Detection and Identification of Pesticides in Fruits Coupling to an Au–Au Nanorod Array SERS Substrate and RF-1D-CNN Model Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Apparatus
2.3. FDTD Model
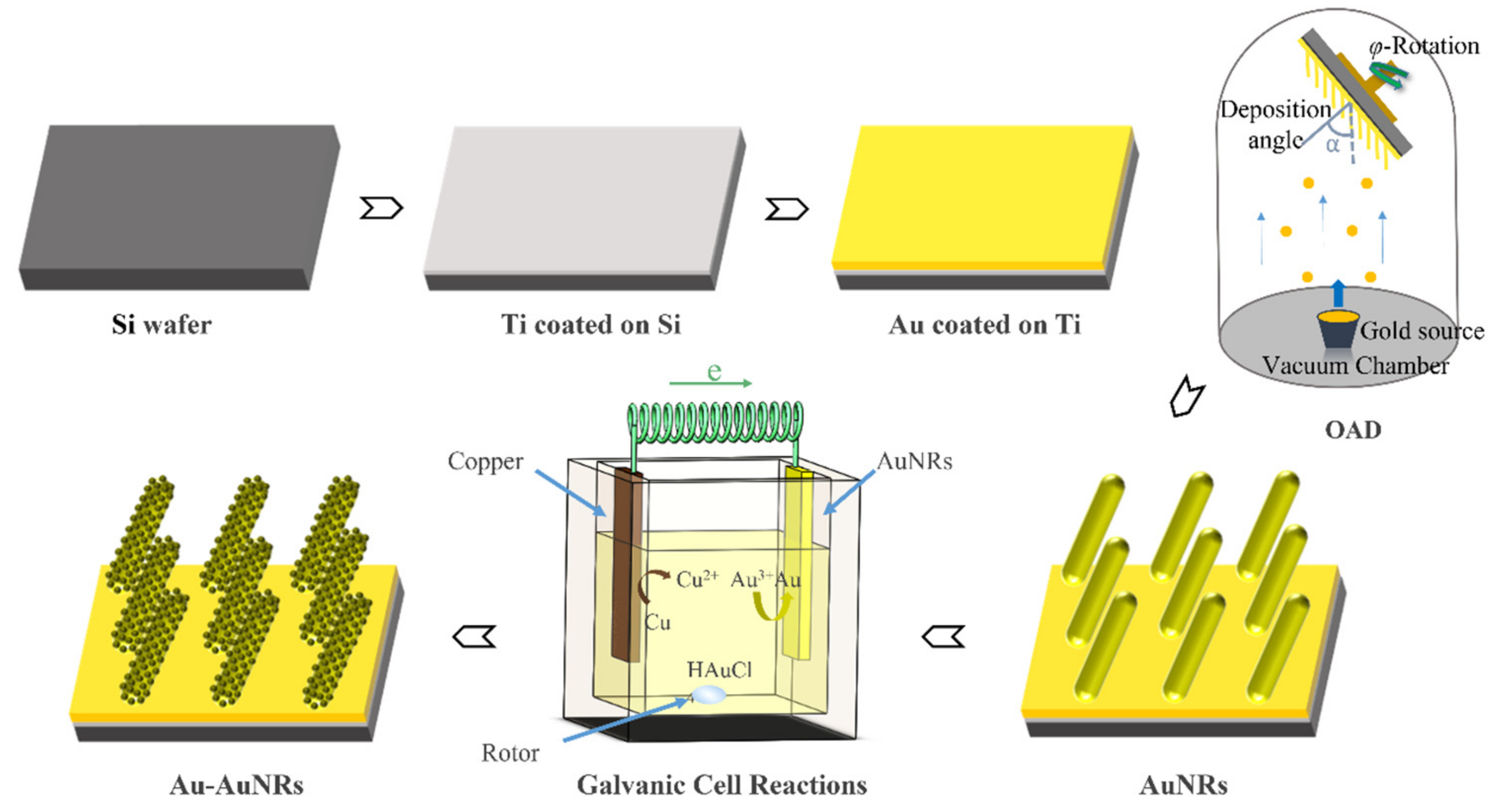
2.4. Preparation of the Au–Au NRA Substrate
2.5. Raman Measurements
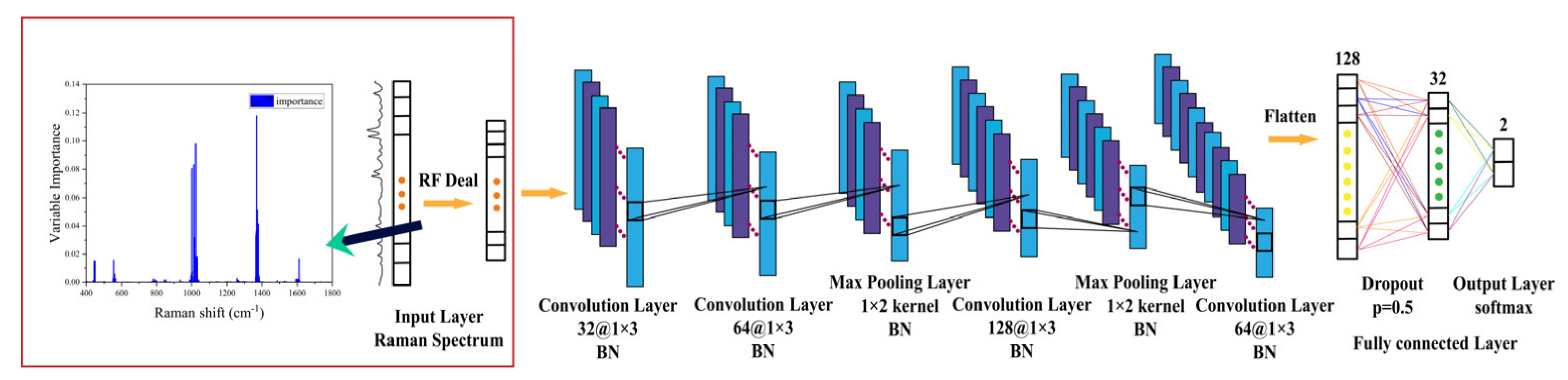
2.6. Data Processing and Division
2.7. RF-1D-CNN Model Construction
2.8. Identification and Evaluation of Models
3. Results and Discussions
3.1. Synthesis and Morphological Characterization of the Au–Au NRA Composite Structure
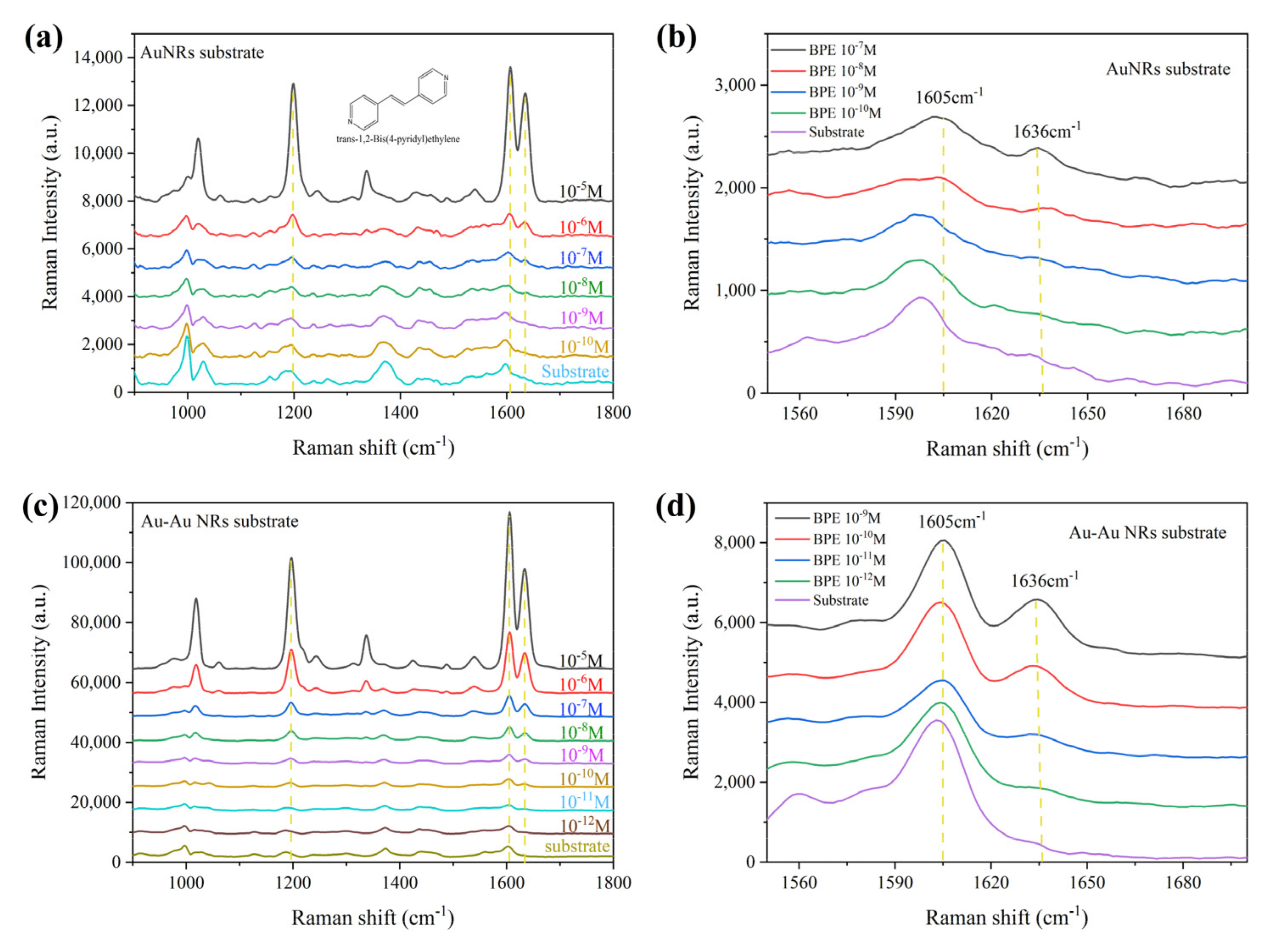
3.2. SERS Performance Characterization
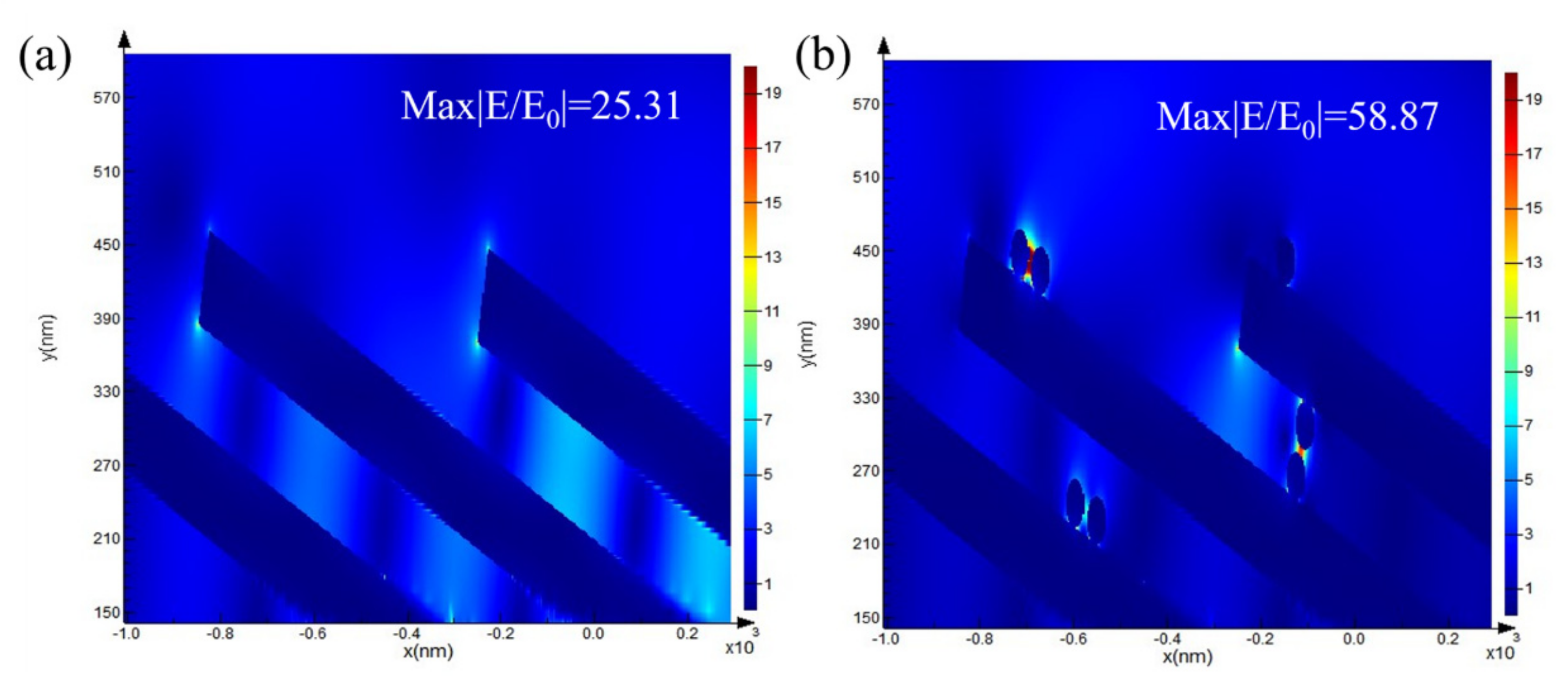
3.3. FDTD Calculation
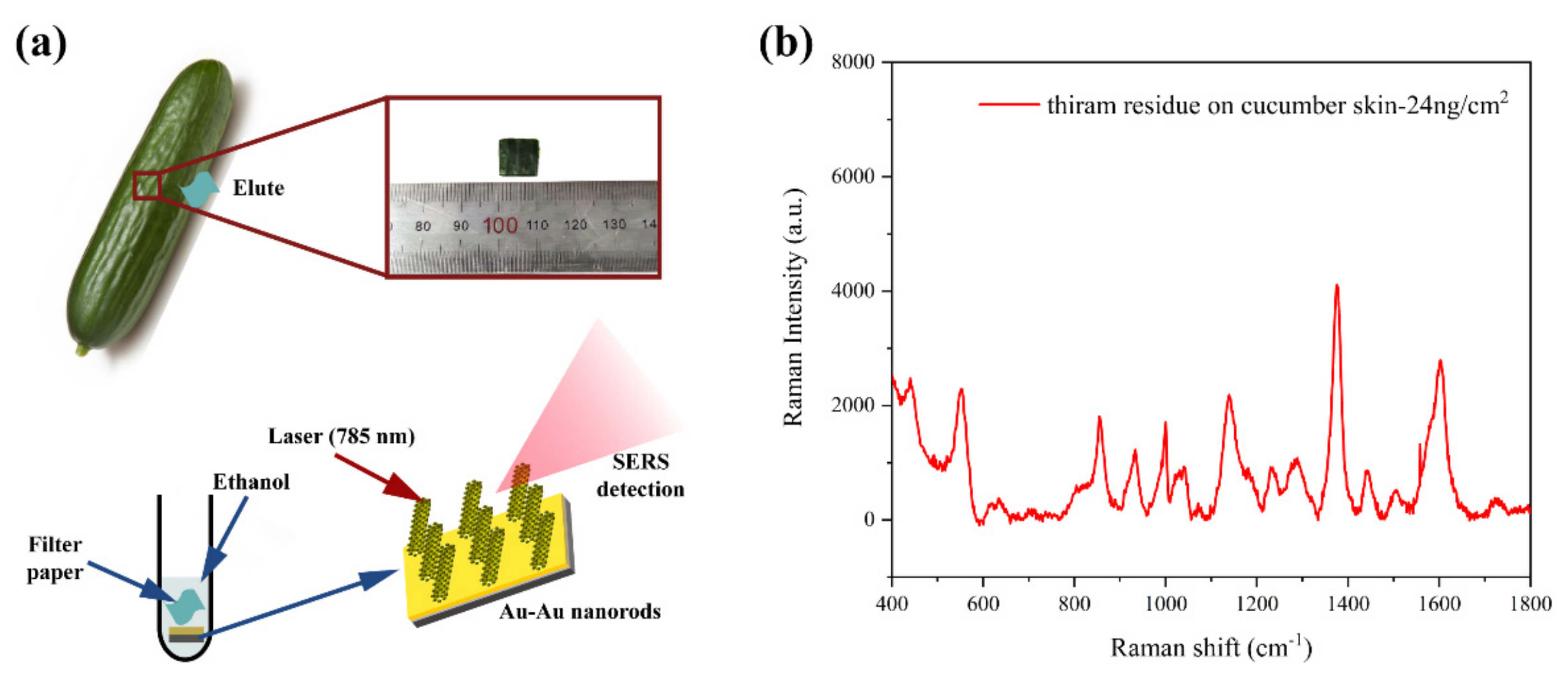
3.4. SERS Detection of Pesticides
3.5. Identification of Pesticides Using RF-1D-CNN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Viehrig, M.; Rajendran, S.T.; Sanger, K.; Schmidt, M.S.; Alstrøm, T.S.; Rindzevicius, T.; Zór, K.; Boisen, A. Quantitative SERS Assay on a Single Chip Enabled by Electrochemically Assisted Regeneration: A Method for Detection of Melamine in Milk. Anal. Chem. 2020, 92, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.E., Jr.; O’Connor, R.; Gallops, C.E.; Kwizera, E.A.; Noroozi, B.; Morshed, B.I.; Wang, Y.; Huang, X. Immunomagnetic Capture and Multiplexed Surface Marker Detection of Circulating Tumor Cells with Magnetic Multicolor Surface-Enhanced Raman Scattering Nanotags. ACS Appl. Mater. Interfaces 2020, 12, 47220–47232. [Google Scholar] [CrossRef] [PubMed]
- Kwizera, E.A.; O’Connor, R.; Vinduska, V.; Williams, M.; Butch, E.R.; Snyder, S.E.; Chen, X.; Huang, X. Molecular Detection and Analysis of Exosomes Using Surface-Enhanced Raman Scattering Gold Nanorods and a Miniaturized Device. Theranostics 2018, 8, 2722–2738. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, B.; Han, F.-Y.; Qin, Z.-N.; Feng, Y.-Q.; Hu, J.-M.; Shen, A.-G. On-Site and Quantitative Detection of Trace Methamphetamine in Urine/Serum Samples with a Surface-Enhanced Raman Scattering-Active Microcavity and Rapid Pretreatment Device. Anal. Chem. 2020, 92, 13539–13549. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Mote, K.R.; MacLaughlin, C.M.; Biswas, N.; Chandra, B.; Basu, J.K.; Walker, G.C.; Madhu, P.K.; Maiti, S. Cell-Membrane-Mimicking Lipid-Coated Nanoparticles Confer Raman Enhancement to Membrane Proteins and Reveal Membrane-Attached Amyloid-β Conformation. ACS Nano 2015, 9, 9070–9077. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-B.; Liu, Q.; Wu, P.; Niu, Q.-F.; Zhang, H.; Zheng, Y.-M. Facile On-Site Aqueous Pollutant Monitoring Using a Flexible, Ultralight, and Robust Surface-Enhanced Raman Spectroscopy Substrate: Interface Self-Assembly of Au@Ag Nanocubes on a Polyvinyl Chloride Template. Environ. Sci. Technol. 2018, 52, 5812–5820. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and Sensitive SERS Platform with Analyte Enrichment and Filtration Function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, P.; Wang, C.; Zhang, C.; Wang, J.; Wu, X. Performance Improving Method of Aligned Silver Nanorod by Grafting Au@Ag Core–Shell Nanoparticles for Surface-Enhanced Raman Scattering. Nano 2017, 12, 1750131. [Google Scholar] [CrossRef]
- Yan, B.; Sun, K.; Chao, K.; Alharbi, N.S.; Li, J.; Huang, Q. Fabrication of a Novel Transparent SERS Substrate Comprised of Ag-nanoparticle Arrays and its Application in Rapid Detection of Ractopamine on Meat. Food Anal. Methods 2018, 11, 2329–2335. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, J.; Zhang, M.; Wang, D.; Wang, Y.; Chen, L.; Zhang, Y.; Yang, J.; Du, Y. Design of Hybrid Nanostructural Arrays to Manipulate SERS-Active Substrates by Nanosphere Lithography. ACS Appl. Mater. Interfaces 2017, 9, 7710–7716. [Google Scholar] [CrossRef]
- Cinel, N.A.; Bütün, S.; Ertaş, G.; Özbay, E. ‘Fairy Chimney’-Shaped Tandem Metamaterials as Double Resonance SERS Substrates. Small 2013, 9, 531–537. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chu, H.Y.; Zhao, Y.P. Silver Nanorod Array Substrates Fabricated by Oblique Angle Deposition: Morphological, Optical, and SERS Characterizations. J. Phys. Chem. C 2010, 114, 8176–8183. [Google Scholar] [CrossRef]
- Singh, J.P.; Lanier, T.E.; Zhu, H.; Dennis, W.M.; Tripp, R.A.; Zhao, Y. Highly Sensitive and Transparent Surface Enhanced Raman Scattering Substrates Made by Active Coldly Condensed Ag Nanorod Arrays. J. Phys. Chem. C 2012, 116, 20550–20557. [Google Scholar] [CrossRef]
- Oh, M.-K.; Shin, Y.-S.; Lee, C.-L.; De, R.; Kang, H.; Yu, N.E.; Kim, B.H.; Kim, J.H.; Yang, J.-K. Morphological and SERS Properties of Silver Nanorod Array Films Fabricated by Oblique Thermal Evaporation at Various Substrate Temperatures. Nanoscale Res. Lett. 2015, 10, 259. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Hou, M.; Li, J.; Xie, Z.; Zhang, Z. Pinhole-Containing, Subnanometer-Thick Al2O3 Shell-Coated Ag Nanorods as Practical Substrates for Quantitative Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2016, 120, 606–615. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Hou, M.; Xie, Z.; Zhang, Z. Ag Nanorods Coated with Ultrathin TiO2 Shells as Stable and Recyclable SERS Substrates. Sci. Rep. 2015, 5, 15442. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, T.; Su, Q.; Wu, X.; Dong, P. Fabrication of Au Nanorods by the Oblique Angle Deposition Process for Trace Detection of Methamphetamine with Surface-Enhanced Raman Scattering. Sensors 2019, 19, 3742. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Dong, P.; Chen, J.; Xiao, R. Hotspots engineering by grafting Au@Ag core-shell nanoparticles on the Au film over slightly etched nanoparticles substrate for on-site paraquat sensing. Biosens. Bioelectron. 2016, 86, 944–950. [Google Scholar] [CrossRef]
- Li, Z.; Sun, K.; Du, Z.; Chen, B.; He, X. Galvanic-Cell-Reaction-Driven Deposition of Large-Area Au Nanourchin Arrays for Surface-Enhanced Raman Scattering. Nanomaterials 2018, 8, 265. [Google Scholar] [CrossRef]
- Li, Z.; Du, Z.; Sun, K.; He, X.; Chen, B. Controllable fabrication of Ag-nanoplate-decorated PAN-nanopillar arrays and their application in surface-enhanced Raman scattering. RSC Adv. 2017, 7, 53157–53163. [Google Scholar] [CrossRef]
- Li, Z.; Meng, G.; Huang, Q.; Hu, X.; He, X.; Tang, H.; Wang, Z.; Li, F. Ag Nanoparticle-Grafted PAN-Nanohump Array Films with 3D High-Density Hot Spots as Flexible and Reliable SERS Substrates. Small 2015, 11, 5452–5459. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.-Y. Shell Thickness-Dependent Raman Enhancement for Rapid Identification and Detection of Pesticide Residues at Fruit Peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zeng, C.; Peng, X.; Qi, W.; Wang, M. Sensitive SERS detection of pesticide residues in beverages based on an extraction integrated plasmonic platform. Sens. Actuators B Chem. 2023, 376, 133042. [Google Scholar] [CrossRef]
- Yusoff, N.N.; Nor Azmi, F.S.; Abu Bakar, N.; Tengku Abdul Aziz, T.H.; Shapter, J.G. Titanium carbide MXene/silver nanostars composite as SERS substrate for thiram pesticide detection. Chem. Pap. 2024, 78, 2855–2865. [Google Scholar] [CrossRef]
- Corvucci, F.; Nobili, L.; Melucci, D.; Grillenzoni, F.-V. The discrimination of honey origin using melissopalynology and Raman spectroscopy techniques coupled with multivariate analysis. Food Chem. 2015, 169, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Thomas, M.R.; Bergholt, M.S.; Pence, I.J.; Seong, H.; Charchar, P.; Todorova, N.; Nagelkerke, A.; Belessiotis-Richards, A.; Payne, D.J.; et al. Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting. Nat. Commun. 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Reokrungruang, P.; Chatnuntawech, I.; Dharakul, T.; Bamrungsap, S. A simple paper-based surface enhanced Raman scattering (SERS) platform and magnetic separation for cancer screening. Sens. Actuators B Chem. 2019, 285, 462–469. [Google Scholar] [CrossRef]
- Uysal Ciloglu, F.; Saridag, A.; Kılıç, İ.; Tokmakci, M.; Kahraman, M.; Aydin, O. Identification of methicillin-resistant Staphylococcus aureus bacteria using surface-enhanced Raman spectroscopy and machine learning techniques. Analyst 2020, 145, 7559–7570. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Wang, Y.; Hua, L.; Chen, A.; Zhang, Y. New method of lung cancer detection by saliva test using surface-enhanced Raman spectroscopy. Thorac. Cancer 2018, 9, 1556–1561. [Google Scholar] [CrossRef]
- Dong, R.; Weng, S.; Yang, L.; Liu, J. Detection and Direct Readout of Drugs in Human Urine Using Dynamic Surface-Enhanced Raman Spectroscopy and Support Vector Machines. Anal. Chem. 2015, 87, 2937–2944. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, R.; Khan, A.; Wahab, N.; Bilal, M.; Ahmed, M. Analysis of dengue infection based on Raman spectroscopy and support vector machine (SVM). Biomed. Opt. Express 2016, 7, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sharma, A.S.; Xu, J.; Xu, Y.; Jiao, T.; Ouyang, Q.; Li, H.; Chen, Q. Rapid on-site identification of pesticide residues in tea by one-dimensional convolutional neural network coupled with surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118994. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, W.; Zhang, J.; Xu, Z. An improved k-nearest neighbour method to diagnose breast cancer. Analyst 2018, 143, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Chen, S.; Liang, Y.-Z. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 2010, 135, 1138–1146. [Google Scholar] [CrossRef]
- Zhou, L.; Poggesi, S.; Casari Bariani, G.; Mittapalli, R.; Adam, P.-M.; Manzano, M.; Ionescu, R.E. Robust SERS Platforms Based on Annealed Gold Nanostructures Formed on Ultrafine Glass Substrates for Various (Bio)Applications. Biosensors 2019, 9, 53. [Google Scholar] [CrossRef]
- Zhao, Y.; Kumar, A.; Yang, Y. Unveiling practical considerations for reliable and standardized SERS measurements: Lessons from a comprehensive review of oblique angle deposition-fabricated silver nanorod array substrates. Chem. Soc. Rev. 2024, 53, 1004–1057. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Soni, R.K. Silver nanodendrites for ultralow detection of thiram based on surface-enhanced Raman spectroscopy. Nanotechnology 2019, 30, 385502. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, J.; Li, M.; Zhang, X.; Luan, L.; Li, P.; Xu, W. Quantitative SERS sensor based on self-assembled Au@Ag heterogeneous nanocuboids monolayer with high enhancement factor for practical quantitative detection. Anal. Bioanal. Chem. 2021, 413, 4207–4215. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Hu, B.; Sun, D.-W. Au@Ag-TGANPs based SERS for facile screening of thiabendazole and ferbam in liquid milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118908. [Google Scholar] [CrossRef]
- Ma, C.-H.; Zhang, J.; Hong, Y.-C.; Wang, Y.-R.; Chen, X. Determination of carbendazim in tea using surface enhanced Raman spectroscopy. Chin. Chem. Lett. 2015, 26, 1455–1459. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Hou, R.; Pang, S.; He, L. In situ SERS detection of multi-class insecticides on plant surfaces. Anal. Methods 2015, 7, 6325–6330. [Google Scholar] [CrossRef]
- Yao, C.; Cheng, F.; Wang, C.; Wang, Y.; Guo, X.; Gong, Z.; Fan, M.; Zhang, Z. Separation, identification and fast determination of organophosphate pesticide methidathion in tea leaves by thin layer chromatography–surface-enhanced Raman scattering. Anal. Methods 2013, 5, 5560–5564. [Google Scholar] [CrossRef]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced Raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Kang, H.; Lee, H.; Jo, A.; Jeong, S.; Jeon, S.-J.; Kim, H.-I.; Lee, H.-Y.; Jeong, D.H.; Kim, J.-H.; et al. Single-Step and Rapid Growth of Silver Nanoshells as SERS-Active Nanostructures for Label-Free Detection of Pesticides. ACS Appl. Mater. Interfaces 2014, 6, 12541–12549. [Google Scholar] [CrossRef] [PubMed]


| Mixture | Models | ACC (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Thiram + TBZ + CBZ ratio: 5%:47.5%:47.5% | SVM | 70 | 100 | 40 |
| RF | 50 | 0 | 100 | |
| KNN | 90 | 100 | 80 | |
| RF-1D-CNN | 100 | 100 | 100 | |
| Thiram + TBZ + CBZ ratio: 10%:45%:45% | SVM | 67.5 | 100 | 30 |
| RF | 97.5 | 95 | 100 | |
| KNN | 90 | 100 | 80 | |
| RF-1D-CNN | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, P.; Zhu, C.; Wang, T.; Dong, P.; Wu, X. Detection and Identification of Pesticides in Fruits Coupling to an Au–Au Nanorod Array SERS Substrate and RF-1D-CNN Model Analysis. Nanomaterials 2024, 14, 717. https://doi.org/10.3390/nano14080717
Sha P, Zhu C, Wang T, Dong P, Wu X. Detection and Identification of Pesticides in Fruits Coupling to an Au–Au Nanorod Array SERS Substrate and RF-1D-CNN Model Analysis. Nanomaterials. 2024; 14(8):717. https://doi.org/10.3390/nano14080717
Chicago/Turabian StyleSha, Pengxing, Chushu Zhu, Tianran Wang, Peitao Dong, and Xuezhong Wu. 2024. "Detection and Identification of Pesticides in Fruits Coupling to an Au–Au Nanorod Array SERS Substrate and RF-1D-CNN Model Analysis" Nanomaterials 14, no. 8: 717. https://doi.org/10.3390/nano14080717







