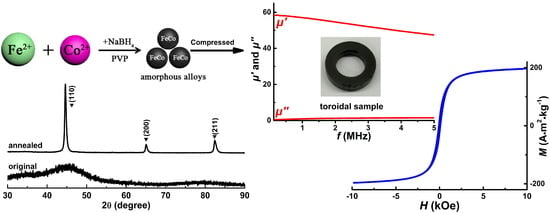
Chemical Synthesis of High-Stable Amorphous FeCo Nanoalloys with Good Magnetic Properties
Abstract
:1. Introduction
2. Experimental
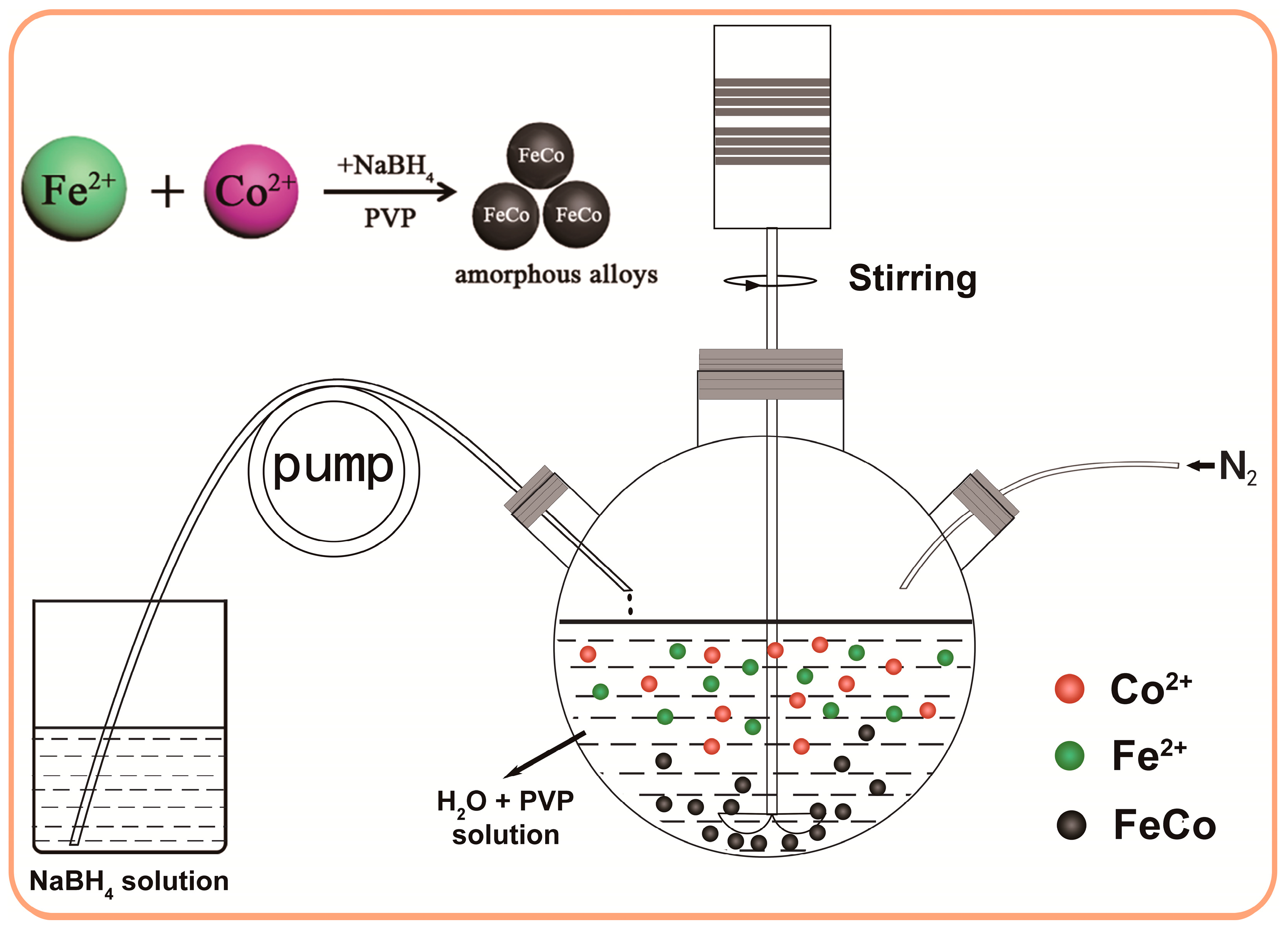
2.1. Synthesis of Amorphous FeCo Nanoalloys
2.2. Materials Characterization
3. Results and Discussion
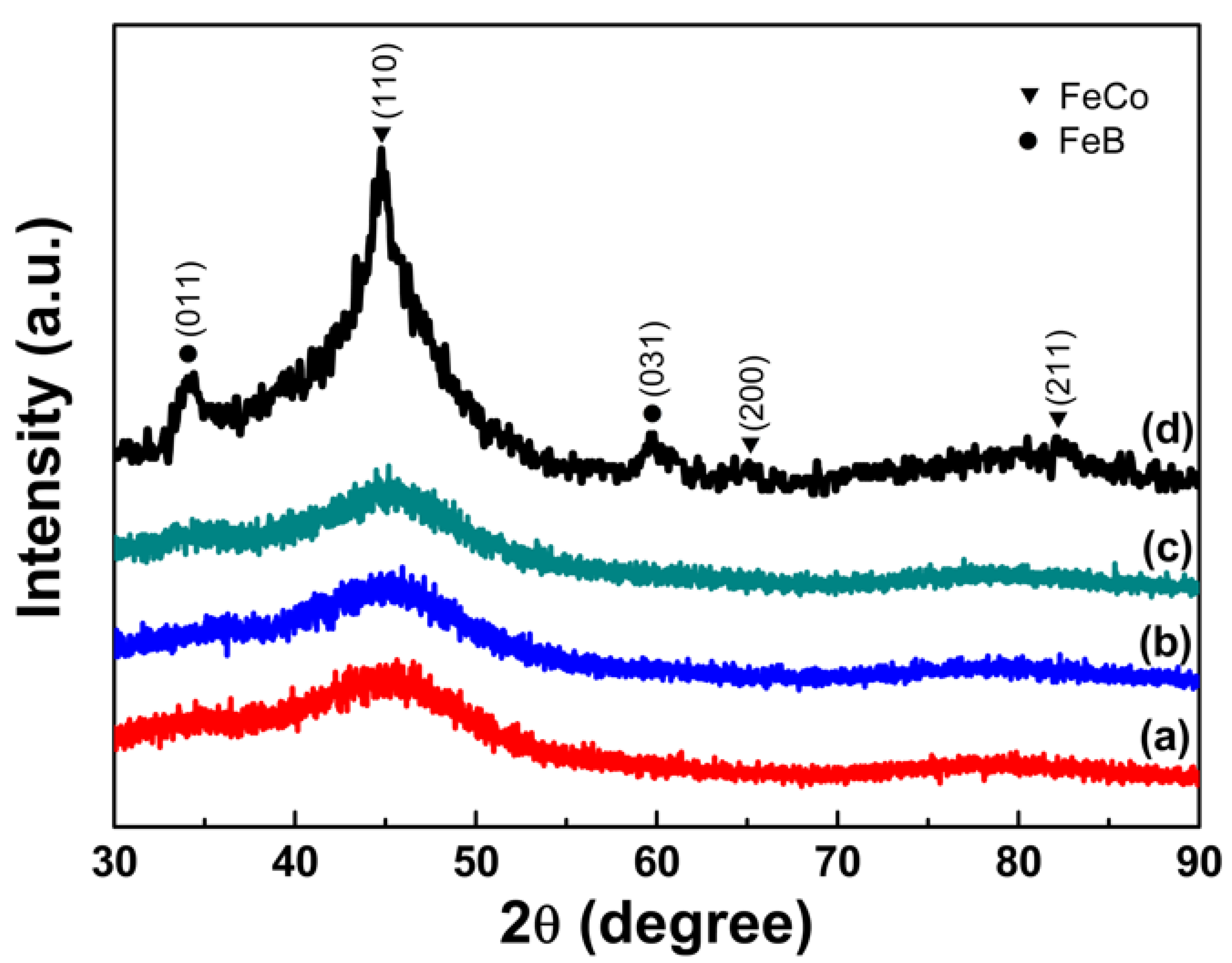
3.1. Structural Analysis
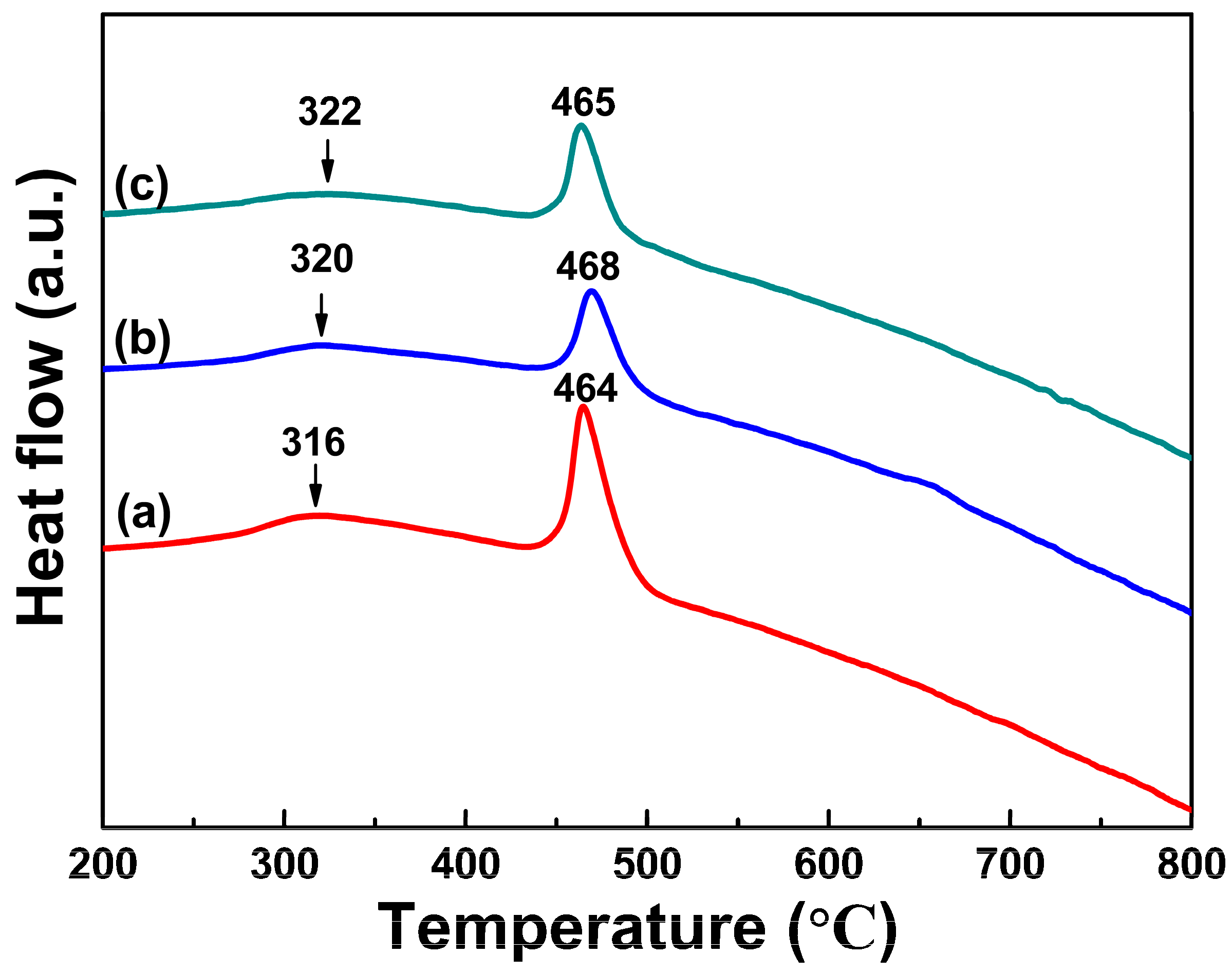
3.2. Crystallization Characteristics
3.3. X-ray Photoelectron Spectroscopy (XPS) Surface Analysis
3.4. Magnetic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kolhatkar, A.G.; Nekrashevich, I.; Litvinov, D.; Willson, R.C.; Lee, T.R. Cubic silica-coated and amine-functionalized FeCo nanoparticles with high saturation magnetization. Chem. Mater. 2013, 25, 1092–1097. [Google Scholar]
- Yang, B.; Li, X.P.; Guo, R.Y.; Yu, R.H. Oxidation fabrication and enhanced soft magnetic properties for core-shell FeCo/CoFe2O4 micron-nano composites. Mater. Des. 2017, 121, 272–279. [Google Scholar]
- Meffre, A.; Mehdaoui, B.; Connord, V.; Carrey, J.; Fazzini, P.F.; Lachaize, S.; Respaud, M.; Chaudret, B. Complex nano-objects displaying both magnetic and catalytic properties: A proof of concept for magnetically induced heterogeneous catalysis. Nano Lett. 2015, 15, 3241–3248. [Google Scholar]
- Martel, S. Magnetic nanoparticles in medical nanorobotics. J. Nanopart. Res. 2015, 17, 75–89. [Google Scholar]
- Bhattacharya, S.; Lass, E.A.; Poon, S.J.; Shiflet, G.J.; Rawlings, M.; Daniil, M.; Willard, M.A. Magnetic properties and thermal stability of (Fe,Co)-Mo-B-P-Si metallic glasses. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Bruna, P.; Stoica, M.; Roth, S.; Eckert, J. Glass forming ability, thermal stability, crystallization and magnetic properties of [(Fe,Co,Ni)0.75Si0.05B0.20]95Nb4Zr1 metallic glasses. J. Non-Cryst. Solids 2013, 367, 30–36. [Google Scholar]
- Liu, L.; Zhu, J.B.; Hou, C.; Li, J.C.; Jiang, Q. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering. Mater. Des. 2013, 46, 675–679. [Google Scholar]
- Han, Y.M.; Wang, Z.; Che, X.H.; Chen, X.G.; Li, W.R.; Li, Y.L. Influence of Co content on the structure and magnetic permeability of nanocrystalline (Fe1−xCox)73.5Cu1Nb3Si13.5B9 alloys. Mater. Sci. Eng. B 2009, 156, 57–61. [Google Scholar]
- Inoue, A.; Shen, B.L.; Chang, C.T. Super-high strength of over 4000 MPa for Fe-based bulk glassy alloys in [(Fe1−xCox)0.75B0.2Si0.05]96Nb4 system. Acta Mater. 2004, 52, 4093–4099. [Google Scholar]
- Han, M.; Qin, J.; Deng, L. Microwave permeability of nanocrystalline alloys (Fe0.67Co0.33)78Nb6B15Cu1 and (Fe0.67Co0.33)78Nb6Al6B9Cu1. J. Alloys Compd. 2012, 543, 79–83. [Google Scholar]
- Han, Y.; Wang, Z. Excellent high-temperature magnetic softness in a wide temperature for FeCo-based nanocrystalline alloy. J. Non-Cryst. Solids 2016, 434, 92–95. [Google Scholar]
- Platt, C.L.; Minor, M.K.; Klemmer, T.J. Magnetic and structural properties of FeCoB thin films. IEEE Trans. Magn. 2001, 7, 2302–2304. [Google Scholar]
- Ogawa, H.; Miura, H. Compositional dependence of amorphization of M–C–Si (M=Fe, Co or Ni) materials by mechanical alloying. J. Mater. Process. Technol. 2003, 143, 256–260. [Google Scholar]
- Guo, W.; Zhang, J.; Wu, Y.; Hong, S.; Qin, Y. Fabrication and characterization of Fe-based amorphous coatings prepared by high-velocity arc spraying. Mater. Des. 2015, 78, 118–124. [Google Scholar]
- Li, X.; Chen, J.; Zhang, K. High temperature deformation behavior of amorphous Fe78Si9B13/nano-Ni laminated composite. Mater. Des. 2009, 30, 2665–2669. [Google Scholar]
- Corrias, A.; Ennas, G.; Musinu, A.; Marongiu, G.; Paschina, G. Amorphous transition metal-boron ultrafine particles prepared by chemical methods. Chem. Mater. 1993, 5, 1722–1726. [Google Scholar]
- Li, F.S.; Zhang, T.; Inoue, A.; Guan, S.K.; Shen, N.F. (Fe,Co)–Zr–Nd–B bulk amorphous alloys with good soft magnetic properties. Intermetallics 2004, 12, 1139–1142. [Google Scholar]
- Jian, H.; Luo, W.; Tao, S.; Yan, M. Mechanical and magnetic properties of (Fe72Mo4B24)100−xTbx, (x = 4, 5, 6, 7 at. %) bulk glassy alloys. J. Alloys Compd. 2010, 505, 315–318. [Google Scholar]
- Taghvaei, A.H.; Bednarčik, J.; Eckert, J. Atomic structure and thermal behavior of (Co0.65Fe0.35)72Ta8B20 metallic glass with excellent soft magnetic properties. Intermetallics 2016, 69, 21–27. [Google Scholar]
- Yang, B.; Yang, X.Y.; Li, X.P.; Cao, Y.; Yu, R.H. Surface modification and enhanced performance of chemically synthesized nanosized amorphous Fe particles. J. Supercond. Nov. Magn. 2015, 28, 2177–2182. [Google Scholar]
- Yang, B.; Cao, Y.; Zhang, L.; Li, R.F.; Yang, X.Y.; Yu, R.H. Controlled chemical synthesis and enhanced performance of micron-sized FeCo particles. J. Alloys Compd. 2014, 615, 322–326. [Google Scholar]
- Klencsár, Z.; Németh, P.; Sándor, Z.; Horváth, T.; Sajó, I.E.; Mészáros, S.; Mantilla, J.; Coaquira, J.A.H.; Garg, V.K.; Kuzmann, E.; et al. Structure and magnetism of Fe–Co alloy nanoparticles. J. Alloys Compd. 2016, 674, 153–161. [Google Scholar]
- Li, X.P.; Yang, B.; Yang, X.Y.; Cao, Y.; Yu, R.H. Structural formation and improved performances of chemically synthesized composition-controlled micron-sized Fe100−xCox particles. J. Supercond. Nov. Magn. 2016, 29, 417–422. [Google Scholar]
- Liaw, B.J.; Chiang, S.J.; Tsai, C.H.; Chen, Y.Z. Preparation and catalysis of polymer-stabilized NiB catalysts on hydrogenation of carbonyl and olefinic groups. Appl. Catal. A Gen. 2005, 284, 239–246. [Google Scholar]
- Fuller, R.O.; Goh, B.M.; Koutsantonis, G.A.; Loedolff, M.J.; Saunders, M.; Woodward, R.C. A simple procedure for the production of large ferromagnetic cobalt nanoparticles. Dalton Trans. 2016, 45, 11983–11989. [Google Scholar]
- Joseyphus, R.J.; Shinoda, K.; Kodama, D.; Jeyadevan, B. Size controlled Fe nanoparticles through polyol process and their magnetic properties. Mater. Chem. Phys. 2010, 123, 487–493. [Google Scholar]
- Arun, T.; Prakash, K.; Joseyphus, R.J. Synthesis and magnetic properties of Prussian blue modified Fe nanoparticles. J. Magn. Magn. Mater. 2013, 345, 100–105. [Google Scholar]
- Zhang, S.; Jiang, G.M.; Filsinger, G.T.; Wu, L.H.; Zhu, H.Y.; Lee, J.H.; Wu, Z.B.; Sun, S.H. Halide ion-mediated growth of single crystalline Fe nanoparticles. Nanoscale 2014, 6, 4852–4856. [Google Scholar]
- Wang, C.; Peng, S.; Lacroix, L.M.; Sun, S.H. Synthesis of high magnetic moment CoFe nanoparticles via interfacial diffusion in core/shell structured Co/Fe nanoparticles. Nano Res. 2009, 2, 380–385. [Google Scholar]
- Zheng, Z.; Zhao, G.; Xu, L.; Wang, L.; Yan, B. Influence of Ni addition on nanocrystallization kinetics of FeCo-based amorphous alloys. J. Non-Cryst. Solids 2016, 434, 23–27. [Google Scholar]
- Kai, W.; Wu, Y.H.; Chen, W.S.; Tsay, L.W.; Jia, H.L.; Liaw, P.K. Air-oxidation behavior of a [(Fe50Co50)75B20Si5]96Nb4 bulk metallic glass at 500–650 °C. Corros. Sci. 2013, 66, 26–32. [Google Scholar]
- Song, Y.J.; Modrow, H.; Henry, L.L.; Saw, C.K.; Doomes, E.E.; Palshin, V.; Hormes, J.; Kumar, C.S.S.R. Microfluidic synthesis of cobalt nanoparticles. Chem. Mater. 2006, 18, 2817–2827. [Google Scholar]
- Zhang, Z.D.; Wang, H.M.; Qin, C.; Chen, S.H.; Ji, X.J.; Sun, K.; Chen, M.; Fan, R.H.; Han, X. Fabrication and magnetic properties of electrospun cobalt nanofibers. Mater. Des. 2016, 89, 543–548. [Google Scholar]
- Ram, S. Allotropic phase transformations in HCP, FCC and BCC metastable structures in Co-nanoparticles. Mater. Sci. Eng. A 2001, 304, 923–927. [Google Scholar]
- Yang, X.Y.; Yang, B.; Li, X.P.; Cao, Y.; Yu, R.H. Structural-controlled chemical synthesis of nanosized amorphous Fe particles and their improved performances. J. Alloys Compd. 2015, 651, 551–556. [Google Scholar]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar]
- Sheu, H.H.; Lu, C.E.; Lee, H.B.; Pu, N.W.; Wu, P.F.; Hsieh, S.H.; Ger, M.D. Electrodeposition of black chromium-cobalt alloy based on trivalent sulfate electrolyte. J. Taiwan Inst. Chem. Eng. 2015, 59, 496–505. [Google Scholar]
- Li, J.; Lu, G.; Wu, G.; Mao, D.; Guo, Y.; Wang, Y.; Guo, Y. Effect of TiO2 crystal structure on the catalytic performance of Co3O4/TiO2 catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2014, 4, 1268–1275. [Google Scholar]
- Shen, W.; Mu, Y.; Xiao, T.; Ai, Z. Magnetic Fe3O4-FeB nanocomposites with promoted Cr (VI) removal performance. Chem. Eng. J. 2016, 285, 57–68. [Google Scholar]
- Shen, X.C.; Dai, M.; Gao, M.; Zhao, B.; Ding, W. Solvent effects in the synthesis of CoB catalysts on hydrogen generation from hydrolysis of sodium borohydride. Chin. J. Catal. 2013, 34, 979–985. [Google Scholar]
- Meng, Y.D.; Chen, D.R.; Jiao, X.L. Synthesis and characterization of CoFe2O4 hollow spheres. Eur. J. Inorg. Chem. 2008, 25, 4019–4023. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar]
- Ahmed, J.; Kumar, B.; Mugweru, A.M.; Trinh, P.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Binary Fe–Co alloy nanoparticles showing significant enhancement in electrocatalytic activity compared with bulk alloys. J. Phys. Chem. C 2010, 114, 18779–18784. [Google Scholar]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar]
- Costa, T.M.H.; Gallas, M.R.; Benvenutti, E.V.; Da-Jornada, J.A.H. Study of nanocrystalline γ-Al2O3 produced by high-pressure compaction. J. Phys. Chem. B 1999, 103, 4278–4284. [Google Scholar]
- Inoue, A.; Takeuchi, A.; Shen, B.L. Formation and functional properties of Fe-based bulk glassy alloys. Mater. Trans. 2001, 42, 970–978. [Google Scholar]
- Inoue, A.; Zhang, T.; Koshiba, H.; Makino, A. New bulk amorphous Fe–(Co,Ni)–M–B (M = Zr, Hf, Nb, Ta, Mo, W) alloys with good soft magnetic properties. J. Appl. Phys. 1998, 83, 6326–6328. [Google Scholar]
- Redjdal, N.; Salah, H.; Hauet, T.; Menari, H.; Chérif, S.M.; Gabouze, N.; Azzaz, M. Microstructural, electrical and magnetic properties of Fe35Co65 thin films grown by thermal evaporation from mechanically alloyed powders. Thin Solid Films 2014, 552, 164–169. [Google Scholar]



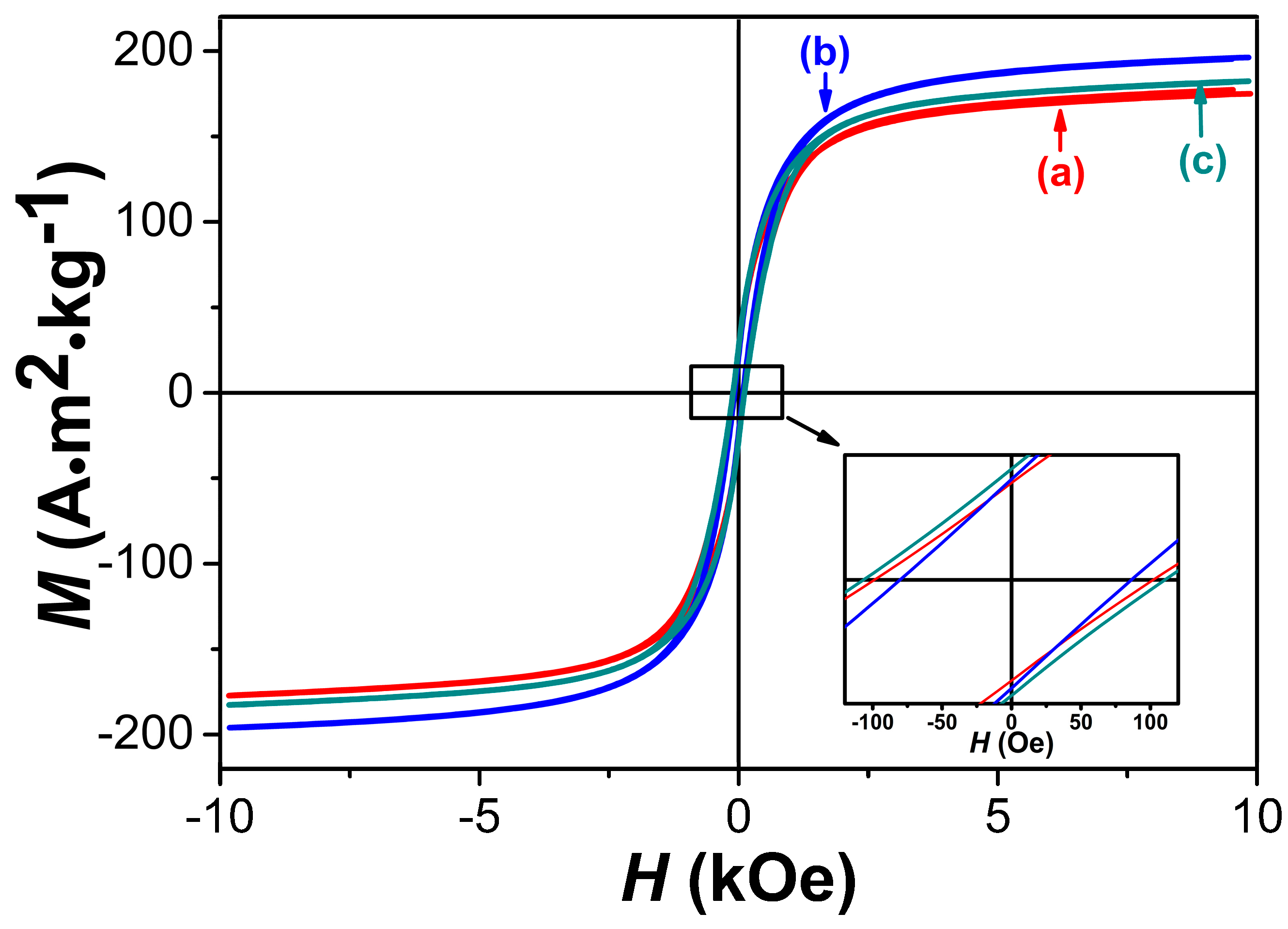
| Samples Prepared with Different PVP Addition (g) | The Fe/Co/O Ratio (at. %) Measured by EDS | The Fe/Co/B Ratios (at. %) Detected by ICP-OES | Average Particle Sizes (nm) | Ms (A·m2/kg) | iHc (Oe) |
|---|---|---|---|---|---|
| (a) 1 g | 60.01:32.31:7.68 | 59.84:31.60:8.56 | 20 | 175.0 | 100.4 |
| (b) 1.5 g | 60.78:33.46:5.76 | 62.77:30.11:7.12 | 16 | 196.2 | 83.3 |
| (d) 2 g | 62.12:30.98:6.90 | 59.67:32.25:7.98 | 25 | 182.3 | 108.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Wu, Y.; Li, X.; Yu, R. Chemical Synthesis of High-Stable Amorphous FeCo Nanoalloys with Good Magnetic Properties. Nanomaterials 2018, 8, 154. https://doi.org/10.3390/nano8030154
Yang B, Wu Y, Li X, Yu R. Chemical Synthesis of High-Stable Amorphous FeCo Nanoalloys with Good Magnetic Properties. Nanomaterials. 2018; 8(3):154. https://doi.org/10.3390/nano8030154
Chicago/Turabian StyleYang, Bai, Yue Wu, Xiaopan Li, and Ronghai Yu. 2018. "Chemical Synthesis of High-Stable Amorphous FeCo Nanoalloys with Good Magnetic Properties" Nanomaterials 8, no. 3: 154. https://doi.org/10.3390/nano8030154