Spatial and Temperature Resolutions of Magnetic Nanoparticle Temperature Imaging with a Scanning Magnetic Particle Spectrometer
Abstract
:1. Introduction
2. Experimental Description
2.1. Experimental Methods for MNP Temperature Imaging
2.2. Experimental Setup and Materials
3. Results and Discussion
3.1. Calibration of Temperature-Dependent Harmonic Ratio
3.2. MNP Temperature Imaging
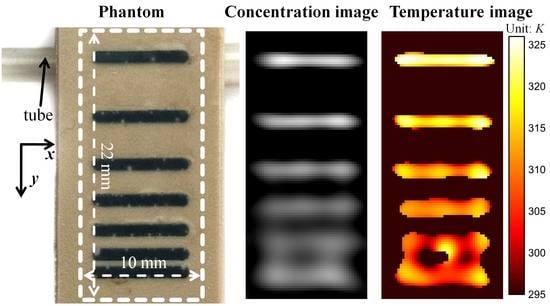
3.3. Spatial Resolution
3.4. Temperature Resolution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vreugdenburg, T.D.; Willis, C.D.; Mundy, L.; Hiller, J.E. A systematic review of elastography, electrical impedance scanning, and digital infrared thermography for breast cancer screening and diagnosis. Breast Cancer Res. Treat. 2013, 137, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Ng, E.Y.-K.; Tan, J.-H.; Sree, S.V. Thermography based breast cancer detection using texture features and support vector machine. J. Med. Syst. 2012, 36, 1503. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldöfner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387. [Google Scholar]
- Li, L.; Hagen, T.L.T.; Schipper, D.; Wijnberg, T.M.; van Rhoon, G.C.; Eggermont, A.M.; Lindner, L.H.; Koning, G.A. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release 2010, 143, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, W.; Du, Z.; de Morais, P.C.; Xiang, Q.; Xie, Q. A noninvasive, remote and precise method for temperature and concentration estimation using magnetic nanoparticles. Nanotechnology 2012, 23, 075703. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, W.; Jiang, L.; Yang, M.; Morais, P.C. Real-time magnetic nanothermometry: The use of magnetization of magnetic nanoparticles assessed under low frequency triangle-wave magnetic fields. Rev. Sci. Instrum. 2014, 85, 094905. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, W.; Kong, L.; Morais, P.C. A new approach for highly accurate, remote temperature probing using magnetic nanoparticles. Sci. Rep. 2014, 4, 6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.B.; Rauwerdink, A.M.; Hansen, E.W. Magnetic nanoparticle temperature estimation. Med. Phys. 2009, 36, 1822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhong, J.; Liu, W.; Du, Z.; Huang, Z.; Yang, M.; Morais, P.C. Study of magnetic nanoparticle spectrum for magnetic nanothermometry. IEEE Trans. Magn. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- He, L.; Liu, W.; Xie, Q.; Pi, S.; Morais, P. A fast and remote magnetonanothermometry for a liquid environment. Meas. Sci. Technol. 2015, 27, 025901. [Google Scholar] [CrossRef] [Green Version]
- Garaio, E.; Collantes, J.-M.; Garcia, J.A.; Plazaola, F.; Sandre, O. Harmonic phases of the nanoparticle magnetization: An intrinsic temperature probe. Appl. Phys. Lett. 2015, 107, 123103. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecke, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214. [Google Scholar] [CrossRef] [PubMed]
- Sattel, T.F.; Knopp, T.; Biederer, S.; Gleich, B.; Weizenecker, J.; Borgert, J.; Buzug, T.M. Single-sided device for magnetic particle imaging. J. Phys. D Appl. Phys. 2008, 42, 022001. [Google Scholar] [CrossRef] [Green Version]
- Vogel, P.; Ruckert, M.A.; Klauer, P.; Kullmann, W.H.; Jakob, P.M.; Behr, V.C. Traveling wave magnetic particle imaging. IEEE Trans. Med. Imaging 2014, 33, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, P.W.; Conolly, S.M. The X-space formulation of the magnetic particle imaging process: 1-D signal, resolution, bandwidth, SNR, SAR, and magnetostimulation. IEEE Trans. Med. Imaging 2010, 29, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Weizenecker, J.; Gleich, B.; Borgert, J. Magnetic particle imaging using a field free line. J. Phys. D Appl. Phys. 2008, 41, 105009. [Google Scholar] [CrossRef]
- Goodwill, P.W.; Konkle, J.J.; Zheng, B.; Saritas, E.U.; Conolly, S.M. Projection x-space magnetic particle imaging. IEEE Trans. Med. Imaging 2012, 31, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Liu, W.; Jiang, T. Real-time and quantitative isotropic spatial resolution susceptibility imaging for magnetic nanoparticles. Meas. Sci. Technol. 2018, 29, 035402. [Google Scholar] [CrossRef] [Green Version]
- Rahmer, J.; Halkola, A.; Gleich, B.; Schmale, I.; Borgert, J. First experimental evidence of the feasibility of multi-color magnetic particle imaging. Phys. Med. Biol. 2015, 60, 1775. [Google Scholar] [CrossRef] [PubMed]
- Viereck, T.; Kuhlmann, C.; Draack, S.; Schilling, M.; Ludwig, F. Dual-frequency magnetic particle imaging of the Brownian particle contribution. J. Magn. Magn. Mater. 2017, 427, 156–161. [Google Scholar] [CrossRef]
- Stehning, C.; Gleich, B.; Rahmer, J. Simultaneous magnetic particle imaging (MPI) and temperature mapping using multi-color MPI. Int. J. Magn. Part. Imaging 2016, 2, 1612001. [Google Scholar]
- Richter, H.; Kettering, M.; Wiekhorst, F.; Steinhoff, U.; Hilger, I.; Trahms, L. Magnetorelaxometry for localization and quantification of magnetic nanoparticles for thermal ablation studies. Phys. Med. Biol. 2010, 55, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Fodil, K.; Denoual, M.; Dolabdjian, C. Experimental and analytical investigation of a 2-D magnetic imaging method using magnetic nanoparticles. IEEE Trans. Magn. 2016, 52, 1–9. [Google Scholar] [CrossRef]
- Zhong, J.; Schilling, M.; Ludwig, F. Magnetic nanoparticle temperature imaging with a scanning magnetic particle spectrometer. Meas. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Zhong, J.; Dieckhoff, J.; Schilling, M.; Ludwig, F. Influence of static magnetic field strength on the temperature resolution of a magnetic nanoparticle thermometer. J. Appl. Phys. 2016, 120, 143902. [Google Scholar] [CrossRef]
- Zhong, J.; Schilling, M.; Ludwig, F. Magnetic nanoparticle thermometry independent of Brownian relaxation. J. Phys. D Appl. Phys. 2017, 51, 015001. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.; Kak, A.C. Simultaneous algebraic reconstruction technique (SART): A superior implementation of the ART algorithm. Ultrason. Imaging 1984, 6, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Dieckhoff, J.; Eberbeck, D.; Schilling, M.; Ludwig, F. Magnetic-field dependence of Brownian and Neel relaxation times. J. Appl. Phys. 2016, 119, 043903. [Google Scholar] [CrossRef]
- Draack, S.; Viereck, T.; Kuhlmann, C.; Schilling, M.; Ludwig, F. Temperature-dependent MPS measurements. Int. J. Magn. Part. Imaging 2017, 3, 1703018. [Google Scholar]
- Wells, J.; Paysen, H.; Kosch, O.; Trahms, L.; Wiekhorst, F. Temperature dependence in magnetic particle imaging. AIP Adv. 2018, 8, 056703. [Google Scholar] [CrossRef]
- Judy, P. The line spread function and modulation transfer function of a computed tomographic scanner. Med. Phys. 1976, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Knopp, T.; Biederer, S.; Sattel, T.F.; Erbe, M.; Buzug, T.M. Prediction of the spatial resolution of magnetic particle imaging using the modulation transfer function of the imaging process. IEEE Trans. Med. Imaging 2011, 30, 1284–1292. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Schilling, M.; Ludwig, F. Spatial and Temperature Resolutions of Magnetic Nanoparticle Temperature Imaging with a Scanning Magnetic Particle Spectrometer. Nanomaterials 2018, 8, 866. https://doi.org/10.3390/nano8110866
Zhong J, Schilling M, Ludwig F. Spatial and Temperature Resolutions of Magnetic Nanoparticle Temperature Imaging with a Scanning Magnetic Particle Spectrometer. Nanomaterials. 2018; 8(11):866. https://doi.org/10.3390/nano8110866
Chicago/Turabian StyleZhong, Jing, Meinhard Schilling, and Frank Ludwig. 2018. "Spatial and Temperature Resolutions of Magnetic Nanoparticle Temperature Imaging with a Scanning Magnetic Particle Spectrometer" Nanomaterials 8, no. 11: 866. https://doi.org/10.3390/nano8110866