Cellular Response to Titanium Dioxide Nanoparticles in Intestinal Epithelial Caco-2 Cells is Dependent on Endocytosis-Associated Structures and Mediated by EGFR
Abstract
:1. Introduction
2. Results
2.1. Characterization of TiO2 Particles in Cell Medium
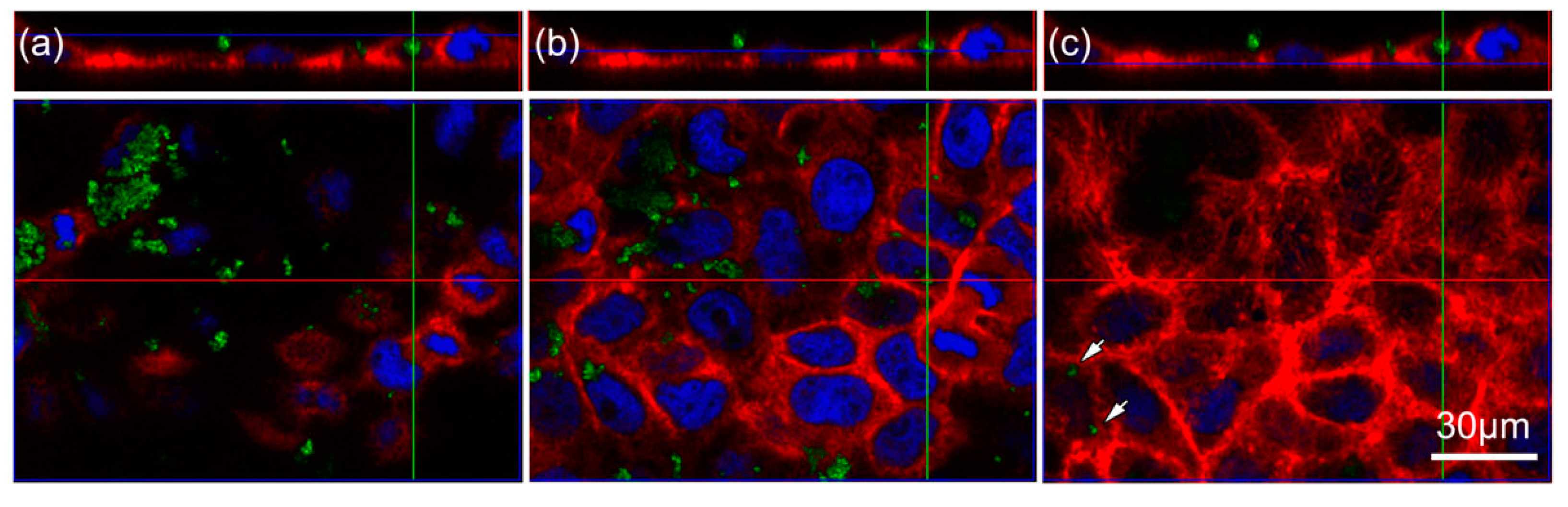
2.2. TiO2 Particles Are Located within the Cell
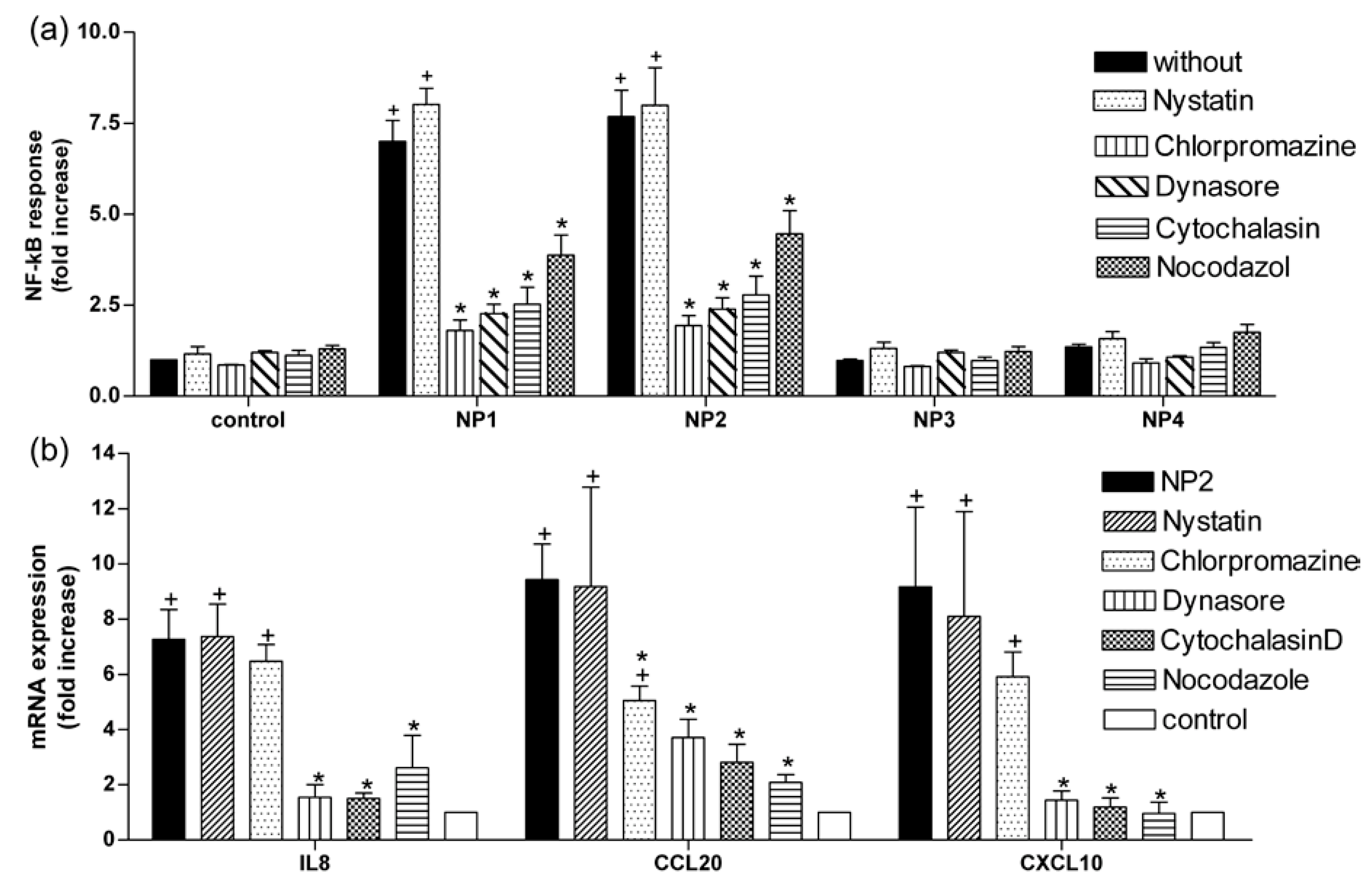
2.3. TiO2 NP-Induced Inflammatory Response Is Reduced by Inhibitors of Endocytosis
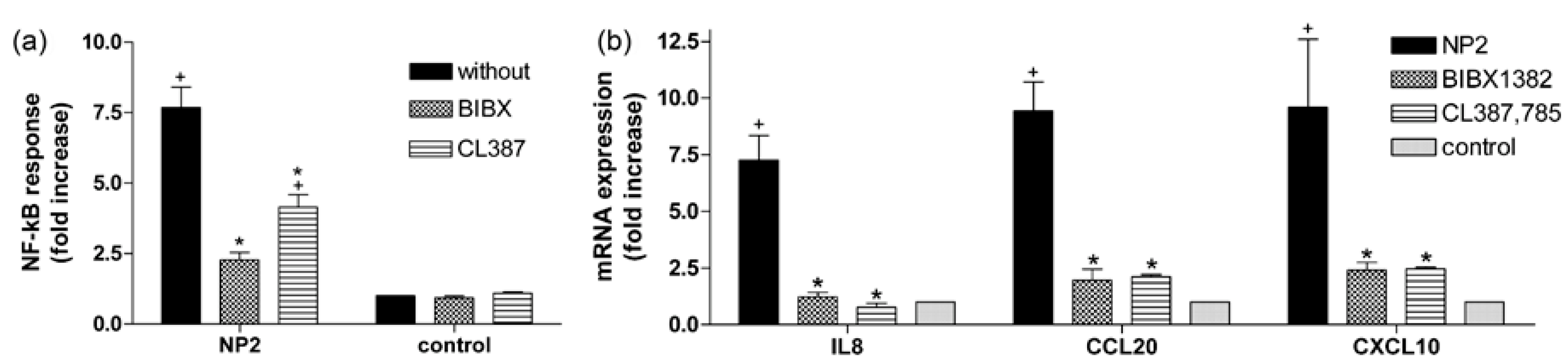
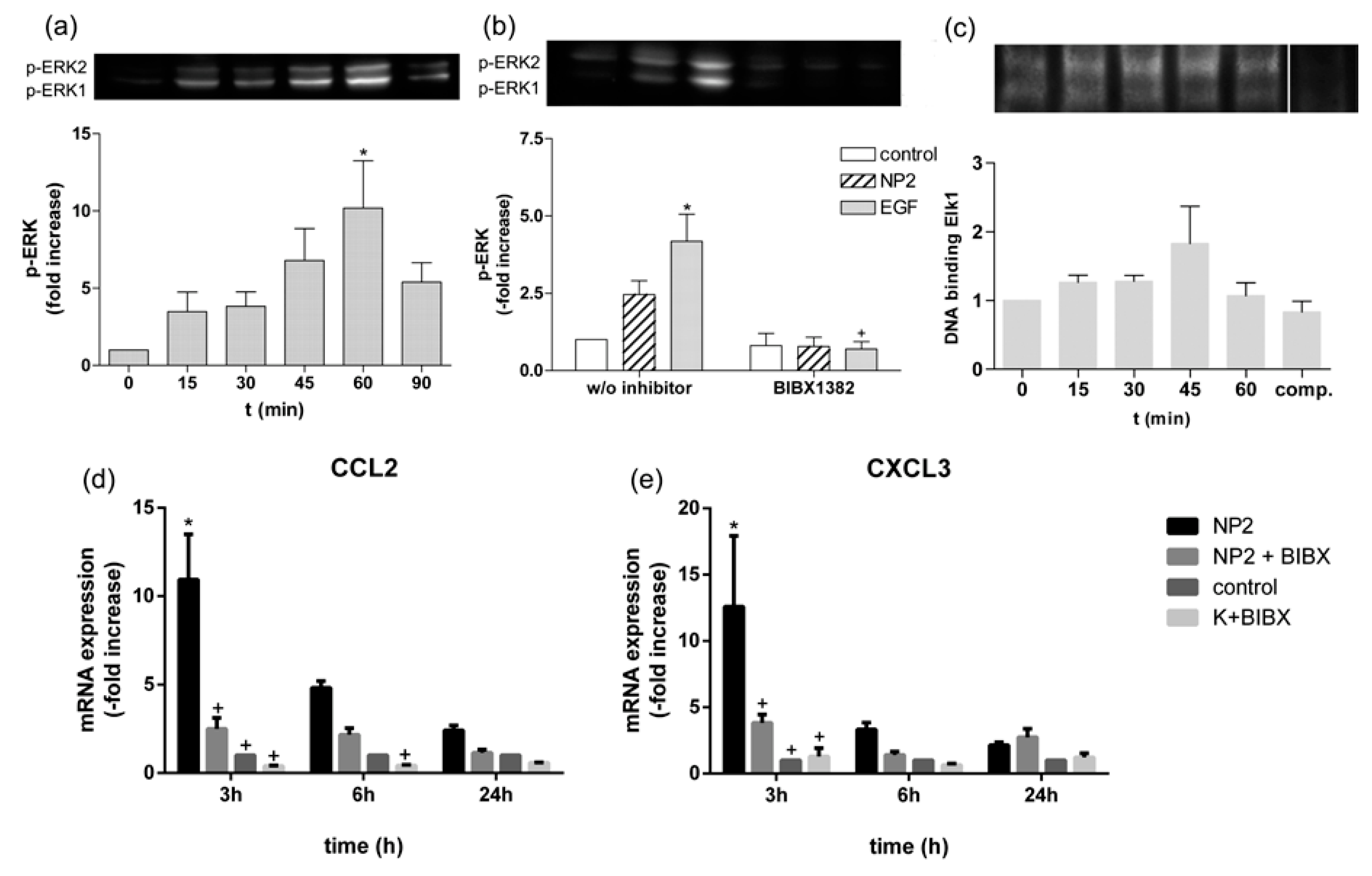
2.4. Activation of EGFR Is Essential for the Cellular Response to TiO2 NPs
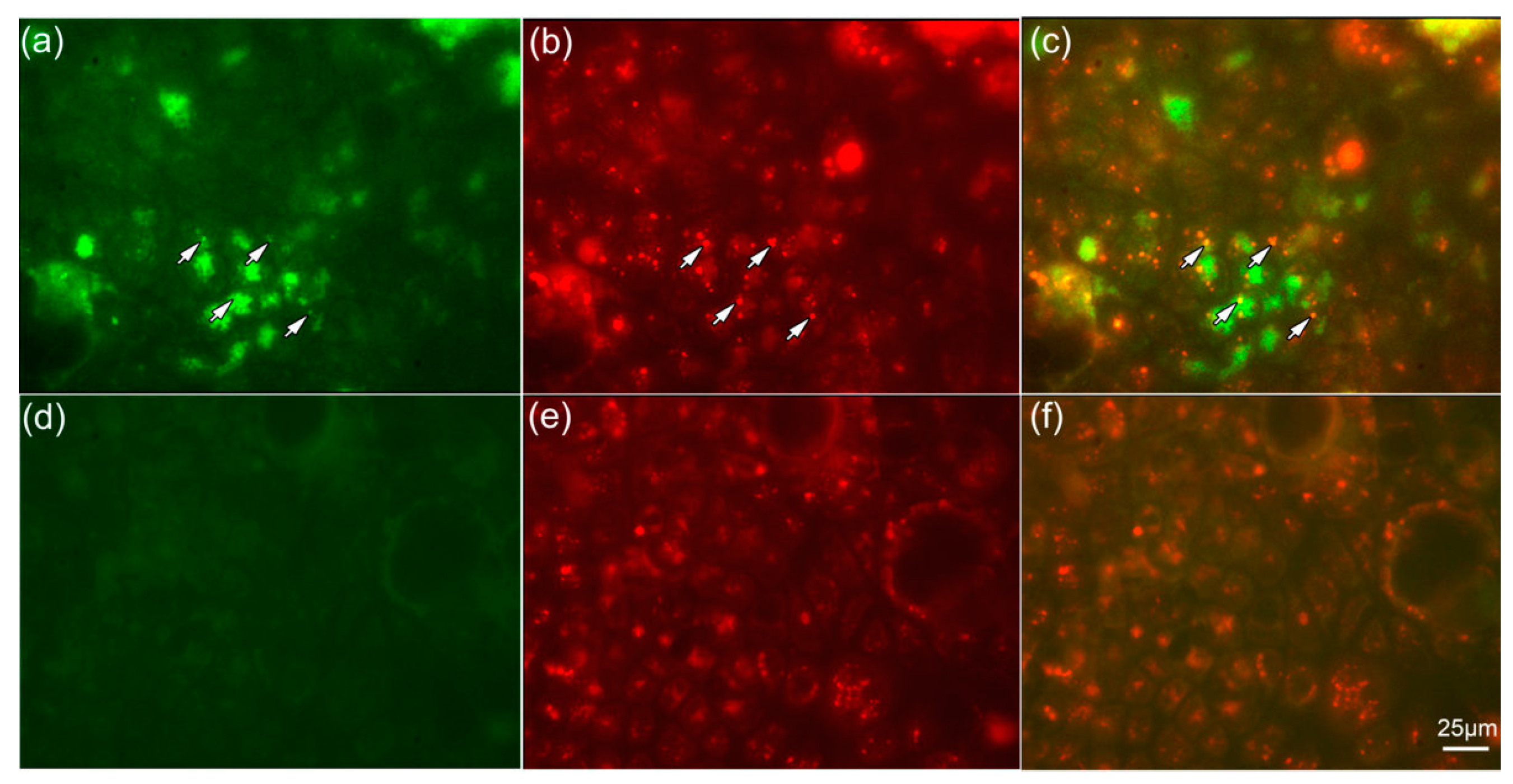
2.5. NPs Are Colocalized with Lysosomes
2.6. TiO2 NPs Activate EGFR/ERK/ELK Signaling Pathway
3. Discussion
4. Material and Methods
4.1. Preparation of TiO2 NPs
4.2. Nanoparticle Characterization
4.3. Cell Culture
4.4. mRNA Expression Analysis
4.5. NF-κB Reporter Gene Assay
4.6. Fluorescence and Confocal Laser Scanning Microscopy (CLSM)
4.7. Western Blot Analyses
4.8. Electrophoretic Mobility Shift Assay (EMSA)
4.9. Cell Treatment
4.10. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 132242–132250. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.; de Berardis, B.; Bizzarri, L.; Degan, P.; Andreoli, C.; Zijno, A.; de Angelis, I. Physico-chemical characteristics and cyto-genotoxic potential of ZnO and TiO2 nanoparticles on human colon carcinoma cells. J. Phys. Conf. Ser. 2011, 304, 012047. [Google Scholar] [CrossRef]
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.W.; Förster, I.; Schins, R.P.F. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, K.; Albrecht, C.; Boots, A.W.; Förster, I.; Schins, R.P.F. Cytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco-2 cells. Nanotoxicology 2009, 3, 355–364. [Google Scholar] [CrossRef]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Käkinen, A.; Sihtmäe, M.; Pokhrel, S.; Mädler, L.; Heinlaan, M.; Kisand, V.; et al. Toxicity of 11 Metal Oxide Nanoparticles to Three Mammalian Cell Types In 9 itro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, M.; Berenguer, F.; Steinmetz, G.; Auffan, M.; Rose, J.; Prat, O. Intestinal toxicity evaluation of TiO2 degraded surface-treated nanoparticles: A combined physico-chemical and toxicogenomics approach in caco-2 cells. Part. Fibre Toxicol. 2012, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Chalew, T.E.A.; Schwab, K.J. Toxicity of commercially available engineered nanoparticles to Caco-2 and SW480 human intestinal epithelial cells. Cell Biol. Toxicol. 2013, 29, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Cossais, F.; Neve, H.; Klempt, M. Titanium dioxide nanoparticles activate IL8-related inflammatory pathways in human colonic epithelial Caco-2 cells. J. Nanopart. Res. 2014, 16, 2402. [Google Scholar] [CrossRef]
- Mano, S.S.; Kanehira, K.; Taniguchi, A. Comparison of Cellular Uptake and Inflammatory Response via Toll-Like Receptor 4 to Lipopolysaccharide and Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2013, 14, 13154–13170. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Migita, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Development of sensor cells using NF-κB pathway activation for detection of nanoparticle-induced inflammation. Sensors 2010, 11, 7219–7230. [Google Scholar] [CrossRef] [PubMed]
- Gitrowski, C.; Al-Jubory, A.R.; Handy, R.D. Uptake of different crystal structures of TiO2 nanoparticles by Caco-2 intestinal cells. Toxicol. Lett. 2014, 226, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schürch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Hof, V.I.; Heyder, J.; Gehr, P. Ultrafine Particles Cross Cellular Membranes by Nonphagocytic Mechanisms in Lungs and in Cultured Cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lin, P.; Jia, Z.; Du, W.; Qu, W.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The transport mechanisms of polymer nanoparticles in Caco-2 epithelial cells. Biomaterials 2013, 34, 6082–6098. [Google Scholar] [CrossRef] [PubMed]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of Nanoparticle Internalization and Transport across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.; del Molino, E.M.; Fernández-Rosas, E.; Fernández, A.; Vázquez-Campos, S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014, 228, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2009, 26, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 2006, 24, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.J.; Doudrick, K.; Yang, Y.; Westerhoff, P.; Capco, D.G. Food grade titanium dioxide disrupts intestinal brush border microvilli in vitro independent of sedimentation. Cell Biol. Toxicol. 2014, 30, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Apodaca, G. Endocytic Traffic in Polarized Epithelial Cells: Role of the Actin and Microtubule Cytoskeleton. Traffic 2001, 2, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Solca, F.F.; Baum, A.; Langkopf, E.; Dahmann, G.; Heider, K.; Himmelsbach, F.; van Meel, J.C.A. Inhibition of Epidermal Growth Factor Receptor Activity by Two Pyrimidopyrimidine Derivatives. J. Pharmacol. Exp. Ther. 2004, 311, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Qualmann, B.; Kessels, M.M.; Kelly, R.B. Molecular Links between Endocytosis and the Actin Cytoskeleton. J. Cell Biol. 2000, 150, 111–116. [Google Scholar] [CrossRef]
- Schafer, D.A. Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 2002, 14, 76–81. [Google Scholar] [CrossRef]
- Goddettes, D.W.; Frieden, C. Actin Polymerization. The mechanism of action of cytochalasin D. J. Biol. Chem. 1986, 261, 15974–15980. [Google Scholar]
- Baker, N.T.; Graham, L.L. Campylobacter fetus translocation across Caco-2 cell monolayers. Microb. Pathog. 2010, 49, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ze’ev, A.; Farmer, S.R.; Penman, S. Mechanisms of Regulating Tubulin Synthesis in Cultured Mammalian Cells. Cell 1979, 17, 319–325. [Google Scholar] [CrossRef]
- Vasquez, R.J.; Howell, B.; Yvon, A.M.; Wadsworth, P.; Cassimeris, L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 1997, 8, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N.; Richmond, A. The role of chemokines in intestinal inflammation and cancer. Curr. Opin. Pharmacol. 2009, 9, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Busch, W.; Bastian, S.; Trahorsch, U.; Iwe, M.; Kühnel, D.; Meißner, T.; Springer, A.; Gelinsky, M.; Richter, V.; Ikonomidou, C.; et al. Internalisation of engineered nanoparticles into mammalian cells in vitro: Influence of cell type and particle properties. J. Nanopart. Res. 2010, 13, 293–310. [Google Scholar] [CrossRef]
- Scherbart, A.M.; Langer, J.; Bushmelev, A.; van Berlo, D.; Haberzettl, P.; van Schooten, F.-J.; Schmidt, A.M.; Rose, C.R.; Schins, R.P.; Albrecht, C. Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms. Part. Fibre Toxicol. 2011, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Allouni, Z.E.; Høl, P.J.; Cauqui, M.A.; Gjerdet, N.R.; Cimpan, M.R. Role of physicochemical characteristics in the uptake of TiO2 nanoparticles by fibroblasts. Toxicol. In Vitro 2012, 26, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, M.L.; Skretting, G.; van Deurs, B.; Sandvig, K. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 2001, 114, 3737–3747. [Google Scholar] [PubMed]
- Krieger, S.E.; Kim, C.; Zhang, L.; Marjomaki, V.; Bergelson, J.M. Echovirus 1 entry into polarized Caco-2 cells depends on dynamin, cholesterol, and cellular factors associated with macropinocytosis. J. Virol. 2013, 87, 8884–8895. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nagai, J.; Mitsui, N.; Yumoto, R.; Takano, M. Effects of endocytosis inhibitors on internalization of human IgG by Caco-2 human intestinal epithelial cells. Life Sci. 2009, 85, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.-S.; Glenney, J.R.; Anderson, R.G.W. Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Al-Jubory, A.R.; Handy, R.D. Uptake of titanium from TiO2 nanoparticle exposure in the isolated perfused intestine of rainbow trout: Nystatin, vanadate and novel CO2—Sensitive components. Nanotoxicology 2013, 7, 1282–1301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rothberg, K.G.; Anderson, R.G.W. Mis-Assembly of Clathrin Lattices on Endosomes Reveals a Regulatory Switch for Coated Pit Formation Materials and Methods. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, J.E. Dynamin and its role in Membrane Fission. Annu. Rev. Cell Dev. Biol. 2000, 16, 483–519. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, J.; Isono, K.; Takeyama, K.; Tagaya, E.; Nakata, J.; Nagai, A. Ultrafine carbon black particles stimulate proliferation of human airway epithelium via EGF receptor-mediated signaling pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Unfried, K.; Sydlik, U.; Bierhals, K.; Weissenberg, A.; Abel, J. Carbon nanoparticle-induced lung epithelial cell proliferation is mediated by receptor-dependent Akt activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L358–L367. [Google Scholar] [CrossRef] [PubMed]
- Discafani, C.M.; Carroll, M.L.; Floyd, M.B.; Hollander, I.J.; Husain, Z.; Johnson, B.D.; Kitchen, D.; May, M.K.; Malo, M.S.; Minnick, A.A.; et al. Irreversible Inhibition of Epidermal Growth Factor Receptor Tyrosine Kinase with In Vivo Activity by N-[4-[(3-Bromophenyl) Amino]-6-Quinazolinyl]-2-Butynamide (CL-387,785). Biochem. Pharmacol. 1999, 57, 917–925. [Google Scholar] [CrossRef]
- Giudice, M.C.L.; Herda, L.M.; Polo, E.; Dawson, K.A. In situ characterization of nanoparticle biomolecular interactions in complex biological media by flow cytometry. Nat. Commun. 2016, 7, 13475. [Google Scholar] [CrossRef] [PubMed]
- Strojan, K.; Leonardi, A.; Bregar, V.B.; KrizÏaj, I.; Svete, J.; Pavlin, M. Dispersion of Nanoparticles in Different Media Importantly Determines the Composition of Their Protein Corona. PLoS ONE 2017, 12, e0169552. [Google Scholar] [CrossRef] [PubMed]
- Marucco, A.; Gazzano, E.; Ghigo, D.; Enrico, E.; Fenoglio, I. Fibrinogen enhances the inflammatory response of alveolar macrophages to TiO2, SiO2 and carbon nanomaterials. Nanotoxicology 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Schweppe, R.E.; Tom, H.C.; Ahn, N.G. Global gene expression analysis of ERK5 and ERK1/2 signaling reveals a role for HIF-1 in ERK5-mediated responses. J. Biol. Chem. 2006, 281, 20993–21003. [Google Scholar] [CrossRef] [PubMed]
- Totlandsdal, A.I.; Refsnes, M.; Låg, M. Mechanisms involved in ultrafine carbon black- induced release of IL-6 from primary rat epithelial lung cells. Toxicol. In Vitro 2010, 24, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Chung, K.-H.; Ryu, D.-Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Comfort, K.K.; Maurer, E.I.; Braydich-Stolle, L.K.; Hussain, S.M. Interference of Silver, Gold, and Iron Oxide Nanoparticles on Epidermal Growth Factor Signal Transduction in Epithelial Cells. ACS Nano 2011, 5, 10000–10008. [Google Scholar] [CrossRef] [PubMed]
- Weissenberg, A.; Sydlik, U.; Peuschel, H.; Schroeder, P.; Schneider, M.; Schins, R.P.F.; Abel, J.; Unfried, K. Reactive oxygen species as mediators of membrane-dependent signaling induced by ultrafine particles. Free Radic. Biol. Med. 2010, 49, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Peuschel, H.; Sydlik, U.; Haendeler, J.; Büchner, N.; Stöckmann, D.; Kroker, M.; Wirth, R.; Brock, W.; Unfried, K. C-Src-mediated activation of Erk1/2 is a reaction of epithelial cells to carbon nanoparticle treatment and may be a target for a molecular preventive strategy. Biol. Chem. 2010, 391, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Sydlik, U.; Bierhals, K.; Soufi, M.; Abel, J.; Schins, R.P.F.; Unfried, K. Ultrafine carbon particles induce apoptosis and proliferation in rat lung epithelial cells via specific signaling pathways both using EGF-R. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L725–L733. [Google Scholar] [CrossRef] [PubMed]
- Skuland, T.; Øvrevik, J.; Låg, M.; Schwarze, P.; Refsnes, M. Silica nanoparticles induce cytokine responses in lung epithelial cells through activation of a p38/TACE/TGF-α/EGFR- pathway and NF-κΒ signaling. Toxicol. Appl. Pharmacol. 2014, 279, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Mariotti, F.; Dattilo, C.; Mariani, V.; Pastore, S.; Mascia, F.; Mariotti, F.; Dattilo, C.; Mariani, V.; et al. ERK1/2 Regulates Epidermal Chemokine Expression and Skin Inflammation. J. Immunol. 2005, 174, 5047–5056. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Gardina, P.; Myers, T.G.; Leto, T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011, 4, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Tang, Y.; Ng, H.S.; Zhao, X.; Jiang, Z.; Chen, Z.; Ng, K.W.; Loo, S.C.J. Specific surface area of titanium dioxide (TiO2) particles influences cyto- and photo-toxicity. Toxicology 2013, 304, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kaulfuss, S.; Burfeind, P.; Gaedcke, J.; Scharf, J.-G. Dual silencing of insulin-like growth factor-I receptor and epidermal growth factor receptor in colorectal cancer cells is associated with decreased proliferation and enhanced apoptosis. Mol. Cancer Ther. 2009, 8, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [PubMed]
- Hennenberg, M.; Strittmatter, F.; Beckmann, C.; Rutz, B.; Füllhase, C.; Waidelich, R.; Montorsi, F.; Hedlund, P.; Andersson, K.E.; Stief, C.G.; et al. Silodosin Inhibits Noradrenaline-Activated Transcription Factors Elk1 and SRF in Human Prostate Smooth 7 Muscle. PLoS ONE 2012, 7, e50904. [Google Scholar] [CrossRef] [PubMed]
- Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Brunner, C.; Kirchhausen, T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 2006, 10, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bergelson, M.J. Echovirus 7 Entry into Polarized Intestinal Epithelial Cells Requires. Am. Soc. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Broeck, D.V.; de Wolf, M. Selective blocking of clathrin-mediated endocytosis by RNA interference: Epsin as target protein. Biotechniques 2006, 41, 475–484. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]


| Inhibitor | Effect | Reference | Concentration | Preincubated Time |
|---|---|---|---|---|
| Cytochalasin D | Inhibits actin polymerization | [26,27] | 0.1 µg mL−1 | 30 min |
| Nocodazole | Depolymerizes microtubules | [28,29] | 2 µM | 30 min |
| Nystatin | Inhibits Caveolae-dependent endocytosis | [37] | 50 µg mL−1 | 30 min |
| Dynasore | Inhibits dynamin | [62,63] | 100 µM | 1 h |
| Chlorpromazine | Inhibitor of clathrin-mediated endocytosis | [35,64] | 100 µM | 1 h |
| BIBX 1382 | EGFR kinase inhibitor | [23] | 46 µM | 1 h |
| CL-387785 | EGFR kinase inhibitor | [43] | 20 µM | 3 h |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, K.; Schrader, K.; Klempt, M. Cellular Response to Titanium Dioxide Nanoparticles in Intestinal Epithelial Caco-2 Cells is Dependent on Endocytosis-Associated Structures and Mediated by EGFR. Nanomaterials 2017, 7, 79. https://doi.org/10.3390/nano7040079
Krüger K, Schrader K, Klempt M. Cellular Response to Titanium Dioxide Nanoparticles in Intestinal Epithelial Caco-2 Cells is Dependent on Endocytosis-Associated Structures and Mediated by EGFR. Nanomaterials. 2017; 7(4):79. https://doi.org/10.3390/nano7040079
Chicago/Turabian StyleKrüger, Kristin, Katrin Schrader, and Martin Klempt. 2017. "Cellular Response to Titanium Dioxide Nanoparticles in Intestinal Epithelial Caco-2 Cells is Dependent on Endocytosis-Associated Structures and Mediated by EGFR" Nanomaterials 7, no. 4: 79. https://doi.org/10.3390/nano7040079





