Biocompatible Materials Based on Self-Assembling Peptides on Ti25Nb10Zr Alloy: Molecular Structure and Organization Investigated by Synchrotron Radiation Induced Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.1.1. Ti25Nb10Zr Alloy Preparation and Preliminary Characterization
2.1.2. Ti25Nb10Zr Alloy Surfaces Functionalization
- -
- MLs: Ti25Nb10Zr discs were sonicated in acetone for 5 min, dried, incubated in the peptide solution for 18 h, washed three times with NaCl 0.10 M at pH 7 and finally three times with distilled water. In these samples the set pH 7 solution was buffered with Hank’s solution to mimic the extracellular physiological environment. Unfortunately, Hank’s solution altered the ionic strength and interfered with peptide deposition. For this reason, the pH 7 sample was prepared again, avoiding the addition of sodium phosphate and other salts, except NaCl 10 mM, and maintaining the 10 mM NaCl washing treatment.
- -
- MULs: Ti25Nb10Zr discs were sonicated in acetone for 5 min and dried. Peptide films were cast by covering the alloy surface with 2–3 drops of 1 mg/mL solutions of EAbuK16 oligopeptide prepared at different pHs, then dried in a low vacuum glass line.
2.2. Spectroscopic Techniques
2.2.1. X-ray Photoelectron Spectroscopy
2.2.2. Near Edge X-ray Absorption Fine Structure Spectroscopy
2.3. Microscopy Techniques
2.3.1. Field Emission Scanning Electron Microscopy
2.3.2. Atomic Force Microscopy
3. Results
3.1. Characterization of Pristine Ti25Nb10Zr
3.1.1. Assessment of the Elemental Composition and Homogeneity
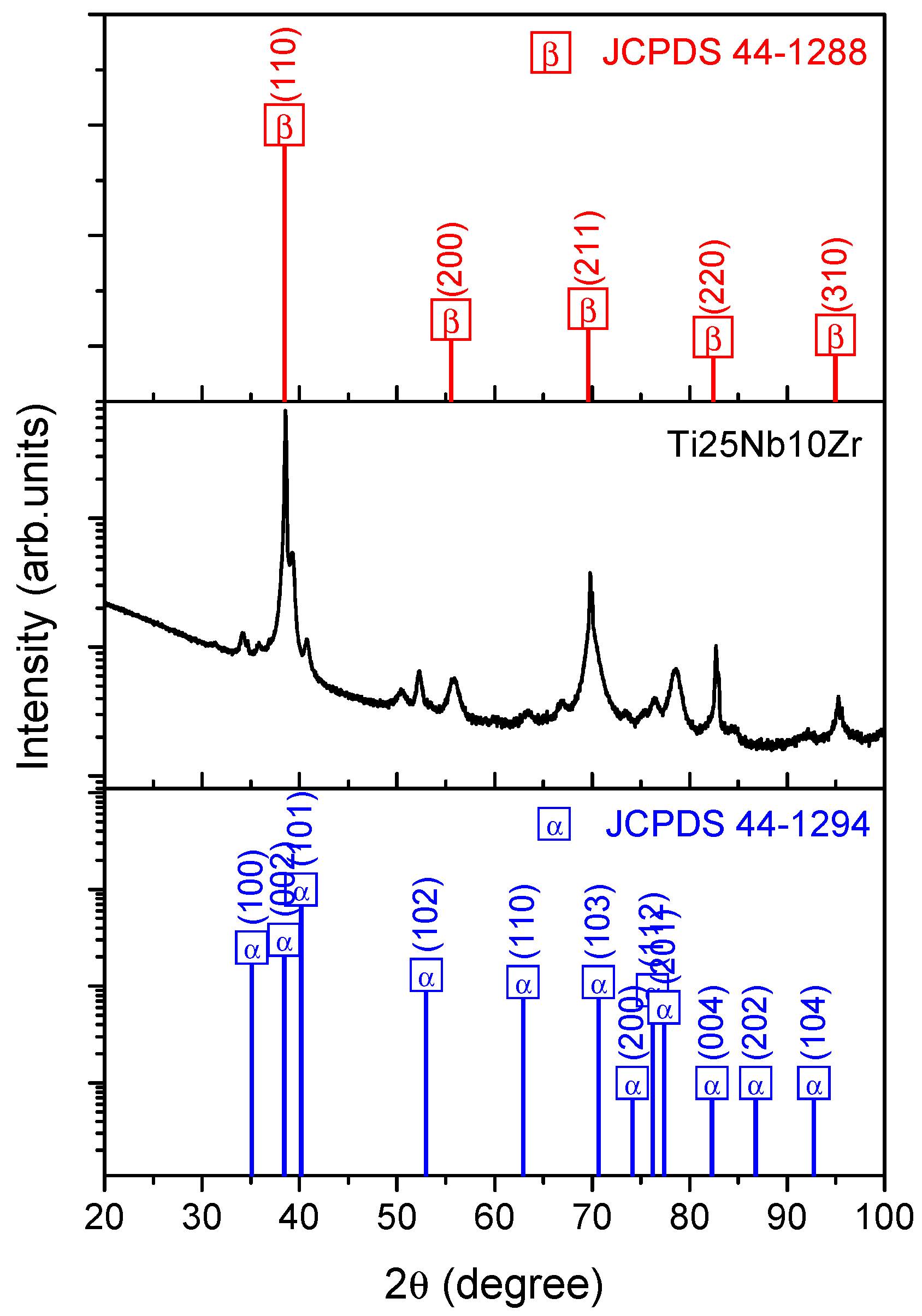
3.1.2. Crystallographic Structure
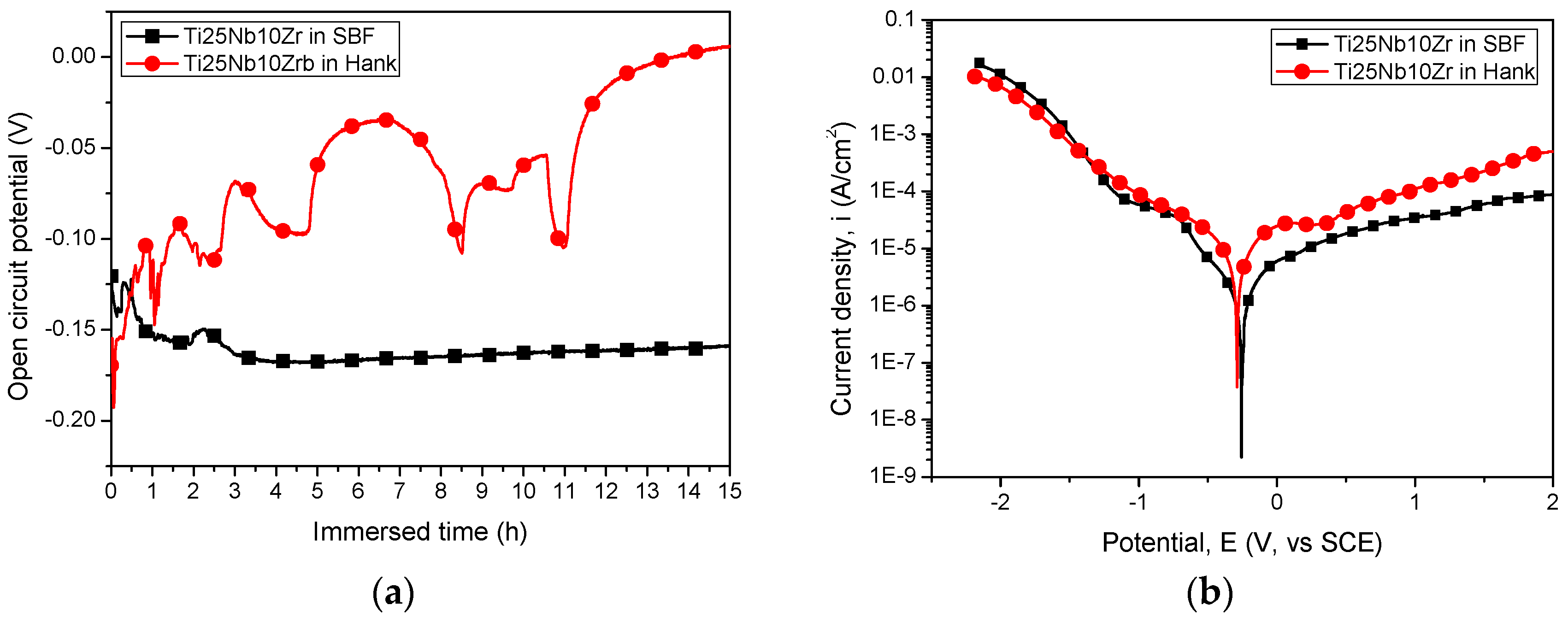
3.1.3. Electrochemical Tests
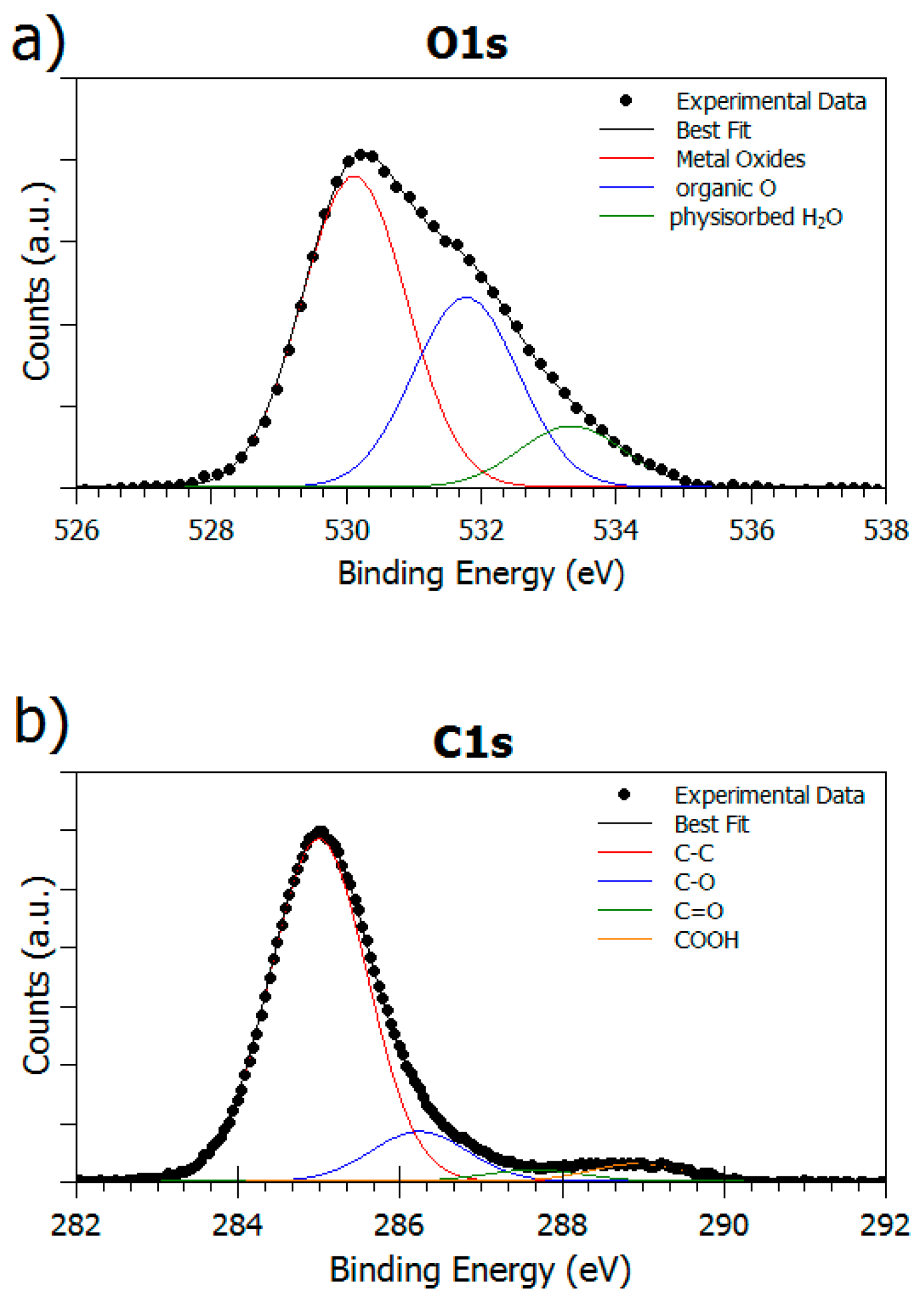
3.1.4. Surface Composition
3.2. Characterization of Ti25Nb10Zr Surfaces Functionalized with EAbuK16
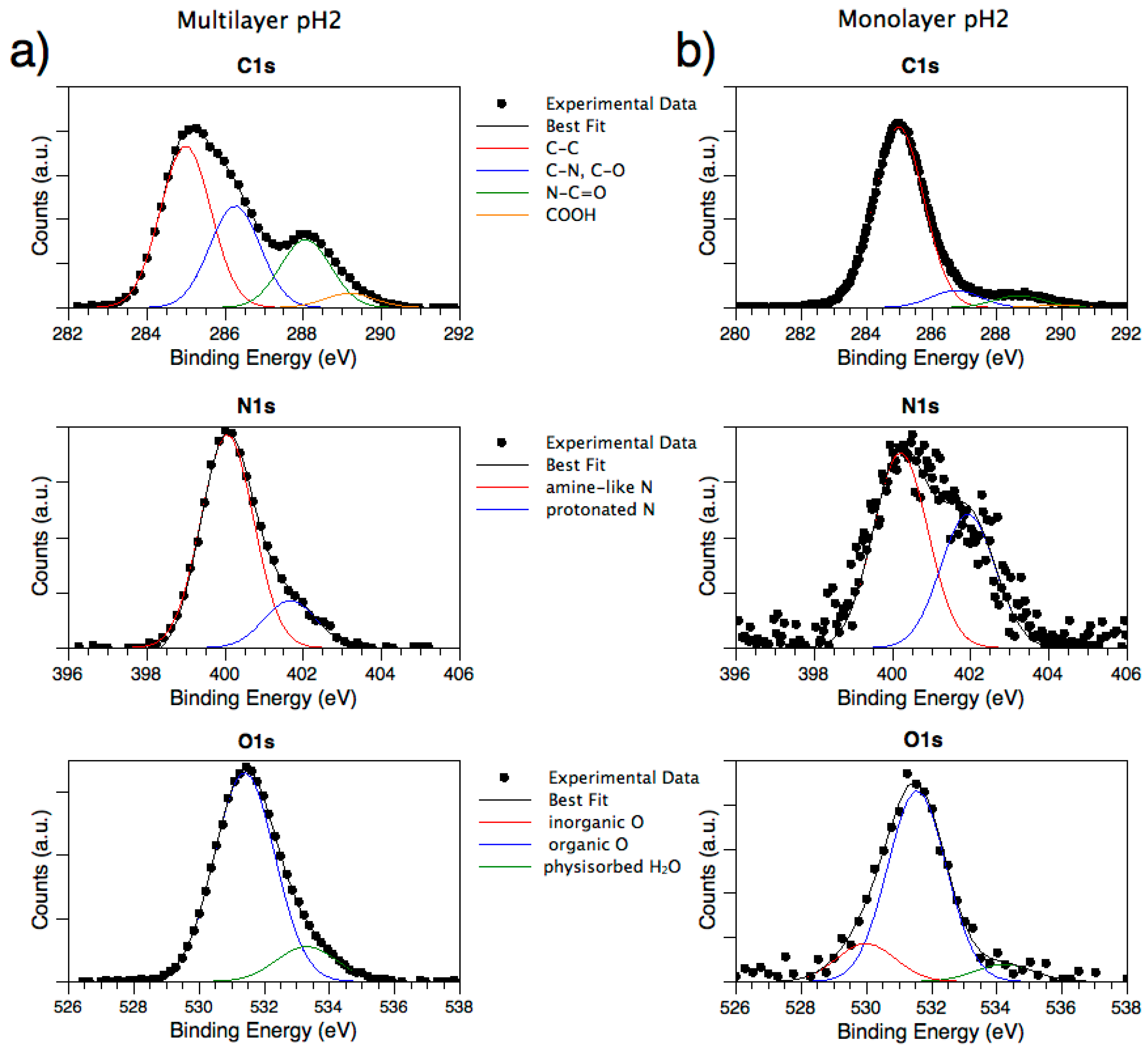
3.2.1. Analysis of SAP Multilayer (MUL) Adsorbed at Different pH Values
3.2.2. Analysis of SAP Monolayer (ML) Adsorbed at Different pH Values
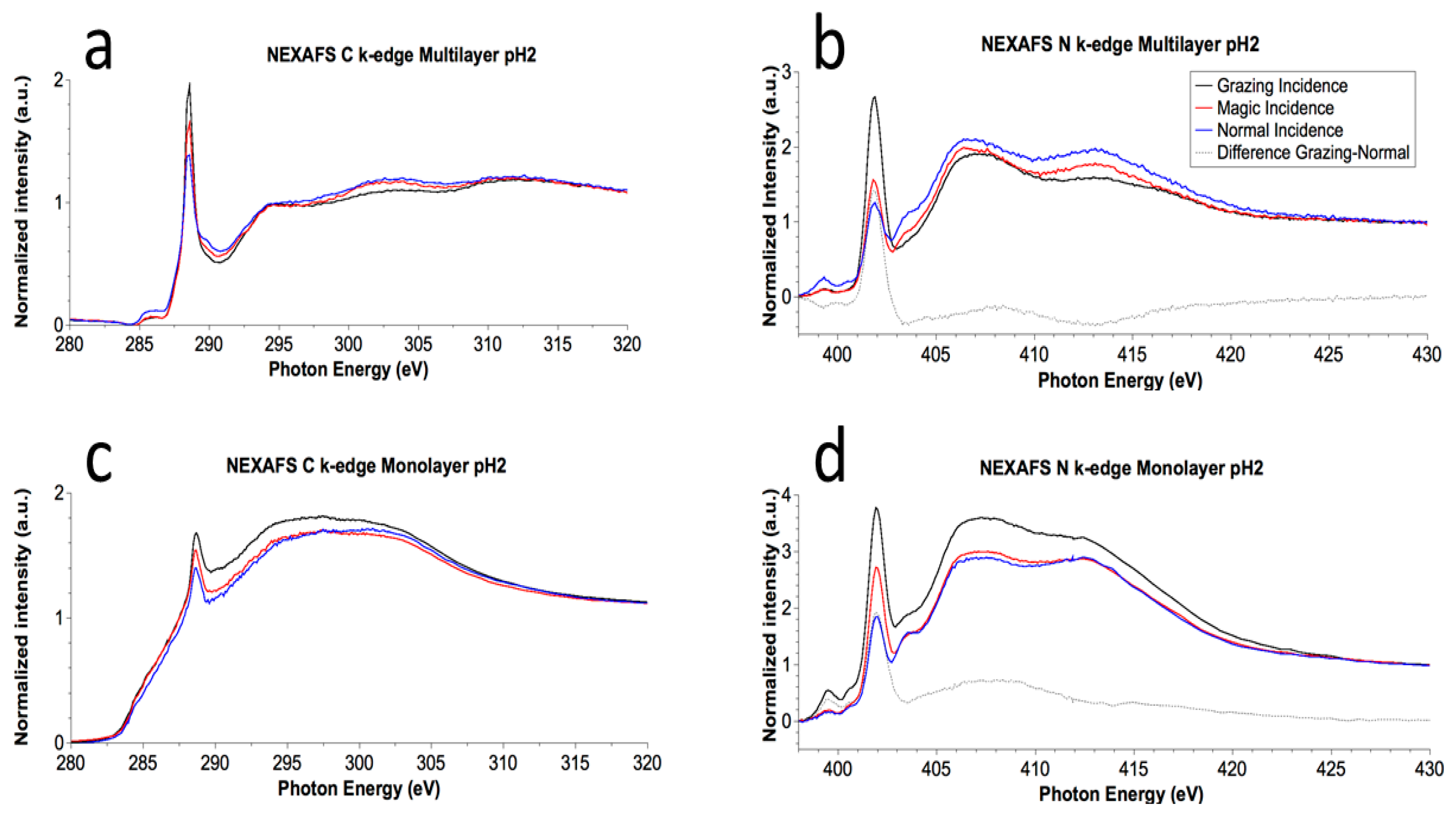
3.2.3. Near Edge X-ray Absorption Fine Structure Spectroscopy
3.3. Microscopy Analysis
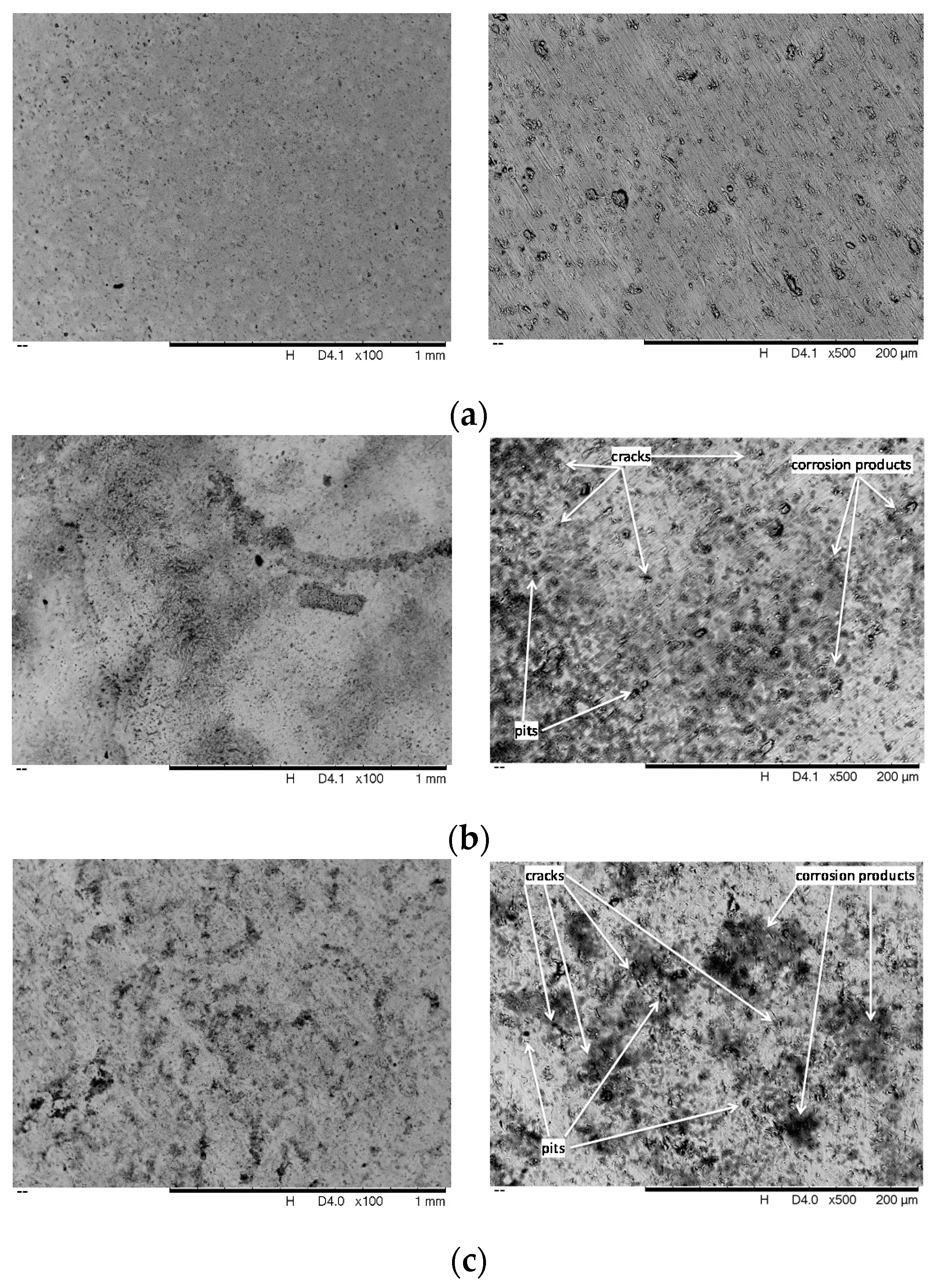
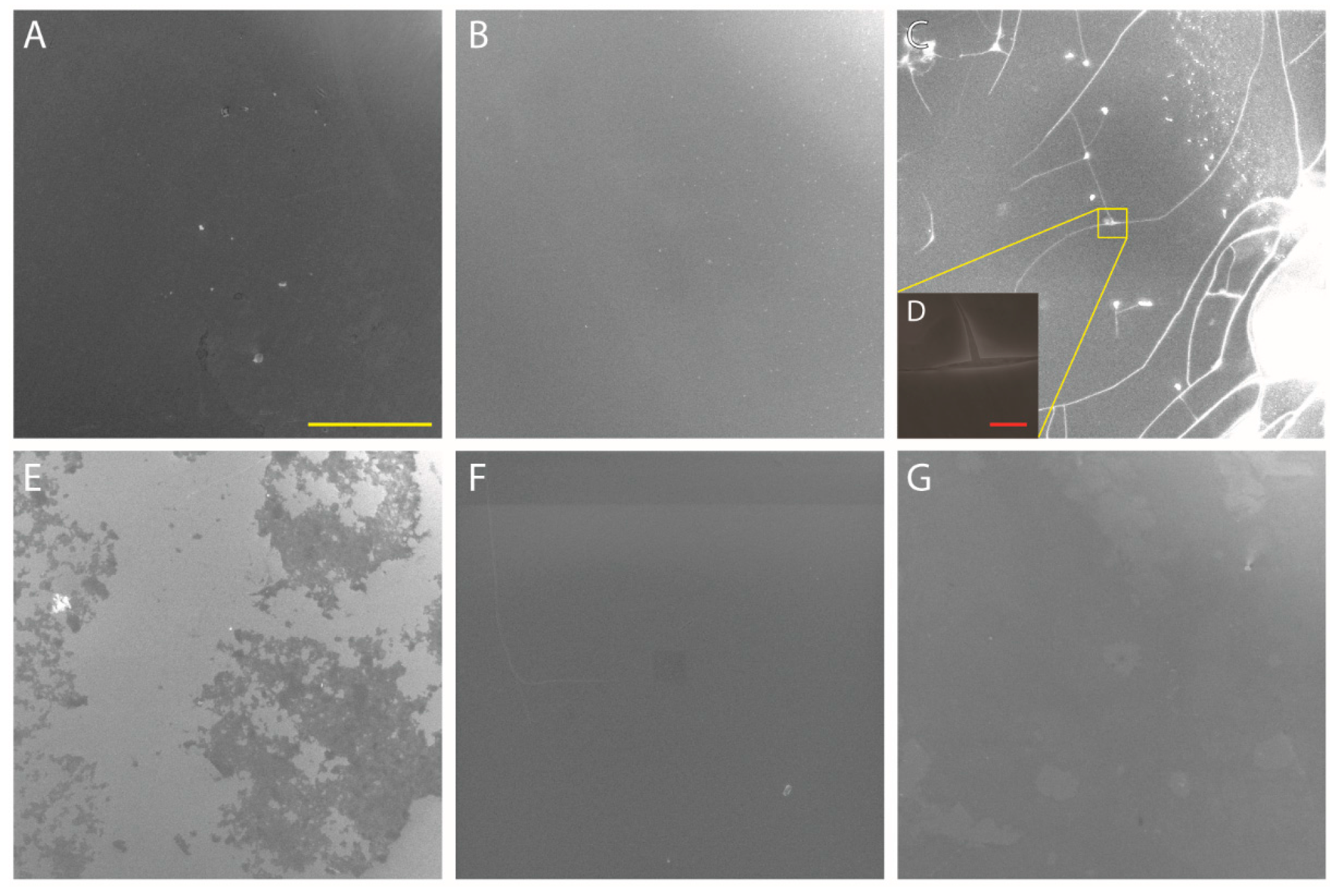
3.3.1. FESEM Analysis
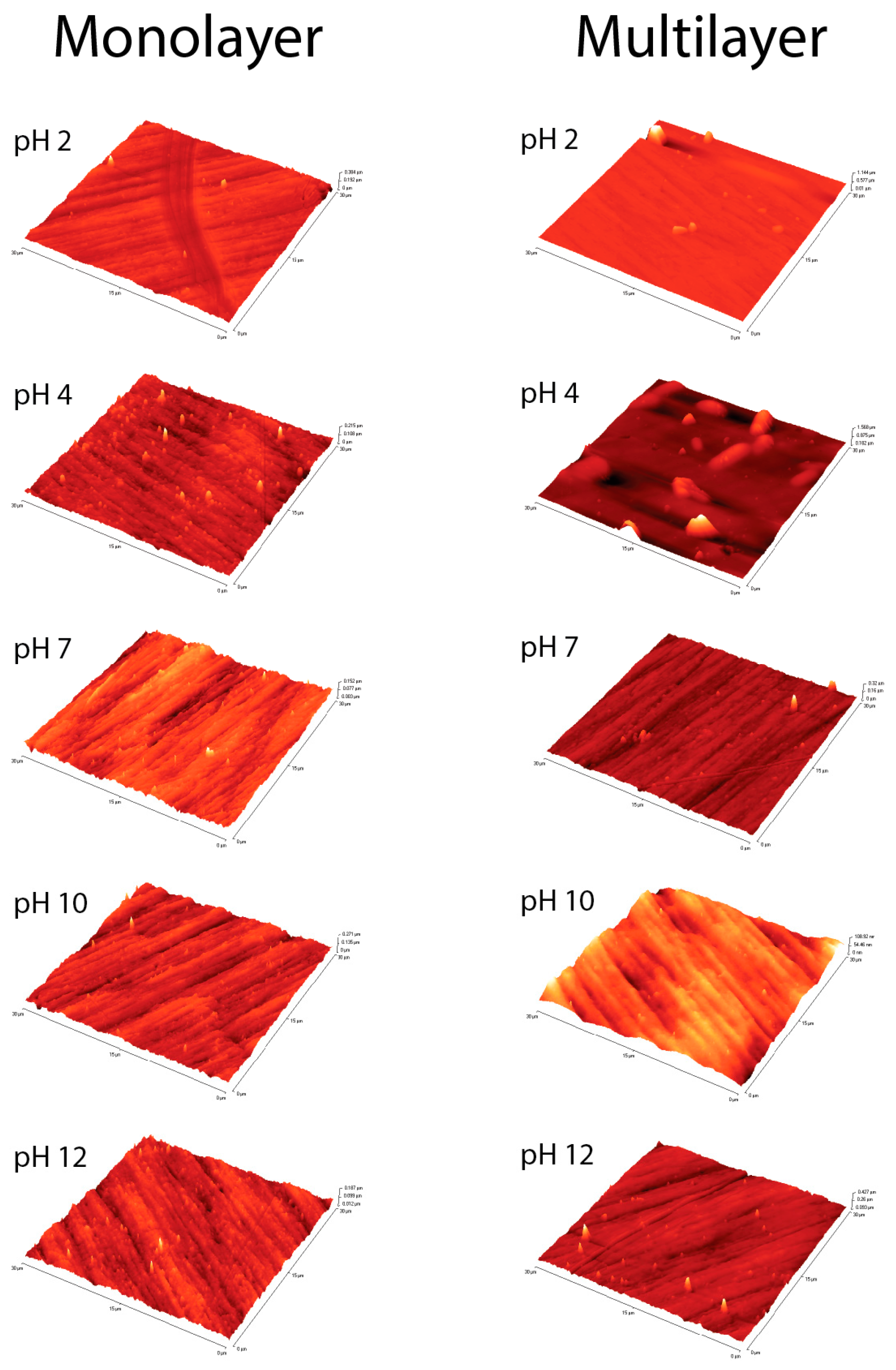
3.3.2. AFM Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Faria, A.C.L.; Rodrigues, R.C.S.; Rosa, A.L.; Ribeiro, R.F. Experimental titanium alloys for dental applications. J. Prosthet. Dent. 2014, 112, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Textor, M.; Sittig, C.; Frauchiger, V.; Tosatti, S.; Brunette, D.M. Properties and biological significance of natural oxide films on titanium and its alloys. In Titanium in Medicine; Springer: Berlin/Heidelberg, Germany, 2001; pp. 171–230. [Google Scholar]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci. Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Dettin, M.; Zamuner, A.; Brun, P.; Castagliuolo, I.; Iucci, G.; Battocchio, C.; Messina, M.; Marletta, G. Covalent grafting of Ti surfaces with peptide hydrogel decorated with growth factors and self-assembling adhesive sequences. J. Pept. Sci. 2014, 20, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C. Review: Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.A.; Lemons, J.E. Medical Applications of Titanium and Its Alloys: The Material and Biological Issues; ASTM: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Katzer, A.; Hockertz, S.; Buchhorn, G.H.; Loehr, J.F. In vitro toxicity and mutagenicity of CoCrMo and Ti6Al wear particles. Toxicology 2003, 190, 145–154. [Google Scholar] [CrossRef]
- Satoh, K.; Sato, S.; Wagatsuma, K. Formation mechanism of toxic-element-free oxide layer on Ti–6Al–4V alloy in d.c. glow discharge plasma with pure oxygen gas. Surf. Coat. Technol. 2016, 302, 82–87. [Google Scholar] [CrossRef]
- Lecocq, M.; Félix, M.S.; Linares, J.-M.; Chaves-Jacob, J.; Decherchi, P.; Dousset, E. Titanium implant impairment and surrounding muscle cell death following neuro-myoelectrostimulation: An in vivo study. J. Biomed. Mater. Res. Part B 2015, 103, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear Characteristics of Metallic Biomaterials: A Review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Mahapatro, A.J. Metals for biomedical applications and devices. J. Biomater. Tissue Eng. 2012, 2, 259–268. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Rao, S.; Asao, S.; Tateishi, T.; Katsuda, S.; Furuki, Y. Effects of Ti, Al and V concentrations on cell viability. Mater. Trans. JIM 1998, 39, 1053–1062. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Attar, H.; Calin, M.; Eckert, J. Review on manufacture by selective laser melting and properties of titanium based materials for biomedical applications. J. Mater. Technol. 2016, 31, 66–76. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-L.; Li, S.-J.; Yang, R. Biomedical titanium alloys and their additive manufacturing. Rare Met. 2016, 35, 661–671. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Qin, J.; Zhang, F.; Zhang, D. Influence of cold deformation on martensite transformation and mechanical properties of Ti–Nb–Ta–Zr alloy. J. Alloys Compd. 2009, 469, 512–518. [Google Scholar] [CrossRef]
- Tane, M.; Hagihara, K.; Ueda, M.; Nakano, T.; Okuda, Y. Elastic-modulus enhancement during room-temperature aging and its suppression in metastable Ti–Nb-Based alloys with low body-centered cubic phase stability. Acta Mater. 2016, 102, 373–384. [Google Scholar] [CrossRef]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti–Ta–Nb–Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y. A New Ti–15Zr–4Nb–4Ta alloy for medical applications. Curr. Opin. Solid State Mater. Sci. 2001, 5, 45–53. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of fatigue strengths of biocompatible Ti-15Zr-4Nb-4Ta alloy and other titanium materials. Mater. Sci. Eng. C 2011, 31, 325–333. [Google Scholar] [CrossRef]
- Banerjee, R.; Nag, S.; Stechschulte, J.; Fraser, H.L. Strengthening mechanisms in Ti–Nb–Zr–Ta and Ti–Mo–Zr–Fe orthopaedic alloys. Biomaterials 2004, 25, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Banerjee, R.; Fraser, H.L. Microstructural evolution and strengthening mechanisms in Ti–Nb–Zr–Ta, Ti–Mo–Zr–Fe and Ti–15Mo biocompatible alloys. Mater. Sci. Eng. C 2005, 25, 357–362. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zu, X.T.; Qiu, S.Y.; Wang, L.; Ma, W.G.; Wei, C.F. Surface characterization and corrosion resistance of Ti–Al–Zr alloy by niobium ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 244, 397–402. [Google Scholar] [CrossRef]
- Chaves, J.M.; Florêncio, O.; Silva, P.S.; Marques, P.W.B.; Afonso, C.R.M. Influence of phase transformations on dynamical elastic modulus and anelasticity of beta Ti–Nb–Fe alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2015, 46, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Kim, H.Y.; Hosoda, H. Development and characterization of Ni-free Ti-base shape memory and superelastic alloys. Mater. Sci. Eng. A 2006, 438–440, 18–24. [Google Scholar] [CrossRef]
- Bai, Y.; Hao, Y.L.; Li, S.J.; Hao, Y.Q.; Yang, R.; Prima, F. Corrosion behavior of biomedical Ti–24Nb–4Zr–8Sn alloy in different simulated body solutions. Mater. Sci. Eng. C 2013, 33, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Okazaki, Y.; Rao, S.; Asao, S.; Tateishi, T. Effects of metallic concentrations other than Ti, Al and V on cell viability. Mater. Trans. JIM 1998, 39, 1070–1079. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.; Xiong, J.; Hodgson, P.; Wen, C. Cytotoxicity of titanium and titanium alloying elements. J. Dent. Res. 2010, 89, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Hickman, J.W.; Gulbransen, E.A. Oxide films formed on titanium, zirconium, and their alloys with nickel, copper, and cobalt. Anal. Chem. 1948, 20, 158–165. [Google Scholar] [CrossRef]
- Yilmazbayhan, A.; Motta, A.T.; Comstock, R.J.; Sabol, G.P.; Laid, B.; Cai, Z. Structure of zirconium alloy oxides formed in pure water studied with synchrotron radiation and optical microscopy: Relation to corrosion rate. J. Nucl. Mater. 2004, 324, 6–22. [Google Scholar] [CrossRef]
- Motta, A.T.; Gomes da Silva, M.J.; Yilmazbayhan, A.; Comstock, R.J.; Cai, Z.; Lai, B. Microstructural characterization of oxides formed on model Zr alloys using synchrotron radiation. J. ASTM Int. 2008, 5, 1–20. [Google Scholar] [CrossRef]
- Wang, B.L.; Zheng, Y.F.; Zhao, L.C. Electrochemical corrosion behavior of biomedical Ti–22Nb and Ti–22Nb–6Zr alloys in saline medium. Mater. Corros. 2009, 60, 788–794. [Google Scholar] [CrossRef]
- Ishii, M.; Kaneko, M.; Oda, T. Titanium and Its Alloys as Key Materials for Corrosion Protection Engineering; Shin-Nittetsu Giho: Tokyo, Japan, 2002; pp. 49–56. [Google Scholar]
- Abdel-Hady, M.; Fuwa, H.; Hinoshita, K.; Kimura, H.; Shinzato, Y.; Morinaga, M. Phase stability change with Zr content in β-type Ti–Nb alloys. Scr. Mater. 2007, 57, 1000–1003. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, H.Y.; Inamura, T.; Hosoda, H.; Miyazaki, S. Shape memory characteristics of Ti–22Nb–(2–8)Zr(at %) biomedical alloys. Mater. Sci. Eng. A 2005, 403, 334–339. [Google Scholar] [CrossRef]
- Wang, B.L.; Li, L.; Zheng, Y.F. In vitro cytotoxicity and hemocompatibility studies of Ti-Nb, Ti-Nb-Zr and Ti-Nb-Hf biomedical shape memory alloys. Biomed. Mater. 2010, 5, 44102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, H.Y.; Inamura, T.; Hosoda, H.; Miyazaki, S. Effect of annealing temperature on microstructure and shape memory characteristics of Ti–22Nb–6Zr (at %) biomedical alloy. Mater. Trans. 2006, 47, 505–512. [Google Scholar] [CrossRef]
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Franchi, S.; Battocchio, C.; Galluzzi, M.; Navisse, E.; Zamuner, A.; Dettin, M.; Iucci, G. Self-assembling peptide hydrogels immobilized on silicon surfaces. Mater. Sci. Eng. C 2016, 69, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Gambaretto, R.; Tonin, L.; Di Bello, C.; Dettin, M. Self-assembling peptides: Correlation among sequence, secondary structure in solution and film formation. Biopolymers 2008, 89, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Iucci, G.; Battocchio, C.; Dettin, M.; Gambaretto, R.; Polzonetti, G. A NEXAFS and XPS study of the adsorption of self-assembling peptides on TiO2: The influence of the side chains. Surf. Interface Anal. 2008, 40, 210–214. [Google Scholar] [CrossRef]
- Battocchio, C.; Iucci, G.; Dettin, M.; Carravetta, V.; Monti, S.; Polzonetti, G. Self-assembling behaviour of self-complementary oligopeptides on biocompatible substrates. Mater. Sci. Eng. B 2010, 169, 36–42. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Gilewicz, A.; Chmielewska, P.; Murzynski, D.; Dobruchowska, E.; Warcholinski, B. Corrosion resistance of CrN and CrCN/CrN coatings deposited using cathodic arc evaporation in Ringer’s and Hank’s solutions. Surf. Coat. Technol. 2016, 299, 7–14. [Google Scholar] [CrossRef]
- Mansfeld, F.; Oldham, K.B. A modification of the Stern—Geary linear polarization equation. Corros. Sci. 1971, 11, 787–796. [Google Scholar] [CrossRef]
- Erika, G.; Ruslan, O.; Florian, S.; Hikmet, S.; Alexander, F. LowDosePES: An End-Station for Low-Dose, Angular-Resolved and Time-Resolved Photoelectron Spectroscopy at BESSY II. In Proceedings of the Scientific Opportunities with Electron Spectroscopy and RIXS, HZB/BESSY II, Berlin, Germany, 16–18 October 2017. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1996. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers, The Scienta ESCA300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Briggs, D.; Seah, M.P. Practical Surface Analysis, Vol. 1, Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]
- Nannarone, S.; Borgatti, F.; De Luisa, A.; Doyle, B.P.; Gazzadi, G.C.; Gigli, A.; Finetti, P.; Mahne, N.; Pasquali, L.; Pedio, M.; et al. The BEAR Beamline at Elettra. AIP Conf. Proc. 2004, 705, 450–453. [Google Scholar]
- Kim, H.Y.; Ikehara, Y.; Kim, J.I.; Hosoda, H.; Miyazaki, S. Martensitic transformation, shape memory effect and superelasticity of Ti–Nb binary alloys. Acta Mater. 2006, 54, 2419–2429. [Google Scholar] [CrossRef]
- Bowen, A.W. Omega phase embrittlement in aged Ti-15%Mo. Scr. Metall. 1971, 5, 709–715. [Google Scholar] [CrossRef]
- Sass, S.L. The ω phase in a Zr-25 at % Ti alloy. Acta Metall. 1969, 17, 813–820. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Ishikawa, Y. Current and Potential Transients during Localized Corrosion of Stainless Steel. J. Electrochem. Soc. 1985, 132, 1288–1293. [Google Scholar] [CrossRef]
- Thirumalaikumarasamy, D.; Shanmugam, K.; Balasubramanian, V. Comparison of the corrosion behaviour of AZ31B magnesium alloy under immersion test and potentiodynamic polarization test in NaCl solution. J. Magnes. Alloys 2014, 2, 36–49. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Parau, A.C.; Gherghilescu, A.I.; Titorencu, I.; Pana, I.; Cojocaru, D.V.; Pruna, V.; Constantin, L.; Dan, I.; Vranceanu, D.M.; et al. Mechanical, in vitro corrosion resistance and biological compatibility of casted and annealed Ti25Nb10Zr alloy. Metals 2017, 7, 86. [Google Scholar] [CrossRef]
- Stohr, J. NEXAFS Spectroscopy; Gomer, C., Ed.; Springer Series in Surface Sciences; Springer: Berlin, Germany, 1991. [Google Scholar]


| Electrolyte | EOC (mV) | Ecorr (mV) | icorr (μA/cm2) | Rp (Ω) | CR (mm/year) |
|---|---|---|---|---|---|
| SBF | −159 | −253 | 1.15 | 45,011 | 0.011 |
| Hank | 6 | −288 | 14.33 | 10,676 | 0.134 |
| MUL Sample | C–N/C–C Ratio | N–C=O/C–C Ratio | COOH/C–C Ratio | Ntot/Ctot Ratio | N/C-SAP Ratio | N+/Ntot (%) Ratio | O–org/Me–Ox Ratio | C-SAP/Ti Ratio | SAP Thickness (nm) |
|---|---|---|---|---|---|---|---|---|---|
| pH 2 | 0.63 | 0.42 | 0.09 | 0.18 | 0.90 | 18.11 | ---- | ---- | ---- |
| pH 4 | 0.57 | 0.37 | 0.05 | 0.14 | 0.77 | 15.57 | ---- | ---- | ---- |
| pH 7 | 0.37 | 0.13 | 0.17 | 0.02 | 0.28 | 0.00 | 1.29 | 4.07 | 1.54 |
| pH 10 | 0.56 | 0.37 | 0.06 | 0.16 | 0.87 | 10.99 | ---- | ---- | ---- |
| pH 12 | 0.48 | 0.27 | 0.10 | 0.11 | 0.76 | 11.33 | 1.75 | 8.17 | 4.31 |
| ML Sample | C–N/C–C Ratio | N–C=O/C–C Ratio | COOH/C–C Ratio | Ntot/Ctot Ratio | N/C-SAP Ratio | N+/Ntot (%) Ratio | O–org /Me–Ox Ratio | C-SAP/Ti Ratio | SAP Thickness (nm) |
|---|---|---|---|---|---|---|---|---|---|
| pH 2 | 0.44 | 0.25 | 0.10 | 0.11 | 0.77 | 13.47 | 24.76 | 2.91 | 1.22 |
| pH 4 | 0.42 | 0.21 | 0.12 | 0.06 | 0.23 | 9.20 | 1.83 | 13.41 | 3.10 |
| pH 7 | 0.34 | 0.12 | 0.10 | 0.04 | 0.58 | 4.13 | 1.04 | 1.76 | 1.28 |
| pH 10 | 0.38 | 0.19 | 0.12 | 0.05 | 0.46 | 0.00 | 1.67 | 4.07 | 2.52 |
| pH 12 | 0.32 | 0.16 | 0.11 | 0.04 | 0.42 | 0.00 | 0.96 | 2.15 | 1.32 |
| Sample | RMS (µm) | |
|---|---|---|
| Clean Surface | 0.0143 | |
| MUL RMS (µm) | ML RMS (µm) | |
| pH 2 | 0.0181 | 0.0446 |
| pH 4 | 0.0136 | 0.1067 |
| pH 7 | 0.0155 | 0.0136 |
| pH 10 | 0.0201 | 0.0141 |
| pH 12 | 0.0127 | 0.0174 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secchi, V.; Franchi, S.; Santi, M.; Vladescu, A.; Braic, M.; Skála, T.; Nováková, J.; Dettin, M.; Zamuner, A.; Iucci, G.; et al. Biocompatible Materials Based on Self-Assembling Peptides on Ti25Nb10Zr Alloy: Molecular Structure and Organization Investigated by Synchrotron Radiation Induced Techniques. Nanomaterials 2018, 8, 148. https://doi.org/10.3390/nano8030148
Secchi V, Franchi S, Santi M, Vladescu A, Braic M, Skála T, Nováková J, Dettin M, Zamuner A, Iucci G, et al. Biocompatible Materials Based on Self-Assembling Peptides on Ti25Nb10Zr Alloy: Molecular Structure and Organization Investigated by Synchrotron Radiation Induced Techniques. Nanomaterials. 2018; 8(3):148. https://doi.org/10.3390/nano8030148
Chicago/Turabian StyleSecchi, Valeria, Stefano Franchi, Marta Santi, Alina Vladescu, Mariana Braic, Tomáš Skála, Jaroslava Nováková, Monica Dettin, Annj Zamuner, Giovanna Iucci, and et al. 2018. "Biocompatible Materials Based on Self-Assembling Peptides on Ti25Nb10Zr Alloy: Molecular Structure and Organization Investigated by Synchrotron Radiation Induced Techniques" Nanomaterials 8, no. 3: 148. https://doi.org/10.3390/nano8030148




.jpg)