; Yuan, B.; Zhou, Y.; Min, L.; Xiao, Z.; Zhu, X.; Tu, C.; Zhang, X. Electrochemical Deposition of Nanostructured Hydroxyapatite Coating on Titanium with Enhanced Early Stage Osteogenic Activity and Osseointegration. Int. J. Nanomed. 2020, 15, 6605–6618. [Google Scholar] [CrossRef]
- Sawada, M.; Sridhar, K.; Kanda, Y.; Yamanaka, S. Pure Hydroxyapatite Synthesis Originating from Amorphous Calcium Carbonate. Sci. Rep. 2021, 11, 11546. [Google Scholar] [CrossRef] [PubMed]
- Roohani, I.; Cheong, S.; Wang, A. How to Build a Bone?—Hydroxyapatite or Posner’s Clusters as Bone Minerals. Open Ceram. 2021, 6, 100092. [Google Scholar] [CrossRef]
- De Yoreo, J.J. Casting a Bright Light on Ostwald’s Rule of Stages. Proc. Natl. Acad. Sci. USA 2022, 119, e2121661119. [Google Scholar] [CrossRef] [PubMed]
- Andrusova, N.N.; Zhavoronok, E.S.; Legon’kova, O.A.; Goncharova, A.S.; Kedik, S.A. Polymer–Mineral Compounds for Cementless Hip Replacement. Polym. Sci. Ser. D 2020, 13, 68–72. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Warcaba, M.; Cieniek, Ł.; Sitarz, M.; Gajewska, M.; Boccaccini, A.R. Hydroxyapatite/Sodium Alginate Coatings Electrophoretically Deposited on Titanium Substrates: Microstructure and Properties. Appl. Surf. Sci. 2021, 540, 148353. [Google Scholar] [CrossRef]
- Janson, O.; Gururaj, S.; Pujari-Palmer, S.; Karlsson Ott, M.; Strømme, M.; Engqvist, H.; Welch, K. Titanium Surface Modification to Enhance Antibacterial and Bioactive Properties While Retaining Biocompatibility. Mater. Sci. Eng. C 2019, 96, 272–279. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Chen, D.; Shao, C.; Yi, M.; Zhang, B. Effects of Pore Size and Porosity of Surface-Modified Porous Titanium Implants on Bone Tissue Ingrowth. Trans. Nonferrous Met. Soc. China 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Lee, Y.J. A Facile One-Pot Hydrothermal Synthesis of Hydroxyapatite/Biochar Nanocomposites: Adsorption Behavior and Mechanisms for the Removal of Copper(II) from Aqueous Media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Ribeiro, A.; Manrique, Y.A.; Barreiro, M.F.; Lopes, J.C.B.; Dias, M.M. Effect of Temperature, pH and Ionic Strength on Hydroxyapatite Stabilised Pickering Emulsions Produced in Batch and Continuous Mode. Food Biophys. 2022, 17, 422–436. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Panova, E.G.; Lessovaia, S.N. Processes and Phenomena on the Boundary Between Biogenic and Abiogenic Nature; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Prihanto, A.; Muryanto, S.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Hydrothermal Production of Nanoparticles, Thermostable Hydroxyapatite with Varying Ph and Temperatures; Rochester: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Song, I. Octacalcium Phosphate, a Promising Bone Substitute Material: A Narrative Review. JYMS, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Bakan, F. A Systematic Study of the Effect of pH on the Initialization of Ca-Deficient Hydroxyapatite to β-TCP Nanoparticles. Mater 2019, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, M.; Jirofti, N.; Ebrahimzadeh, M.H.; Moradi, A. Different Methods of Hydroxyapatite-Based Coatings on External Fixator Pin with High Adhesion Approach. Plasma Process. Polym. 2023, 20, e2200219. [Google Scholar] [CrossRef]
- Botterill, J.; Khatkar, H. The Role of Hydroxyapatite Coating in Joint Replacement Surgery—Key Considerations. J. Clin. Orthop. Trauma. 2022, 29, 101874. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a Multifunctional Material for Air, Water and Soil Pollution Control: A Review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Yuan, Z.; Bi, J.; Wang, W.; Sun, X.; Wang, L.; Mao, J.; Yang, F. A Novel Synthesis Method and Properties of Calcium-Deficient Hydroxyapatite/α-TCP Biphasic Calcium Phosphate. J. Biomater. Appl. 2022, 36, 1712–1719. [Google Scholar] [CrossRef]
- Bystrov, V.; Paramonova, E.; Avakyan, L.; Coutinho, J.; Bulina, N. Simulation and Computer Study of Structures and Physical Properties of Hydroxyapatite with Various Defects. Nanomaterials 2021, 11, 2752. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Dhar, B.R. Phosphorus Recovery from Wastewater via Calcium Phosphate Precipitation: A Critical Review of Methods, Progress, and Insights. Chemosphere 2023, 330, 138685. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Garcia-Giralt, N.; Dosta, S.; Cano, I.G.; Nogués, X.; Guilemany, J.M. In-Vitro Comparison of Hydroxyapatite Coatings Obtained by Cold Spray and Conventional Thermal Spray Technologies. Mater. Sci. Eng. C 2020, 107, 110306. [Google Scholar] [CrossRef]
- Su, Y.; Li, K.; Tielens, F.; Wang, J. Effect of Sprayed Techniques on the Surface Microstructure and in Vitro Behavior of Nano-HAp Coatings. Mater. Sci. Eng. C 2020, 117, 111318. [Google Scholar] [CrossRef]





| ID | Concentrate to Solution Dilution [%v/v] | Ionic Strength [mmol·L−1] |
|---|---|---|
| Solution 1 | 3.1 | 163.08 |
| Solution 2 | 4.6 | 244.71 |
| Solution 3 | 6.2 | 326.81 |
| Solution 4 | 7.7 | 407.71 |
| Solution 5 | 9.2 | 489.74 |
| Solution 6 | 10.8 | 570.76 |
| Solution 7 | 12.3 | 652.36 |
| Acronym | Mineral | Formula | Ca/P Ratio | Atomic % Oxygen |
| HA | Hydroxyapatite | Ca10(PO4)6(OH)2 | 1.67 | 34.9 |
| ACP | Amorphous calcium phosphate | CaxHy(PO4)z∙nH2O | 1.2–2.2 | Flexible |
| OCP | Octacalcium phosphate | Ca8H2(PO4)6·5H2O | 1.33 | 39.7 |
| TCP | Tricalcium phosphate | Ca3(PO4)2 | 1.5 | 41.3 |
| CDHA | Calcium deficient hydroxyapatite | Ca9(HPO4)(PO4)5OH | 1.5 | 43.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, B.; Morris, M.A.; Baez, J. Development of Hydroxyapatite Coatings for Orthopaedic Implants from Colloidal Solutions: Part 1—Effect of Solution Concentration and Deposition Kinetics. Nanomaterials 2023, 13, 2577. https://doi.org/10.3390/nano13182577
Murphy B, Morris MA, Baez J. Development of Hydroxyapatite Coatings for Orthopaedic Implants from Colloidal Solutions: Part 1—Effect of Solution Concentration and Deposition Kinetics. Nanomaterials. 2023; 13(18):2577. https://doi.org/10.3390/nano13182577
Chicago/Turabian StyleMurphy, Bríd, Mick A. Morris, and Jhonattan Baez. 2023. "Development of Hydroxyapatite Coatings for Orthopaedic Implants from Colloidal Solutions: Part 1—Effect of Solution Concentration and Deposition Kinetics" Nanomaterials 13, no. 18: 2577. https://doi.org/10.3390/nano13182577