Synthesis and Characterization of MnIn2S4/Single-Walled Carbon Nanotube Composites as an Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
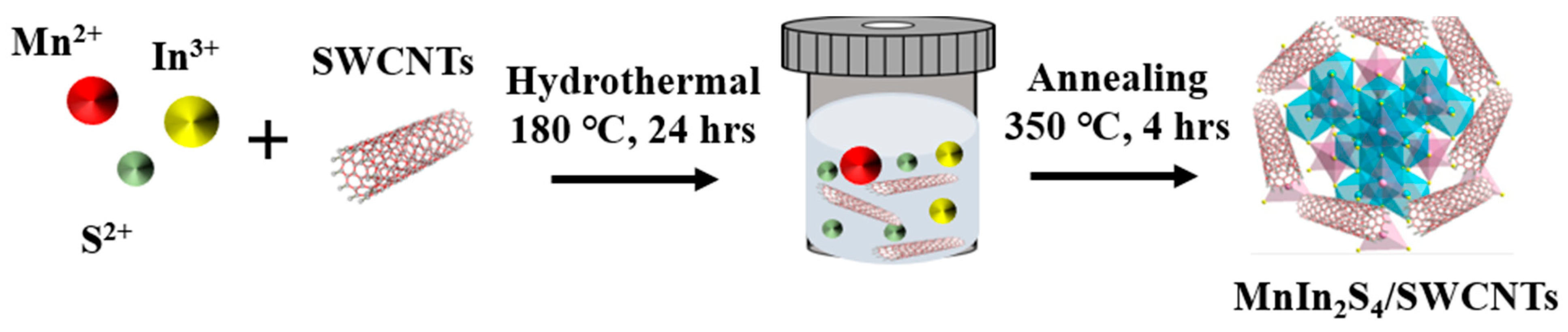
2.1. Synthesis of Pristine MIS and MIS/SWCNT Composites
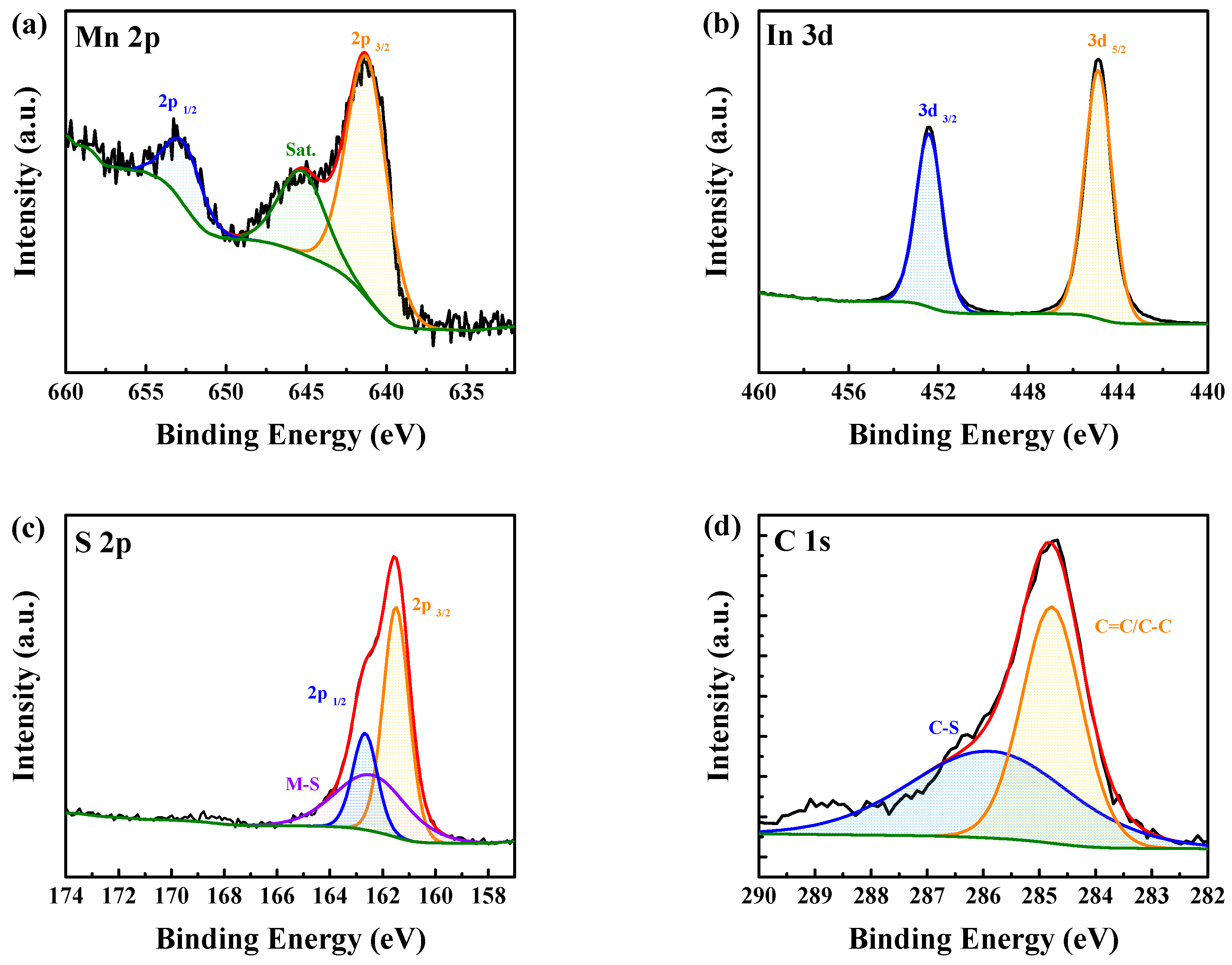
2.2. Characterizations
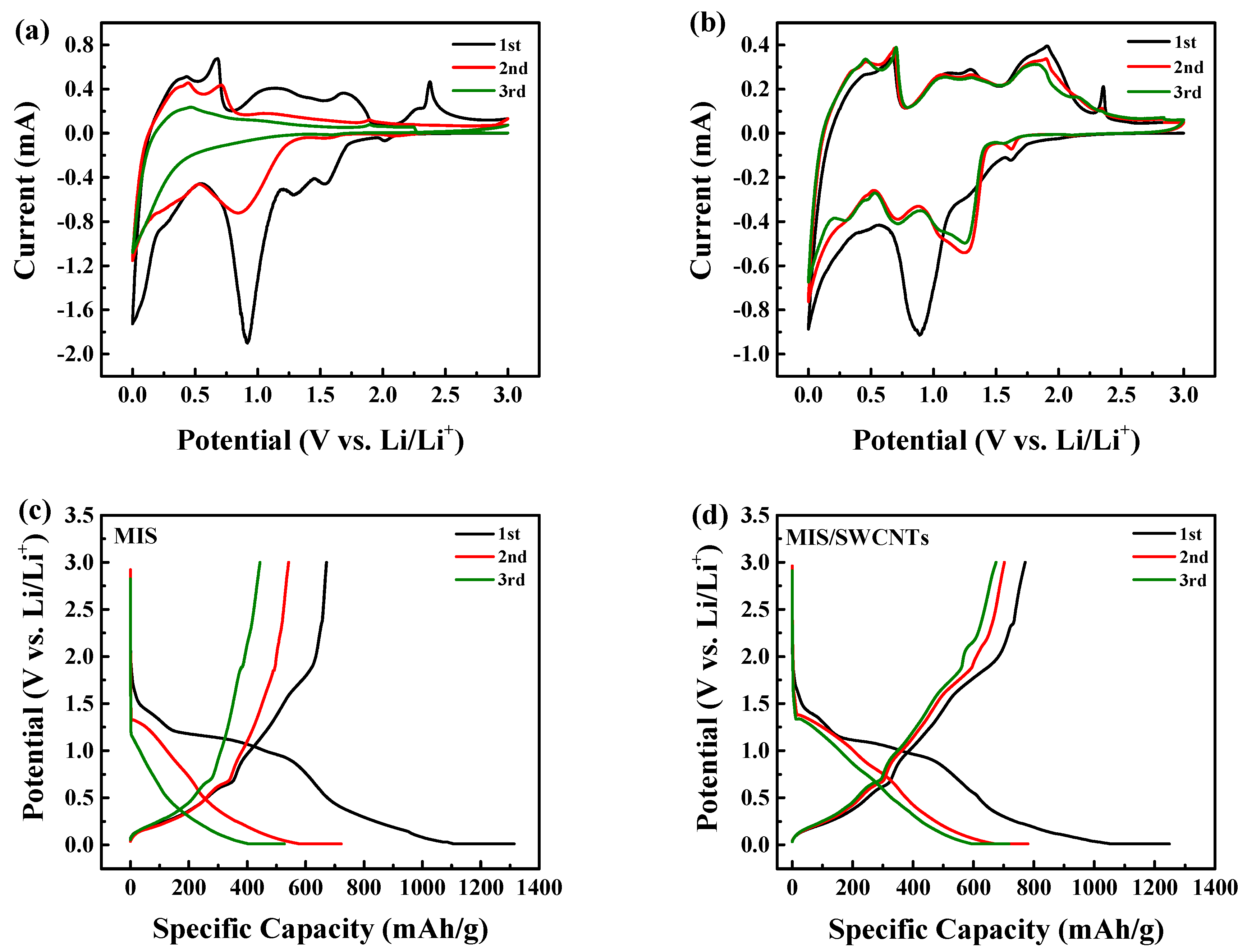
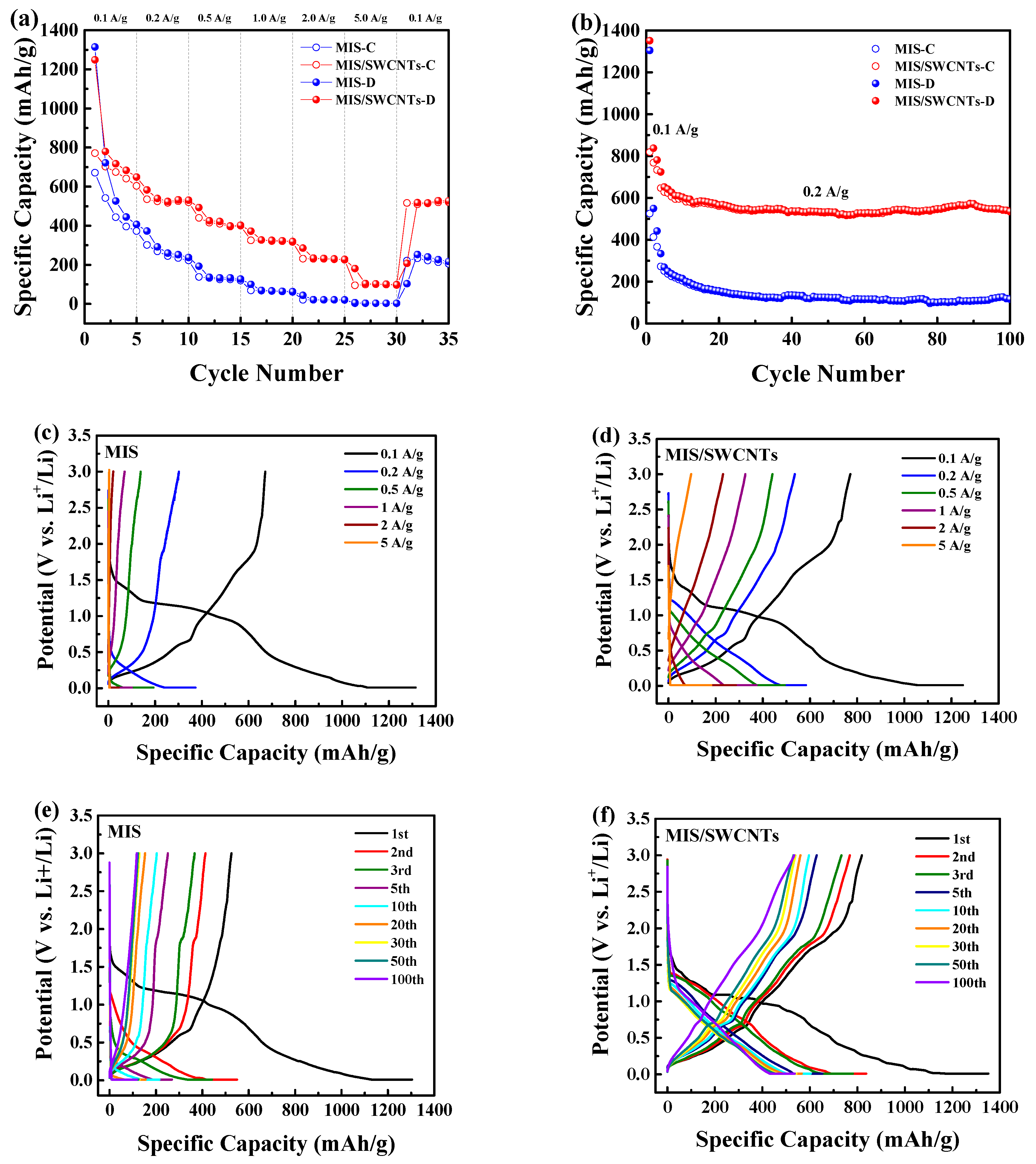
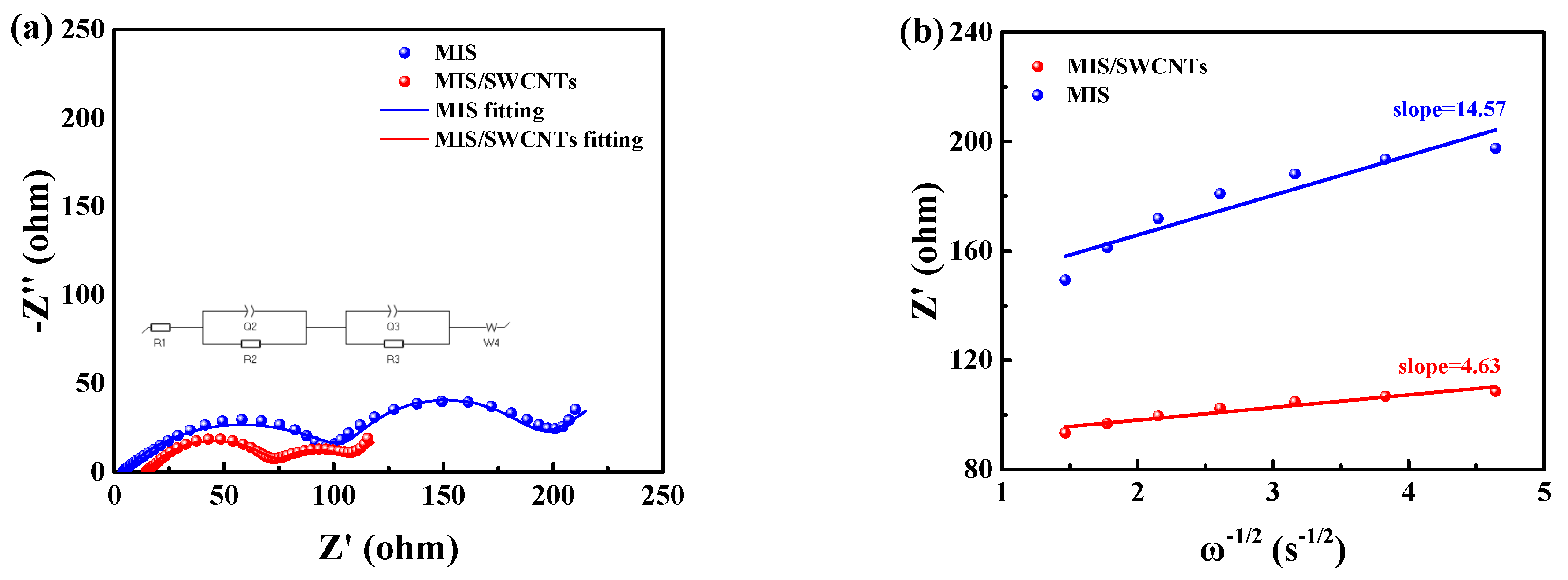
2.3. Electrochemical Measurements
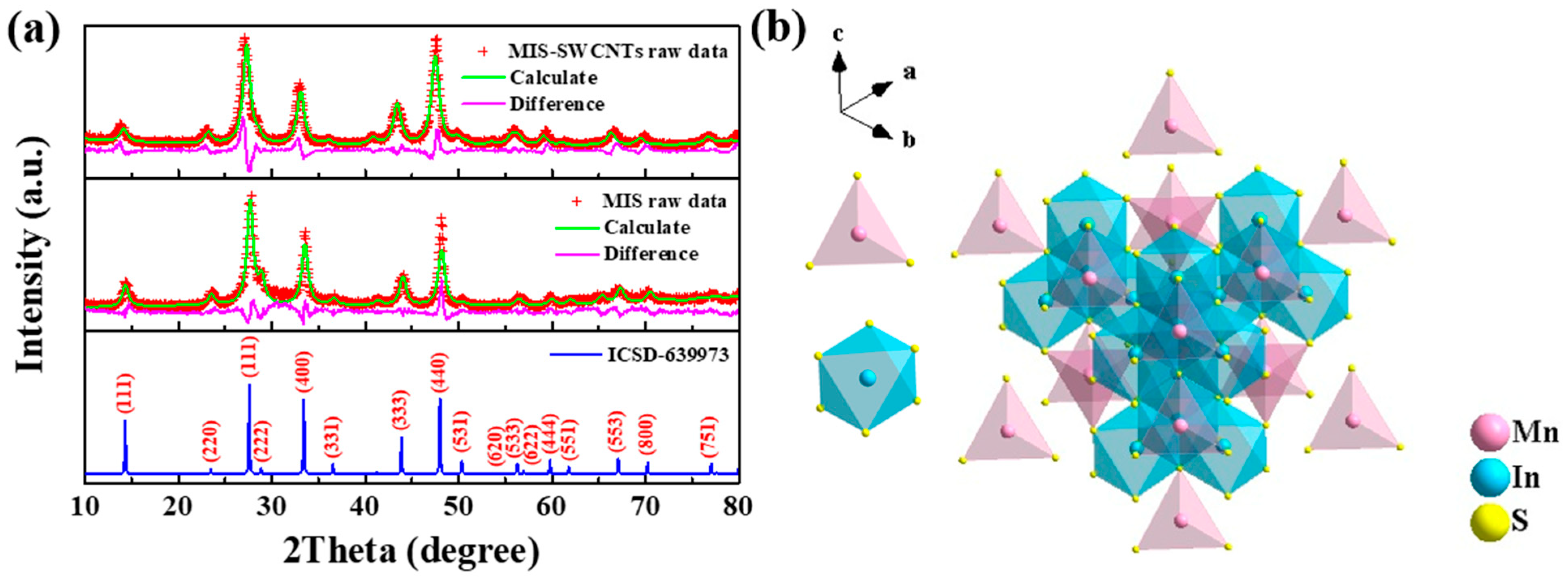
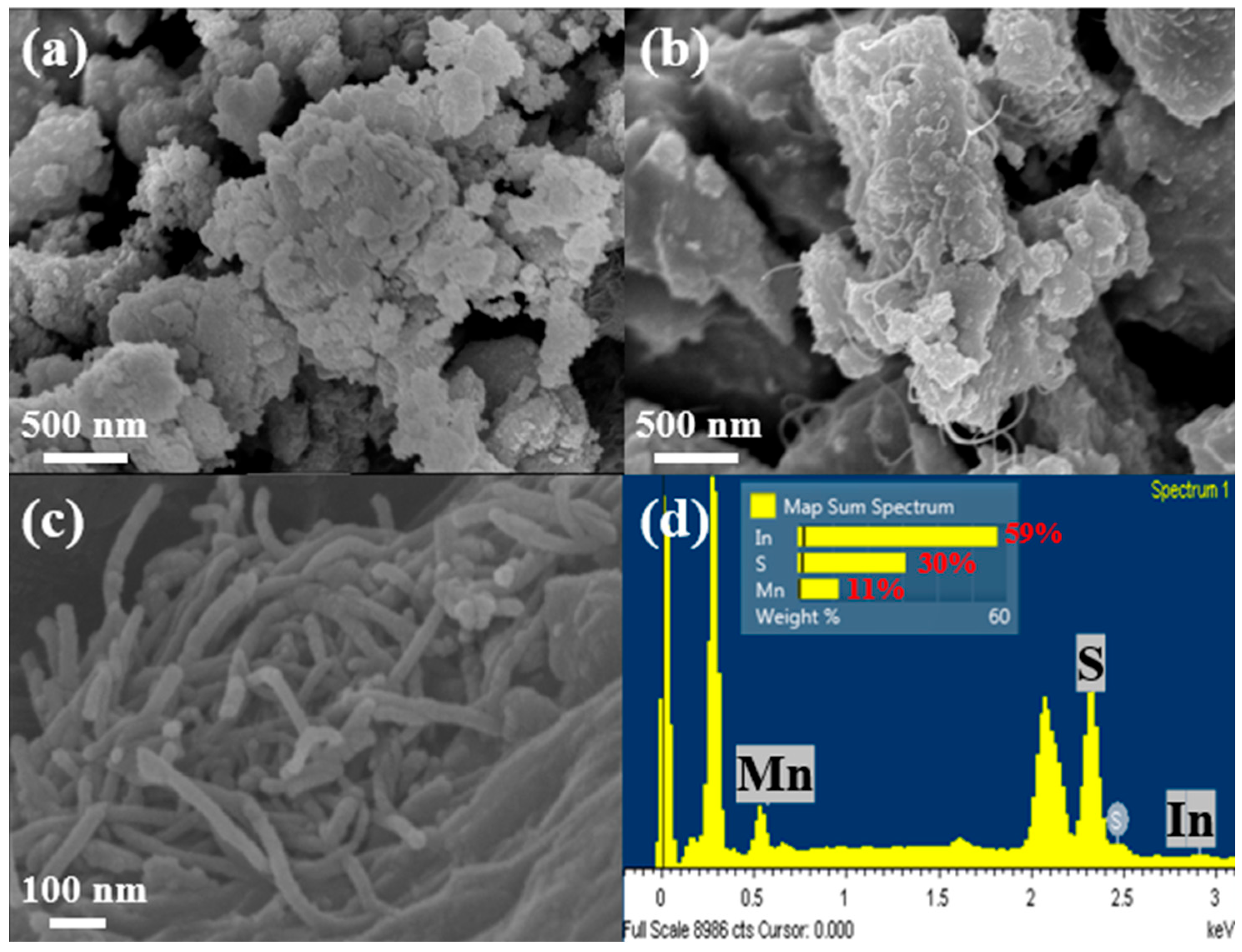
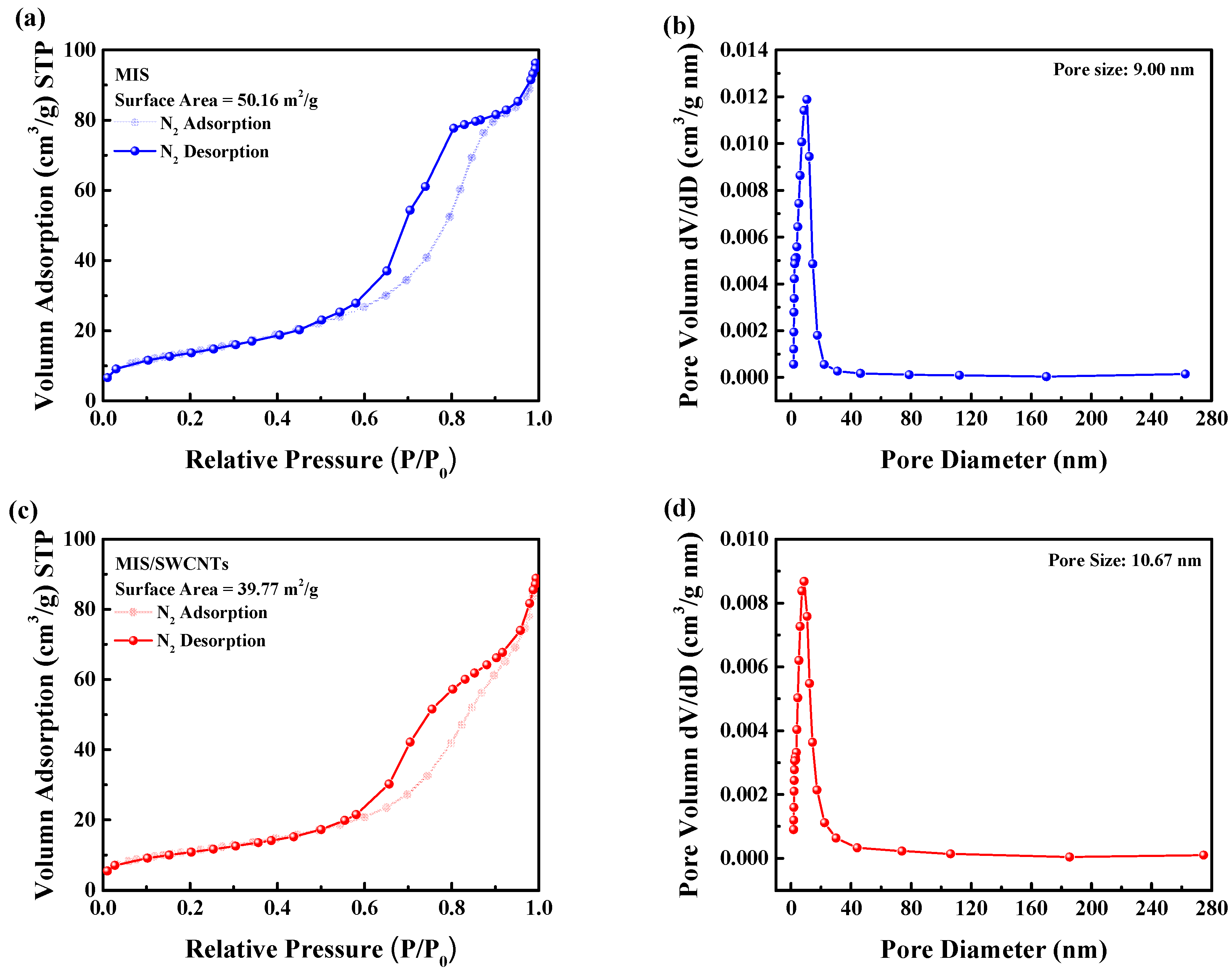
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Li, J.; Daniel, C.; Wood, D. Materials processing for lithium-ion batteries. J. Power Sources 2011, 196, 2452–2460. [Google Scholar] [CrossRef]
- Hu, D.; Chen, L.; Tian, J.; Su, Y.; Li, N.; Chen, G.; Hu, Y.; Dou, Y.; Chen, S.; Wu, F. Research progress of lithium plating on graphite anode in lithium-ion batteries. Chin. J. Chem. 2021, 39, 165–173. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Luo, L.; Pan, Q.; Zhang, P.; Huang, Y.; Zheng, F.; Wang, H.; Li, Q. From spent graphite to recycle graphite anode for high-performance lithium ion batteries and sodium ion batteries. Electrochim. Acta 2020, 356, 136856. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Sun, C.; Lu, Z.; Xue, P.; Tang, B.; Bai, Z.; Dou, S. 3D spongy CoS2 nanoparticles/carbon composite as high-performance anode material for lithium/sodium ion batteries. Chem. Eng. J. 2018, 332, 370–376. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Zhang, H.; Cao, K.; Wang, S.; Jiang, Y.; Jing, Q.; Jiao, L. Lowering the voltage-hysteresis of CuS anode for Li-ion batteries via constructing heterostructure. Chem. Eng. J. 2021, 425, 130548. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.; Ma, T.; Zheng, L.; Xu, Y.; Guo, X.; Zhang, J. In2S3 nanosheets anchored on N-doped carbon fibers for improved lithium storage performances. Solid State Ion. 2019, 329, 8–14. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yang, Y.; Lv, W.; Liang, G.; Golbert, D.; Wang, X.; Zhao, X.; Ding, Y. A MoS2/Carbon hybrid anode for high-performance Li-ion batteries at low temperature. Nano Energy 2020, 70, 104550. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Nong, Y.; Pan, Q.; Huang, Y.; Wang, H.; Zheng, F.; Li, Q. Synthesis of core-shell ZnS@C microrods as advanced anode materials for lithium-ion batteries. New J. Chem. 2022, 46, 18069–18075. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.R.; Ramakrishan, S.; Logeshwaran, N.; Ramasamy, S.K.; Kim, H.J.; Yoo, D.J. Integrating the essence of metal organic framework-derived ZnCoTe–N–C/MoS2 cathode and ZnCo-NPS-N-CNT as anode for high-energy density hybrid supercapacitors. Compos. Part B Eng. 2022, 247, 110339. [Google Scholar] [CrossRef]
- Yan, W.; Liang, K.; Chi, Z.; Liu, T.; Cao, M.; Fan, S.; Xu, T.; Liu, T.; Su, J. Litchi-like structured MnCo2S4@C as a high capacity and long-cycling time anode for lithium-ion batteries. Electrochim. Acta 2021, 376, 138035. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, A.; Sun, Z.; Ning, X.; Guo, J.; Lv, Y.; Jia, D. Boosting the performance of half/full lithium-ion batteries by designing smart architecture anode of SnS2 nanosheet coating on NiCo2S4 hollow spheres. J. Alloy Compd. 2020, 847, 156505. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, L.; Fan, W.; Liu, T. NiCo2S4 nanosheets grown on 3D networks of nitrogen doped graphene/carbon nanotubes: Advanced anode materials for lithium-ion batteries. Chemelectrochem 2016, 3, 1384–1391. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Shen, P.K. NiCo2S4 nanocores in-situ encapsulated in graphene sheets as anode materials for lithium-ion batteries. Chem. Eng. J. 2019, 364, 167–176. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Ye, L.W.; Zhang, D.; Chen, F.; Zhu, M.; Wang, L.N.; Ying, S.M.; Cai, G.S.; Guo, S.Y. NiCo2S4 multi-shelled hollow polyhedrons as high-performance anode materials for lithium-ion batteries. Electrochim. Acta 2019, 299, 289–297. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.; Meng, X.; Li, S.; Ren, M. Porous core–shell CuCo2S4 nanospheres as anode material for enhanced lithium-ion batteries. Chem. A Eur. J. 2019, 25, 885–891. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Yin, Y.; Fan, L.; Zhang, N.; Sun, K. In situ synthesis of CuCo2S4@N/S-doped graphene composites with pseudocapacitive properties for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 11708–11714. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Hou, B.; Pawar, S.M.; Kim, H.; Im, H. Graphene-integrated CuCo2S4 microspheres as a sustainable anode material for high-performance Li-ion batteries. Int. J. Energy Res. 2021, 45, 1613–1626. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Li, J.; Hong, S. Electrochemical properties and reaction mechanism of NiTi2S4 ternary metal sulfide as an anode for lithium ion battery. ACS Sustain. Chem. Eng. 2021, 9, 9680–9688. [Google Scholar] [CrossRef]
- Fan, S.; Liu, H.; Bi, S.; Gao, C.; Meng, X.; Wang, Y. Insight on the conversion reaction mechanism of NiCo2S4@CNTs as anode materials for lithium ion batteries and sodium ion batteries. Electrochim. Acta 2021, 388, 138618. [Google Scholar] [CrossRef]
- Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Design of cuboidal FeNi2S4-rGO-MWCNTs composite for lithium-ion battery anode showing excellent half and full cell performances. Batteries 2022, 8, 261. [Google Scholar] [CrossRef]
- Hao, Z.; Dimov, N.; Chang, J.K.; Okada, S. Synthesis of bimetallic sulfide FeCoS4@carbon nanotube graphene hybrid as a high-performance anode material for sodium-ion batteries. Chem. Eng. J. 2021, 423, 130070. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Li, H.; Zhou, W.; Feng, X.; Qiu, B.; Shi, Q.; Zheng, Y.; Zhao, Y.; Feng, C. In-situ preparation of multi-layered sandwich-like CuCo2S4/rGO architectures as anode material for high-performance lithium and sodium ion batteries. J. Alloys Compd. 2020, 845, 156183. [Google Scholar] [CrossRef]
- Lee, T.Y.; Liu, W.R. Reduced graphene oxide-wrapped novel CoIn2S4 spinel composite anode materials for Li-ion batteries. Nanomaterials 2022, 12, 4367. [Google Scholar] [CrossRef]
- Chen, D.; Quan, H.; Wang, G.S.; Guo, L. Hollow α-MnS spheres and their hybrids with reduced graphene oxide: Synthesis, microwave absorption, and lithium storage properties. Chempluschem 2013, 78, 843–851. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, Z.; Liu, L.; Yang, J.; Zhang, C.; Pan, X.; Chi, F. Spray-drying assisted hydrothermal synthesis of ZnIn2S4@GO as anode material for improved lithium ion batteries. Int. J. Electrochem. Sci. 2020, 15, 8797–8807. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Zhang, J.F.; Zhang, J.; Cao, L.; Zhang, B.; Ou, X. Rational design of bimetal-organic framework-derived ZnSnS3 nanodots incorporated into the nitrogen-doped graphene framework for advanced lithium storage. ACS Sustain. Chem. Eng. 2020, 8, 4464–4473. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, C.; Chen, B.; Zhang, Z.; Wang, X.; Zhao, J.; He, J.; Du, H.; Cui, G. Graphene boosted Cu2GeS3 for advanced lithium-ion batteries. Inorg. Chem. Front. 2017, 4, 541–546. [Google Scholar] [CrossRef]
- Jagadale, A.; Zhou, X.; Blaisdell, D.; Yang, S. Carbon nanofibers (CNFs) supported cobalt- nickel sulfide (CoNi2S4) nanoparticles hybrid anode for high performance lithium ion capacitor. Sci. Rep. 2018, 8, 1602. [Google Scholar] [CrossRef]
- Hsu, T.H.; Muruganantham, R.; Liu, W.R. High-energy ball-milling for fabrication of CuIn2S4/C composite as an anode material for lithium-ion batteries. Ceram. Int. 2022, 48, 11561–11572. [Google Scholar] [CrossRef]
- Lin, Z.; Min, Z.; Chen, W.; Min, F.; Wu, H.; Wang, S.; Feng, C.; Zhang, Y. NiCo2S4/carbon nanotube composites as anode material for lithium-ion batteries. J. Electron. Mater. 2019, 48, 8138–8148. [Google Scholar] [CrossRef]
- Muralidharan, N.; Nallathamby, K. CuCo2S4: Versatile anode for high capacity and high rate for lithium and sodium ion battery application. J. Phys. Chem. Solids 2021, 151, 109902. [Google Scholar] [CrossRef]
- Muruganantham, R.; Chen, J.A.; Yang, C.C.; Wu, P.J.; Wang, F.M.; Liu, W.R. Spinel phase MnIn2S4 enfolded with reduced graphene oxide as composite anode material for lithium-ion storage. Mater. Today Sustain. 2023, 21, 100278. [Google Scholar] [CrossRef]
- Xu, X.; Ji, S.; Gu, M.; Liu, J. In situ synthesis of MnS hollow microspheres on reduced graphene oxide sheets as high-capacity and long-life anodes for Li-and Na-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 20957–20964. [Google Scholar] [CrossRef]
- Hsu, B.H.; Liu, W.R. Synthesis and characterizations of Na4MnCr(PO4)3/rGO as Nasicon-type cathode materials for sodium-ion batteries. Polymers 2022, 14, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhu, J.; Meng, T.; Ma, L.; Zhang, H.; Xu, M.; Jiang, J.; Li, C. One-dimensional integrated MnS@carbon nanoreactors hybrid: An alternative anode for full-cell Li-ion and Na-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 27911–27919. [Google Scholar] [CrossRef]
- Geng, H.; Su, H.; Lin, H.C.; Ma, Y.; Zhang, Y.; Chen, D.; Tan, H.; Rui, X.; Fang, Y.; Li, C. Double-layer N,S-codoped carbon protection of MnS nanoparticles enabling ultralong-life and high-rate lithium ion storage. ACS Appl. Energy Mater. 2018, 1, 4867–4873. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Bresser, D.; Ji, Y.; Geiger, D.; Kaiser, U.; Streb, C.; Varzi, A.; Passerini, S. Cobalt disulfide nanoparticles embedded in porous carbonaceous micro-polyhedrons interlinked by carbon nanotubes for superior lithium and sodium storage. ACS Nano 2018, 12, 7220–7231. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Z.; Yang, X.; Gan, Y.; Chen, W. ZnS nanoparticles embedded in porous carbon matrices as anode materials for lithium ion batteries. RSC Adv. 2015, 5, 86941–86944. [Google Scholar] [CrossRef]
- Ren, J.; Ren, R.P.; Lv, Y.K. Hollow spheres constructed by ultrathin SnS sheets for enhanced lithium storage. J. Mater. Sci. 2020, 55, 7492–7501. [Google Scholar] [CrossRef]
- Yang, X.; Xu, J.; Xi, L.; Yao, Y.; Yang, Q.; Chung, C.; Lee, C. Microwave-assisted synthesis of Cu2ZnSnS4 nanocrystals as a novel anode material for lithium ion battery. J. Nanoparticle Res. 2012, 14, 931. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-J.; Huang, C.-H.; Hsieh, C.-T.; Liu, W.-R. Synthesis and Characterization of MnIn2S4/Single-Walled Carbon Nanotube Composites as an Anode Material for Lithium-Ion Batteries. Nanomaterials 2024, 14, 716. https://doi.org/10.3390/nano14080716
Wu P-J, Huang C-H, Hsieh C-T, Liu W-R. Synthesis and Characterization of MnIn2S4/Single-Walled Carbon Nanotube Composites as an Anode Material for Lithium-Ion Batteries. Nanomaterials. 2024; 14(8):716. https://doi.org/10.3390/nano14080716
Chicago/Turabian StyleWu, Pei-Jun, Chia-Hung Huang, Chien-Te Hsieh, and Wei-Ren Liu. 2024. "Synthesis and Characterization of MnIn2S4/Single-Walled Carbon Nanotube Composites as an Anode Material for Lithium-Ion Batteries" Nanomaterials 14, no. 8: 716. https://doi.org/10.3390/nano14080716




